 Found 5118 hits with Last Name = 'fernandes' and Initial = 's'
Found 5118 hits with Last Name = 'fernandes' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50321606
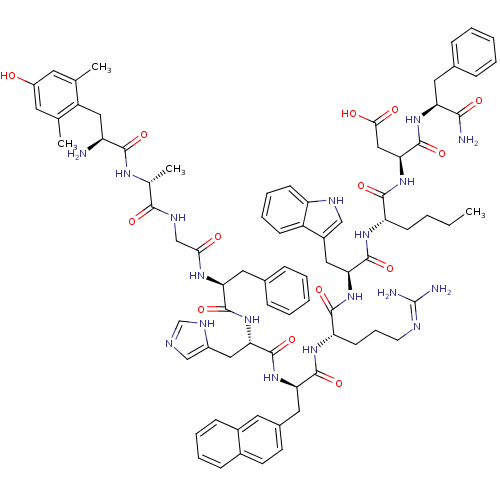
((3S,6S,9S,12S,15R,18S,21S,27R,30S)-18-((1H-imidazo...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,wU:8.20,58.71,93.99,78.84,wD:73.79,48.59,22.31,33.48,4.4,101.107,(24.59,-5.48,;25.93,-4.72,;25.93,-3.18,;24.59,-2.4,;24.59,-.86,;23.26,-.1,;21.93,-.87,;21.93,-2.41,;20.59,-.11,;20.59,1.44,;22,2.07,;23.25,1.19,;24.48,2.12,;23.98,3.58,;24.73,4.92,;23.94,6.24,;22.4,6.22,;21.65,4.87,;22.44,3.55,;19.25,-.87,;17.91,-.12,;17.91,1.43,;16.57,-.89,;16.57,-2.43,;17.91,-3.2,;17.91,-4.73,;19.24,-5.51,;19.24,-7.05,;17.93,-7.8,;20.61,-7.81,;15.25,-.1,;13.92,-.87,;13.92,-2.41,;12.59,-.1,;12.6,1.44,;13.92,2.21,;15.26,1.44,;16.59,2.21,;16.6,3.75,;17.93,4.53,;17.92,6.07,;16.58,6.84,;15.25,6.06,;15.26,4.52,;13.92,3.75,;11.26,-.86,;9.92,-.1,;9.92,1.45,;8.58,-.87,;8.58,-2.41,;9.92,-3.18,;11.32,-2.55,;12.36,-3.69,;11.59,-5.03,;10.08,-4.71,;7.25,-.09,;5.91,-.85,;5.91,-2.39,;4.58,-.08,;4.58,1.46,;5.91,2.23,;7.25,1.47,;8.58,2.24,;8.58,3.78,;7.24,4.55,;5.91,3.78,;3.25,-.85,;1.92,-.07,;1.92,1.47,;.58,-.85,;-.75,-.07,;-2.08,-.85,;-2.08,-2.38,;-3.41,-.07,;-3.41,1.47,;-4.75,-.84,;-6.09,-.08,;-6.09,1.47,;-7.42,-.86,;-7.4,-2.4,;-8.75,-.1,;-10.08,-.88,;-10.06,-2.42,;-8.72,-3.17,;-11.4,-3.2,;-12.73,-2.44,;-14.07,-3.22,;-12.76,-.9,;-11.41,-.13,;-11.43,1.41,;25.93,-.09,;25.93,1.45,;27.26,-.87,;28.59,-.09,;28.59,1.45,;29.93,2.21,;31.25,1.45,;29.93,3.76,;29.93,-.87,;29.93,-2.4,;31.26,-.1,;32.6,-.87,;32.6,-2.4,;33.93,-3.17,;35.28,-2.4,;36.61,-3.17,;36.61,-4.71,;35.27,-5.48,;33.94,-4.71,;33.93,-.1,;35.27,-.86,;33.93,1.45,)| Show InChI InChI=1S/C80H98N18O14/c1-5-6-25-60(73(106)98-67(40-69(101)102)79(112)94-62(70(82)103)34-48-18-9-7-10-19-48)92-77(110)65(37-53-41-87-59-26-16-15-24-56(53)59)96-74(107)61(27-17-30-86-80(83)84)93-76(109)64(36-50-28-29-51-22-13-14-23-52(51)33-50)95-78(111)66(38-54-42-85-44-89-54)97-75(108)63(35-49-20-11-8-12-21-49)91-68(100)43-88-71(104)47(4)90-72(105)58(81)39-57-45(2)31-55(99)32-46(57)3/h7-16,18-24,26,28-29,31-33,41-42,44,47,58,60-67,87,99H,5-6,17,25,27,30,34-40,43,81H2,1-4H3,(H2,82,103)(H,85,89)(H,88,104)(H,90,105)(H,91,100)(H,92,110)(H,93,109)(H,94,112)(H,95,111)(H,96,107)(H,97,108)(H,98,106)(H,101,102)(H4,83,84,86)/t47-,58+,60+,61+,62+,63+,64-,65+,66+,67+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50321606

((3S,6S,9S,12S,15R,18S,21S,27R,30S)-18-((1H-imidazo...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,wU:8.20,58.71,93.99,78.84,wD:73.79,48.59,22.31,33.48,4.4,101.107,(24.59,-5.48,;25.93,-4.72,;25.93,-3.18,;24.59,-2.4,;24.59,-.86,;23.26,-.1,;21.93,-.87,;21.93,-2.41,;20.59,-.11,;20.59,1.44,;22,2.07,;23.25,1.19,;24.48,2.12,;23.98,3.58,;24.73,4.92,;23.94,6.24,;22.4,6.22,;21.65,4.87,;22.44,3.55,;19.25,-.87,;17.91,-.12,;17.91,1.43,;16.57,-.89,;16.57,-2.43,;17.91,-3.2,;17.91,-4.73,;19.24,-5.51,;19.24,-7.05,;17.93,-7.8,;20.61,-7.81,;15.25,-.1,;13.92,-.87,;13.92,-2.41,;12.59,-.1,;12.6,1.44,;13.92,2.21,;15.26,1.44,;16.59,2.21,;16.6,3.75,;17.93,4.53,;17.92,6.07,;16.58,6.84,;15.25,6.06,;15.26,4.52,;13.92,3.75,;11.26,-.86,;9.92,-.1,;9.92,1.45,;8.58,-.87,;8.58,-2.41,;9.92,-3.18,;11.32,-2.55,;12.36,-3.69,;11.59,-5.03,;10.08,-4.71,;7.25,-.09,;5.91,-.85,;5.91,-2.39,;4.58,-.08,;4.58,1.46,;5.91,2.23,;7.25,1.47,;8.58,2.24,;8.58,3.78,;7.24,4.55,;5.91,3.78,;3.25,-.85,;1.92,-.07,;1.92,1.47,;.58,-.85,;-.75,-.07,;-2.08,-.85,;-2.08,-2.38,;-3.41,-.07,;-3.41,1.47,;-4.75,-.84,;-6.09,-.08,;-6.09,1.47,;-7.42,-.86,;-7.4,-2.4,;-8.75,-.1,;-10.08,-.88,;-10.06,-2.42,;-8.72,-3.17,;-11.4,-3.2,;-12.73,-2.44,;-14.07,-3.22,;-12.76,-.9,;-11.41,-.13,;-11.43,1.41,;25.93,-.09,;25.93,1.45,;27.26,-.87,;28.59,-.09,;28.59,1.45,;29.93,2.21,;31.25,1.45,;29.93,3.76,;29.93,-.87,;29.93,-2.4,;31.26,-.1,;32.6,-.87,;32.6,-2.4,;33.93,-3.17,;35.28,-2.4,;36.61,-3.17,;36.61,-4.71,;35.27,-5.48,;33.94,-4.71,;33.93,-.1,;35.27,-.86,;33.93,1.45,)| Show InChI InChI=1S/C80H98N18O14/c1-5-6-25-60(73(106)98-67(40-69(101)102)79(112)94-62(70(82)103)34-48-18-9-7-10-19-48)92-77(110)65(37-53-41-87-59-26-16-15-24-56(53)59)96-74(107)61(27-17-30-86-80(83)84)93-76(109)64(36-50-28-29-51-22-13-14-23-52(51)33-50)95-78(111)66(38-54-42-85-44-89-54)97-75(108)63(35-49-20-11-8-12-21-49)91-68(100)43-88-71(104)47(4)90-72(105)58(81)39-57-45(2)31-55(99)32-46(57)3/h7-16,18-24,26,28-29,31-33,41-42,44,47,58,60-67,87,99H,5-6,17,25,27,30,34-40,43,81H2,1-4H3,(H2,82,103)(H,85,89)(H,88,104)(H,90,105)(H,91,100)(H,92,110)(H,93,109)(H,94,112)(H,95,111)(H,96,107)(H,97,108)(H,98,106)(H,101,102)(H4,83,84,86)/t47-,58+,60+,61+,62+,63+,64-,65+,66+,67+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50610174
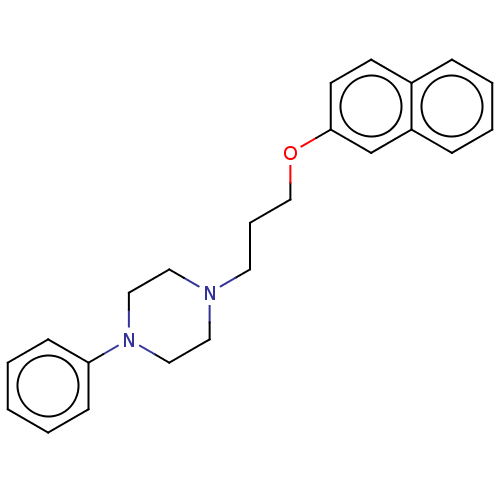
(CHEMBL4293814) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50321607
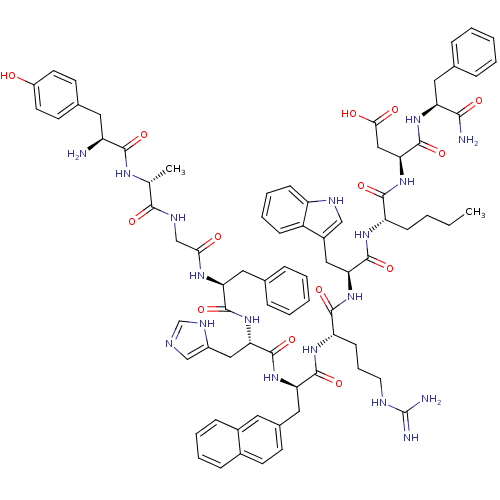
((3S,6S,9S,12S,15R,18S,21S,27R,30S)-18-((1H-imidazo...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C78H94N18O14/c1-3-4-23-58(71(104)96-65(40-67(99)100)77(110)92-60(68(80)101)35-46-16-7-5-8-17-46)90-75(108)63(38-52-41-85-57-24-14-13-22-55(52)57)94-72(105)59(25-15-32-84-78(81)82)91-74(107)62(37-49-26-29-50-20-11-12-21-51(50)33-49)93-76(109)64(39-53-42-83-44-87-53)95-73(106)61(36-47-18-9-6-10-19-47)89-66(98)43-86-69(102)45(2)88-70(103)56(79)34-48-27-30-54(97)31-28-48/h5-14,16-22,24,26-31,33,41-42,44-45,56,58-65,85,97H,3-4,15,23,25,32,34-40,43,79H2,1-2H3,(H2,80,101)(H,83,87)(H,86,102)(H,88,103)(H,89,98)(H,90,108)(H,91,107)(H,92,110)(H,93,109)(H,94,105)(H,95,106)(H,96,104)(H,99,100)(H4,81,82,84)/t45-,56+,58+,59+,60+,61+,62-,63+,64+,65+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50610173
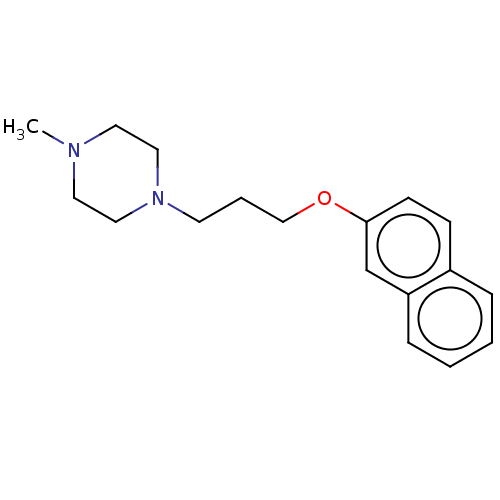
(CHEMBL5280702) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50610180
(CHEMBL5276588) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50610177

(CHEMBL5268329) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50610178

(CHEMBL5289468) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50121205

(CHEBI:18295 | Histamine)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-Histamine from human histamine 4 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115924
BindingDB Entry DOI: 10.7270/Q2833WNJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(3) dopamine receptor
(Homo sapiens) | BDBM50610182
(CHEMBL5287235) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50321604

((3S,6S,9S,12S,15R,18S,21S,24R,27S)-18-((1H-imidazo...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,wU:58.71,74.80,8.20,87.93,69.75,wD:48.59,22.31,4.4,33.48,95.101,(27.14,-11.6,;25.81,-10.83,;25.81,-9.29,;24.47,-8.52,;24.47,-6.97,;23.14,-6.2,;21.8,-6.97,;21.8,-8.5,;20.47,-6.2,;20.47,-4.66,;21.89,-4.07,;23.12,-5,;24.38,-4.1,;23.93,-2.63,;24.71,-1.31,;23.96,.04,;22.43,.05,;21.64,-1.26,;22.38,-2.61,;19.13,-6.97,;17.8,-6.2,;17.8,-4.66,;16.46,-6.97,;16.46,-8.51,;17.8,-9.28,;17.8,-10.82,;19.14,-11.58,;19.13,-13.12,;17.8,-13.89,;20.46,-13.9,;15.12,-6.21,;13.78,-6.97,;13.78,-8.51,;12.45,-6.2,;12.42,-4.66,;13.74,-3.87,;15.09,-4.61,;16.4,-3.81,;16.37,-2.28,;17.69,-1.49,;17.67,.05,;16.32,.8,;15,.01,;15.03,-1.52,;13.71,-2.32,;11.12,-6.97,;9.78,-6.21,;9.78,-4.66,;8.44,-6.98,;8.44,-8.51,;9.78,-9.29,;11.18,-8.65,;12.21,-9.8,;11.45,-11.14,;9.94,-10.82,;7.1,-6.21,;5.76,-6.97,;5.76,-8.5,;4.43,-6.2,;4.43,-4.66,;5.76,-3.89,;7.1,-4.65,;8.42,-3.88,;8.42,-2.34,;7.09,-1.57,;5.75,-2.34,;3.1,-6.99,;1.76,-6.22,;1.76,-4.68,;.42,-6.99,;.42,-8.53,;-.9,-6.21,;-2.25,-6.97,;-2.25,-8.51,;-3.58,-6.2,;-4.91,-6.98,;-3.58,-4.66,;-2.25,-3.89,;-.92,-4.66,;.41,-3.89,;.4,-2.35,;1.77,-1.59,;-.92,-1.58,;-2.25,-2.35,;25.81,-6.21,;25.81,-4.67,;27.15,-6.98,;28.48,-6.21,;28.48,-4.66,;29.82,-3.89,;31.15,-4.66,;29.82,-2.35,;29.82,-6.97,;29.82,-8.51,;31.15,-6.2,;32.49,-6.97,;32.49,-8.5,;33.83,-9.28,;35.16,-8.5,;36.5,-9.28,;36.5,-10.82,;35.16,-11.58,;33.83,-10.81,;33.83,-6.2,;35.15,-6.96,;33.83,-4.65,)| Show InChI InChI=1S/C76H91N17O13/c1-3-4-23-57(69(100)93-64(40-65(95)96)75(106)88-59(66(78)97)35-45-16-7-5-8-17-45)86-73(104)62(38-51-41-83-56-24-14-13-22-54(51)56)91-70(101)58(25-15-32-82-76(79)80)87-71(102)61(37-48-26-29-49-20-11-12-21-50(49)33-48)90-74(105)63(39-52-42-81-43-84-52)92-72(103)60(36-46-18-9-6-10-19-46)89-67(98)44(2)85-68(99)55(77)34-47-27-30-53(94)31-28-47/h5-14,16-22,24,26-31,33,41-44,55,57-64,83,94H,3-4,15,23,25,32,34-40,77H2,1-2H3,(H2,78,97)(H,81,84)(H,85,99)(H,86,104)(H,87,102)(H,88,106)(H,89,98)(H,90,105)(H,91,101)(H,92,103)(H,93,100)(H,95,96)(H4,79,80,82)/t44-,55+,57+,58+,59+,60+,61-,62+,63+,64+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8(SO3) from human CCK1 receptor expressed in human HEK293 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50321604

((3S,6S,9S,12S,15R,18S,21S,24R,27S)-18-((1H-imidazo...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,wU:58.71,74.80,8.20,87.93,69.75,wD:48.59,22.31,4.4,33.48,95.101,(27.14,-11.6,;25.81,-10.83,;25.81,-9.29,;24.47,-8.52,;24.47,-6.97,;23.14,-6.2,;21.8,-6.97,;21.8,-8.5,;20.47,-6.2,;20.47,-4.66,;21.89,-4.07,;23.12,-5,;24.38,-4.1,;23.93,-2.63,;24.71,-1.31,;23.96,.04,;22.43,.05,;21.64,-1.26,;22.38,-2.61,;19.13,-6.97,;17.8,-6.2,;17.8,-4.66,;16.46,-6.97,;16.46,-8.51,;17.8,-9.28,;17.8,-10.82,;19.14,-11.58,;19.13,-13.12,;17.8,-13.89,;20.46,-13.9,;15.12,-6.21,;13.78,-6.97,;13.78,-8.51,;12.45,-6.2,;12.42,-4.66,;13.74,-3.87,;15.09,-4.61,;16.4,-3.81,;16.37,-2.28,;17.69,-1.49,;17.67,.05,;16.32,.8,;15,.01,;15.03,-1.52,;13.71,-2.32,;11.12,-6.97,;9.78,-6.21,;9.78,-4.66,;8.44,-6.98,;8.44,-8.51,;9.78,-9.29,;11.18,-8.65,;12.21,-9.8,;11.45,-11.14,;9.94,-10.82,;7.1,-6.21,;5.76,-6.97,;5.76,-8.5,;4.43,-6.2,;4.43,-4.66,;5.76,-3.89,;7.1,-4.65,;8.42,-3.88,;8.42,-2.34,;7.09,-1.57,;5.75,-2.34,;3.1,-6.99,;1.76,-6.22,;1.76,-4.68,;.42,-6.99,;.42,-8.53,;-.9,-6.21,;-2.25,-6.97,;-2.25,-8.51,;-3.58,-6.2,;-4.91,-6.98,;-3.58,-4.66,;-2.25,-3.89,;-.92,-4.66,;.41,-3.89,;.4,-2.35,;1.77,-1.59,;-.92,-1.58,;-2.25,-2.35,;25.81,-6.21,;25.81,-4.67,;27.15,-6.98,;28.48,-6.21,;28.48,-4.66,;29.82,-3.89,;31.15,-4.66,;29.82,-2.35,;29.82,-6.97,;29.82,-8.51,;31.15,-6.2,;32.49,-6.97,;32.49,-8.5,;33.83,-9.28,;35.16,-8.5,;36.5,-9.28,;36.5,-10.82,;35.16,-11.58,;33.83,-10.81,;33.83,-6.2,;35.15,-6.96,;33.83,-4.65,)| Show InChI InChI=1S/C76H91N17O13/c1-3-4-23-57(69(100)93-64(40-65(95)96)75(106)88-59(66(78)97)35-45-16-7-5-8-17-45)86-73(104)62(38-51-41-83-56-24-14-13-22-54(51)56)91-70(101)58(25-15-32-82-76(79)80)87-71(102)61(37-48-26-29-49-20-11-12-21-50(49)33-48)90-74(105)63(39-52-42-81-43-84-52)92-72(103)60(36-46-18-9-6-10-19-46)89-67(98)44(2)85-68(99)55(77)34-47-27-30-53(94)31-28-47/h5-14,16-22,24,26-31,33,41-44,55,57-64,83,94H,3-4,15,23,25,32,34-40,77H2,1-2H3,(H2,78,97)(H,81,84)(H,85,99)(H,86,104)(H,87,102)(H,88,106)(H,89,98)(H,90,105)(H,91,101)(H,92,103)(H,93,100)(H,95,96)(H4,79,80,82)/t44-,55+,57+,58+,59+,60+,61-,62+,63+,64+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8(SO3) from human CCK1 receptor expressed in human HEK293 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50121205

(CHEBI:18295 | Histamine)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-Histamine from human histamine 3 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115924
BindingDB Entry DOI: 10.7270/Q2833WNJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM21123
((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C23H29N5O5/c1-14(27-23(33)18(24)11-16-7-9-17(29)10-8-16)22(32)26-13-20(30)28-19(21(25)31)12-15-5-3-2-4-6-15/h2-10,14,18-19,29H,11-13,24H2,1H3,(H2,25,31)(H,26,32)(H,27,33)(H,28,30)/t14-,18+,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM21123

((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C23H29N5O5/c1-14(27-23(33)18(24)11-16-7-9-17(29)10-8-16)22(32)26-13-20(30)28-19(21(25)31)12-15-5-3-2-4-6-15/h2-10,14,18-19,29H,11-13,24H2,1H3,(H2,25,31)(H,26,32)(H,27,33)(H,28,30)/t14-,18+,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50610169
(CHEMBL5270151) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50321605
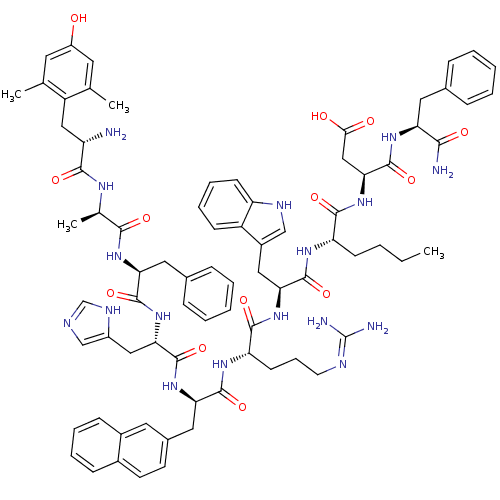
((6R,9S,12S,15R,18S,21S,24S,27S)-12-((1H-imidazol-5...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,wU:69.75,8.20,58.71,89.95,wD:48.59,22.31,33.48,4.4,97.103,74.80,(28.82,-7.95,;30.15,-7.19,;30.15,-5.65,;28.82,-4.88,;28.82,-3.34,;27.48,-2.57,;26.15,-3.35,;26.15,-4.89,;24.82,-2.58,;24.82,-1.03,;26.22,-.4,;27.48,-1.28,;28.71,-.35,;28.21,1.11,;28.95,2.45,;28.16,3.77,;26.62,3.74,;25.87,2.4,;26.66,1.08,;23.48,-3.34,;22.13,-2.59,;22.13,-1.04,;20.8,-3.36,;20.8,-4.9,;22.13,-5.67,;22.13,-7.21,;23.47,-7.98,;23.47,-9.52,;22.16,-10.27,;24.84,-10.28,;19.47,-2.57,;18.15,-3.35,;18.15,-4.88,;16.82,-2.57,;16.82,-1.03,;18.15,-.26,;19.48,-1.03,;20.81,-.26,;20.82,1.28,;22.15,2.06,;22.15,3.6,;20.8,4.36,;19.47,3.58,;19.48,2.05,;18.14,1.28,;15.48,-3.33,;14.14,-2.57,;14.14,-1.02,;12.8,-3.34,;12.8,-4.88,;14.14,-5.65,;15.55,-5.02,;16.59,-6.17,;15.81,-7.5,;14.31,-7.18,;11.47,-2.57,;10.13,-3.32,;10.13,-4.86,;8.81,-2.55,;8.81,-1.01,;10.13,-.25,;11.47,-1.01,;12.81,-.24,;12.8,1.31,;11.47,2.07,;10.14,1.3,;7.47,-3.33,;6.13,-2.55,;6.13,-1.01,;4.8,-3.33,;4.8,-4.87,;3.47,-2.56,;2.12,-3.32,;2.13,-4.86,;.8,-2.54,;.81,-1,;-.54,-3.3,;-1.86,-2.52,;-1.85,-.98,;-.51,-.23,;-3.18,-.2,;-4.52,-.96,;-5.84,-.18,;-4.53,-2.49,;-3.2,-3.27,;-3.21,-4.81,;30.15,-2.57,;30.15,-1.03,;31.49,-3.34,;32.82,-2.57,;32.82,-1.02,;34.15,-.26,;35.48,-1.03,;34.15,1.29,;34.15,-3.34,;34.15,-4.88,;35.49,-2.57,;36.82,-3.34,;36.82,-4.88,;38.16,-5.65,;39.5,-4.87,;40.83,-5.65,;40.84,-7.18,;39.5,-7.95,;38.16,-7.19,;38.16,-2.57,;39.49,-3.33,;38.16,-1.02,)| Show InChI InChI=1S/C78H95N17O13/c1-5-6-25-59(71(102)95-66(40-67(97)98)77(108)90-61(68(80)99)34-47-18-9-7-10-19-47)88-75(106)64(37-52-41-85-58-26-16-15-24-55(52)58)93-72(103)60(27-17-30-84-78(81)82)89-73(104)63(36-49-28-29-50-22-13-14-23-51(50)33-49)92-76(107)65(38-53-42-83-43-86-53)94-74(105)62(35-48-20-11-8-12-21-48)91-69(100)46(4)87-70(101)57(79)39-56-44(2)31-54(96)32-45(56)3/h7-16,18-24,26,28-29,31-33,41-43,46,57,59-66,85,96H,5-6,17,25,27,30,34-40,79H2,1-4H3,(H2,80,99)(H,83,86)(H,87,101)(H,88,106)(H,89,104)(H,90,108)(H,91,100)(H,92,107)(H,93,103)(H,94,105)(H,95,102)(H,97,98)(H4,81,82,84)/t46-,57+,59+,60+,61+,62+,63-,64+,65+,66+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human delta opioid receptor expressed in mouse HN9.10 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50552436

(CHEMBL4749654) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-Histamine from human histamine 3 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115924
BindingDB Entry DOI: 10.7270/Q2833WNJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50321605

((6R,9S,12S,15R,18S,21S,24S,27S)-12-((1H-imidazol-5...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,wU:69.75,8.20,58.71,89.95,wD:48.59,22.31,33.48,4.4,97.103,74.80,(28.82,-7.95,;30.15,-7.19,;30.15,-5.65,;28.82,-4.88,;28.82,-3.34,;27.48,-2.57,;26.15,-3.35,;26.15,-4.89,;24.82,-2.58,;24.82,-1.03,;26.22,-.4,;27.48,-1.28,;28.71,-.35,;28.21,1.11,;28.95,2.45,;28.16,3.77,;26.62,3.74,;25.87,2.4,;26.66,1.08,;23.48,-3.34,;22.13,-2.59,;22.13,-1.04,;20.8,-3.36,;20.8,-4.9,;22.13,-5.67,;22.13,-7.21,;23.47,-7.98,;23.47,-9.52,;22.16,-10.27,;24.84,-10.28,;19.47,-2.57,;18.15,-3.35,;18.15,-4.88,;16.82,-2.57,;16.82,-1.03,;18.15,-.26,;19.48,-1.03,;20.81,-.26,;20.82,1.28,;22.15,2.06,;22.15,3.6,;20.8,4.36,;19.47,3.58,;19.48,2.05,;18.14,1.28,;15.48,-3.33,;14.14,-2.57,;14.14,-1.02,;12.8,-3.34,;12.8,-4.88,;14.14,-5.65,;15.55,-5.02,;16.59,-6.17,;15.81,-7.5,;14.31,-7.18,;11.47,-2.57,;10.13,-3.32,;10.13,-4.86,;8.81,-2.55,;8.81,-1.01,;10.13,-.25,;11.47,-1.01,;12.81,-.24,;12.8,1.31,;11.47,2.07,;10.14,1.3,;7.47,-3.33,;6.13,-2.55,;6.13,-1.01,;4.8,-3.33,;4.8,-4.87,;3.47,-2.56,;2.12,-3.32,;2.13,-4.86,;.8,-2.54,;.81,-1,;-.54,-3.3,;-1.86,-2.52,;-1.85,-.98,;-.51,-.23,;-3.18,-.2,;-4.52,-.96,;-5.84,-.18,;-4.53,-2.49,;-3.2,-3.27,;-3.21,-4.81,;30.15,-2.57,;30.15,-1.03,;31.49,-3.34,;32.82,-2.57,;32.82,-1.02,;34.15,-.26,;35.48,-1.03,;34.15,1.29,;34.15,-3.34,;34.15,-4.88,;35.49,-2.57,;36.82,-3.34,;36.82,-4.88,;38.16,-5.65,;39.5,-4.87,;40.83,-5.65,;40.84,-7.18,;39.5,-7.95,;38.16,-7.19,;38.16,-2.57,;39.49,-3.33,;38.16,-1.02,)| Show InChI InChI=1S/C78H95N17O13/c1-5-6-25-59(71(102)95-66(40-67(97)98)77(108)90-61(68(80)99)34-47-18-9-7-10-19-47)88-75(106)64(37-52-41-85-58-26-16-15-24-55(52)58)93-72(103)60(27-17-30-84-78(81)82)89-73(104)63(36-49-28-29-50-22-13-14-23-51(50)33-49)92-76(107)65(38-53-42-83-43-86-53)94-74(105)62(35-48-20-11-8-12-21-48)91-69(100)46(4)87-70(101)57(79)39-56-44(2)31-54(96)32-45(56)3/h7-16,18-24,26,28-29,31-33,41-43,46,57,59-66,85,96H,5-6,17,25,27,30,34-40,79H2,1-4H3,(H2,80,99)(H,83,86)(H,87,101)(H,88,106)(H,89,104)(H,90,108)(H,91,100)(H,92,107)(H,93,103)(H,94,105)(H,95,102)(H,97,98)(H4,81,82,84)/t46-,57+,59+,60+,61+,62+,63-,64+,65+,66+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50321598
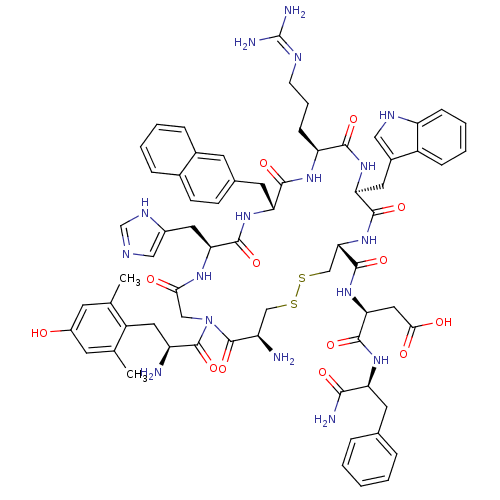
((S)-3-((4R,7S,10S,13R,16S,22S)-16-((1H-imidazol-5-...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CC(=O)N[C@@H](Cc2cnc[nH]2)C(=O)N[C@H](Cc2ccc3ccccc3c2)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CSSC[C@@H](N)C1=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,wU:69.84,89.95,29.31,10.11,wD:44.48,74.80,19.20,81.87,55.59,(1.42,-5.48,;.09,-4.71,;-1.25,-5.49,;-2.59,-4.72,;-3.92,-5.48,;-2.59,-3.17,;-1.25,-2.41,;-1.25,-.87,;.08,-3.17,;1.41,-2.4,;2.75,-3.17,;2.75,-4.71,;4.08,-2.39,;4.08,-.85,;5.42,-3.16,;6.02,-4.57,;5.09,-5.8,;3.56,-5.61,;5.68,-7.22,;4.75,-8.45,;3.42,-7.68,;2.08,-8.45,;.68,-7.83,;-.35,-8.98,;.42,-10.31,;1.93,-9.98,;4.75,-9.99,;3.42,-10.76,;6.09,-10.76,;6.09,-12.3,;4.75,-13.07,;4.75,-14.61,;6.08,-15.37,;6.09,-16.91,;4.75,-17.69,;4.75,-19.22,;3.42,-19.99,;2.08,-19.22,;2.09,-17.68,;3.42,-16.92,;3.42,-15.39,;7.42,-13.07,;7.42,-14.61,;8.75,-12.3,;10.09,-13.07,;10.09,-14.61,;8.75,-15.38,;8.75,-16.92,;7.42,-17.69,;7.42,-19.23,;6.09,-20,;8.75,-20,;11.42,-12.3,;12.75,-13.07,;11.42,-10.76,;12.75,-9.99,;14.09,-10.76,;15.42,-9.99,;15.57,-8.48,;17.08,-8.15,;17.86,-9.48,;19.37,-9.79,;19.85,-11.26,;18.82,-12.42,;17.3,-12.1,;16.82,-10.63,;12.75,-8.45,;11.42,-7.68,;14.09,-7.68,;14.09,-6.14,;12.75,-5.37,;12.75,-3.83,;10,-3.73,;8.47,-3.54,;7.88,-2.12,;8.81,-.89,;6.35,-1.93,;5.75,-.51,;15.42,-5.37,;16.75,-6.14,;15.42,-3.83,;16.75,-3.06,;18.09,-3.83,;19.42,-3.06,;19.42,-1.52,;20.76,-3.83,;16.75,-1.52,;15.42,-.75,;18.09,-.75,;18.09,.78,;16.75,1.55,;16.75,3.09,;18.09,3.85,;18.09,5.39,;16.76,6.16,;15.42,5.39,;15.43,3.86,;19.42,1.55,;19.42,3.09,;20.76,.78,)| Show InChI InChI=1S/C68H81N17O13S2/c1-36-21-44(86)22-37(2)46(36)28-47(69)66(97)85-32-57(87)78-54(27-43-31-74-35-77-43)63(94)81-52(25-39-18-19-40-13-6-7-14-41(40)23-39)61(92)79-50(17-10-20-75-68(72)73)60(91)82-53(26-42-30-76-49-16-9-8-15-45(42)49)62(93)84-56(34-100-99-33-48(70)67(85)98)65(96)83-55(29-58(88)89)64(95)80-51(59(71)90)24-38-11-4-3-5-12-38/h3-9,11-16,18-19,21-23,30-31,35,47-48,50-56,76,86H,10,17,20,24-29,32-34,69-70H2,1-2H3,(H2,71,90)(H,74,77)(H,78,87)(H,79,92)(H,80,95)(H,81,94)(H,82,91)(H,83,96)(H,84,93)(H,88,89)(H4,72,73,75)/t47-,48+,50-,51-,52+,53-,54-,55-,56-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50321608

((3S,6S,9S,12S,15R,18S,21S,27R,30S)-18-((1H-imidazo...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C74H92N18O14/c1-3-4-24-54(67(100)92-61(38-63(95)96)73(106)88-56(64(76)97)33-44-17-8-5-9-18-44)86-71(104)59(36-48-39-81-53-25-15-14-23-51(48)53)90-68(101)55(26-16-31-80-74(77)78)87-70(103)58(35-46-21-12-7-13-22-46)89-72(105)60(37-49-40-79-42-83-49)91-69(102)57(34-45-19-10-6-11-20-45)85-62(94)41-82-65(98)43(2)84-66(99)52(75)32-47-27-29-50(93)30-28-47/h5-15,17-23,25,27-30,39-40,42-43,52,54-61,81,93H,3-4,16,24,26,31-38,41,75H2,1-2H3,(H2,76,97)(H,79,83)(H,82,98)(H,84,99)(H,85,94)(H,86,104)(H,87,103)(H,88,106)(H,89,105)(H,90,101)(H,91,102)(H,92,100)(H,95,96)(H4,77,78,80)/t43-,52+,54+,55+,56+,57+,58-,59+,60+,61+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human delta opioid receptor expressed in mouse HN9.10 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50552434
(CHEMBL4747180) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-Histamine from human histamine 3 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115924
BindingDB Entry DOI: 10.7270/Q2833WNJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50321601
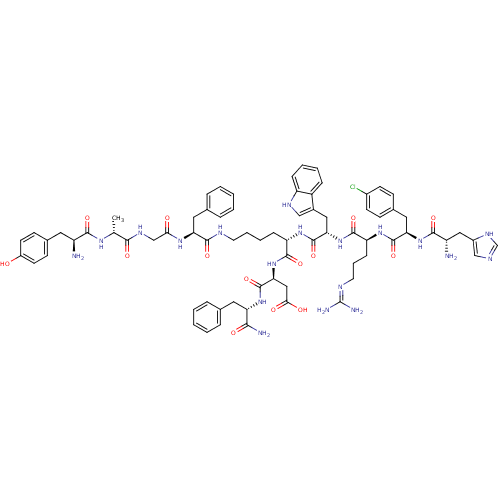
((2S,5R,11S,18S,21S)-2-amino-21-((S)-1-amino-1-oxo-...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)NCCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)[C@@H](N)Cc1cnc[nH]1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,wU:5.5,1.0,78.83,66.79,55.66,37.39,97.102,wD:22.22,41.55,89.94,(17.54,.39,;18.85,-.37,;20.18,.39,;20.18,1.92,;18.85,2.7,;21.5,2.69,;21.5,4.24,;22.81,1.93,;22.81,.41,;21.51,-.37,;21.53,-1.91,;22.87,-2.67,;22.87,-4.2,;24.18,-1.88,;24.18,-.35,;18.85,-1.92,;20.2,-2.69,;17.54,-2.68,;17.54,-4.23,;16.23,-4.98,;14.91,-4.23,;16.23,-6.53,;14.91,-7.3,;13.6,-6.53,;12.26,-7.31,;12.29,-8.88,;10.96,-9.65,;9.62,-8.89,;9.61,-7.35,;10.94,-6.57,;14.91,-8.85,;16.26,-9.62,;13.6,-9.6,;13.6,-11.15,;12.3,-11.91,;12.3,-13.47,;10.99,-14.22,;10.99,-15.77,;9.67,-16.53,;8.36,-15.77,;8.36,-14.26,;7.02,-16.55,;7.02,-18.1,;8.36,-18.88,;9.61,-17.94,;10.87,-18.83,;10.39,-20.31,;11.18,-21.63,;10.42,-22.98,;8.88,-22.99,;8.1,-21.66,;8.85,-20.32,;5.7,-15.79,;4.36,-16.57,;4.36,-18.12,;3.05,-15.81,;3.05,-14.3,;4.36,-13.54,;4.36,-11.99,;5.67,-11.23,;5.67,-9.68,;4.33,-8.91,;6.98,-8.93,;1.71,-16.59,;.4,-15.83,;.4,-14.32,;-.95,-16.61,;-.95,-18.16,;.4,-18.93,;1.74,-18.14,;3.07,-18.9,;3.08,-20.45,;4.42,-21.21,;1.76,-21.23,;.42,-20.46,;-2.26,-15.85,;-3.61,-16.63,;-3.61,-18.18,;-4.92,-15.87,;-6.26,-16.65,;-4.92,-14.35,;-3.61,-13.6,;-2.21,-14.2,;-1.18,-13.04,;-1.97,-11.71,;-3.47,-12.05,;12.33,-16.55,;12.33,-18.07,;13.67,-15.77,;14.98,-16.53,;14.98,-18.05,;16.33,-18.82,;17.67,-18.05,;16.33,-20.33,;16.33,-15.75,;16.33,-14.2,;17.64,-16.51,;18.98,-15.73,;18.98,-14.18,;20.3,-13.43,;21.63,-14.17,;22.95,-13.39,;22.94,-11.85,;21.61,-11.09,;20.27,-11.87,;20.3,-16.49,;21.64,-15.71,;20.3,-18.01,)| Show InChI InChI=1S/C74H92ClN19O14/c1-42(87-66(101)52(76)31-45-23-27-50(95)28-24-45)65(100)85-40-62(96)88-58(33-44-15-6-3-7-16-44)68(103)82-29-11-10-19-55(69(104)94-61(37-63(97)98)73(108)91-57(64(78)99)32-43-13-4-2-5-14-43)89-72(107)60(35-47-38-84-54-18-9-8-17-51(47)54)93-70(105)56(20-12-30-83-74(79)80)90-71(106)59(34-46-21-25-48(75)26-22-46)92-67(102)53(77)36-49-39-81-41-86-49/h2-9,13-18,21-28,38-39,41-42,52-53,55-61,84,95H,10-12,19-20,29-37,40,76-77H2,1H3,(H2,78,99)(H,81,86)(H,82,103)(H,85,100)(H,87,101)(H,88,96)(H,89,107)(H,90,106)(H,91,108)(H,92,102)(H,93,105)(H,94,104)(H,97,98)(H4,79,80,83)/t42-,52+,53+,55+,56+,57+,58+,59-,60+,61+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50321599
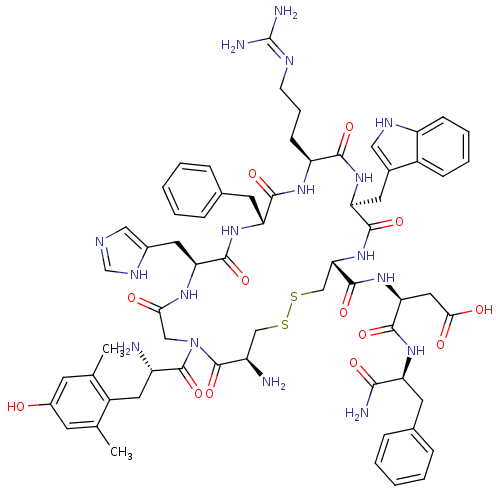
((S)-3-((4R,7S,10S,13R,16S,22S)-16-((1H-imidazol-5-...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CC(=O)N[C@@H](Cc2cnc[nH]2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CSSC[C@@H](N)C1=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,wU:10.11,65.79,85.90,29.31,wD:19.20,70.75,40.43,77.82,51.54,(-6.99,1.44,;-8.33,2.2,;-9.67,1.43,;-11.01,2.2,;-12.34,1.43,;-11,3.74,;-9.66,4.51,;-9.66,6.05,;-8.33,3.74,;-7,4.51,;-5.67,3.75,;-5.66,2.21,;-4.33,4.52,;-4.34,6.06,;-3,3.75,;-2.4,2.34,;-3.33,1.11,;-4.86,1.3,;-2.73,-.31,;-3.66,-1.53,;-5,-.77,;-6.33,-1.54,;-7.73,-.92,;-8.76,-2.06,;-7.99,-3.4,;-6.48,-3.07,;-3.66,-3.08,;-5,-3.85,;-2.33,-3.85,;-2.33,-5.39,;-3.66,-6.16,;-3.66,-7.7,;-5,-8.46,;-5,-10,;-3.67,-10.77,;-2.33,-10,;-2.34,-8.46,;-1,-6.16,;-1,-7.7,;.34,-5.39,;1.67,-6.16,;1.67,-7.7,;.34,-8.47,;.34,-10.01,;-1,-10.78,;-1,-12.32,;-2.33,-13.09,;.34,-13.09,;3,-5.39,;4.34,-6.16,;3,-3.85,;4.34,-3.08,;5.67,-3.85,;7.01,-3.08,;7.16,-1.56,;8.67,-1.24,;9.44,-2.57,;10.95,-2.88,;11.43,-4.34,;10.4,-5.5,;8.89,-5.18,;8.41,-3.71,;4.34,-1.54,;3,-.77,;5.67,-.77,;5.67,.77,;4.34,1.54,;4.34,3.08,;1.59,3.18,;.06,3.38,;-.54,4.79,;.39,6.02,;-2.07,4.99,;-2.66,6.41,;7.01,1.54,;8.34,.77,;7.01,3.08,;8.34,3.85,;9.67,3.08,;11.01,3.85,;11.01,5.39,;12.34,3.08,;8.34,5.39,;7.01,6.16,;9.67,6.16,;9.67,7.7,;8.34,8.47,;8.34,10.01,;9.68,10.78,;9.68,12.32,;8.34,13.09,;7.01,12.31,;7.01,10.77,;11.01,8.47,;11.01,10.01,;12.34,7.7,)| Show InChI InChI=1S/C64H79N17O13S2/c1-34-20-40(82)21-35(2)42(34)26-43(65)62(93)81-30-53(83)74-50(25-39-29-70-33-73-39)59(90)77-48(23-37-14-7-4-8-15-37)57(88)75-46(18-11-19-71-64(68)69)56(87)78-49(24-38-28-72-45-17-10-9-16-41(38)45)58(89)80-52(32-96-95-31-44(66)63(81)94)61(92)79-51(27-54(84)85)60(91)76-47(55(67)86)22-36-12-5-3-6-13-36/h3-10,12-17,20-21,28-29,33,43-44,46-52,72,82H,11,18-19,22-27,30-32,65-66H2,1-2H3,(H2,67,86)(H,70,73)(H,74,83)(H,75,88)(H,76,91)(H,77,90)(H,78,87)(H,79,92)(H,80,89)(H,84,85)(H4,68,69,71)/t43-,44+,46-,47-,48+,49-,50-,51-,52-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50321607

((3S,6S,9S,12S,15R,18S,21S,27R,30S)-18-((1H-imidazo...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C78H94N18O14/c1-3-4-23-58(71(104)96-65(40-67(99)100)77(110)92-60(68(80)101)35-46-16-7-5-8-17-46)90-75(108)63(38-52-41-85-57-24-14-13-22-55(52)57)94-72(105)59(25-15-32-84-78(81)82)91-74(107)62(37-49-26-29-50-20-11-12-21-51(50)33-49)93-76(109)64(39-53-42-83-44-87-53)95-73(106)61(36-47-18-9-6-10-19-47)89-66(98)43-86-69(102)45(2)88-70(103)56(79)34-48-27-30-54(97)31-28-48/h5-14,16-22,24,26-31,33,41-42,44-45,56,58-65,85,97H,3-4,15,23,25,32,34-40,43,79H2,1-2H3,(H2,80,101)(H,83,87)(H,86,102)(H,88,103)(H,89,98)(H,90,108)(H,91,107)(H,92,110)(H,93,109)(H,94,105)(H,95,106)(H,96,104)(H,99,100)(H4,81,82,84)/t45-,56+,58+,59+,60+,61+,62-,63+,64+,65+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8(SO3) from human CCK1 receptor expressed in human HEK293 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50321607

((3S,6S,9S,12S,15R,18S,21S,27R,30S)-18-((1H-imidazo...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C78H94N18O14/c1-3-4-23-58(71(104)96-65(40-67(99)100)77(110)92-60(68(80)101)35-46-16-7-5-8-17-46)90-75(108)63(38-52-41-85-57-24-14-13-22-55(52)57)94-72(105)59(25-15-32-84-78(81)82)91-74(107)62(37-49-26-29-50-20-11-12-21-51(50)33-49)93-76(109)64(39-53-42-83-44-87-53)95-73(106)61(36-47-18-9-6-10-19-47)89-66(98)43-86-69(102)45(2)88-70(103)56(79)34-48-27-30-54(97)31-28-48/h5-14,16-22,24,26-31,33,41-42,44-45,56,58-65,85,97H,3-4,15,23,25,32,34-40,43,79H2,1-2H3,(H2,80,101)(H,83,87)(H,86,102)(H,88,103)(H,89,98)(H,90,108)(H,91,107)(H,92,110)(H,93,109)(H,94,105)(H,95,106)(H,96,104)(H,99,100)(H4,81,82,84)/t45-,56+,58+,59+,60+,61+,62-,63+,64+,65+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8(SO3) from human CCK1 receptor expressed in human HEK293 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50610176
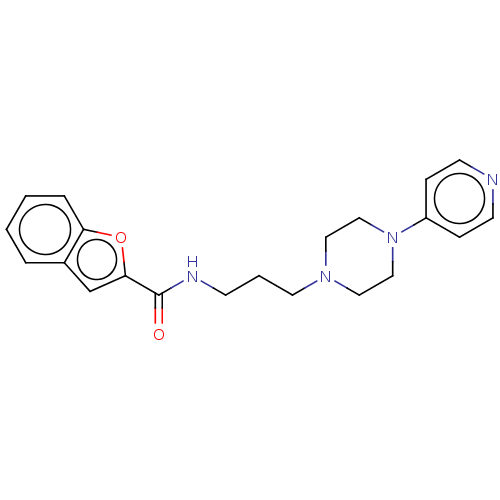
(CHEMBL5265920) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50610170

(CHEMBL5278711) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50610179

(CHEMBL5282002) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50610175
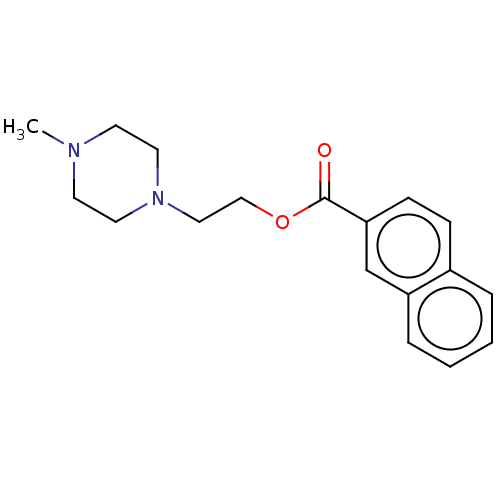
(CHEMBL5283987) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50610181
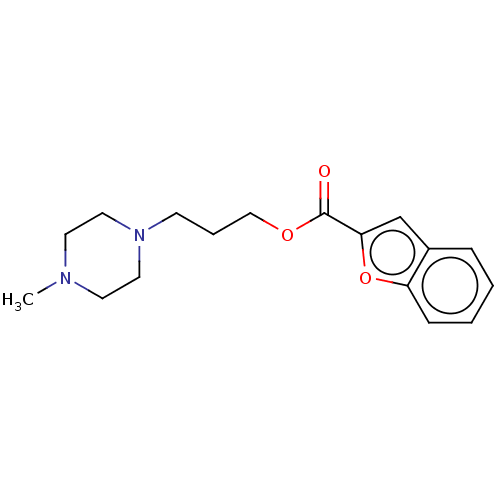
(CHEMBL5270288) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50321608

((3S,6S,9S,12S,15R,18S,21S,27R,30S)-18-((1H-imidazo...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C74H92N18O14/c1-3-4-24-54(67(100)92-61(38-63(95)96)73(106)88-56(64(76)97)33-44-17-8-5-9-18-44)86-71(104)59(36-48-39-81-53-25-15-14-23-51(48)53)90-68(101)55(26-16-31-80-74(77)78)87-70(103)58(35-46-21-12-7-13-22-46)89-72(105)60(37-49-40-79-42-83-49)91-69(102)57(34-45-19-10-6-11-20-45)85-62(94)41-82-65(98)43(2)84-66(99)52(75)32-47-27-29-50(93)30-28-47/h5-15,17-23,25,27-30,39-40,42-43,52,54-61,81,93H,3-4,16,24,26,31-38,41,75H2,1-2H3,(H2,76,97)(H,79,83)(H,82,98)(H,84,99)(H,85,94)(H,86,104)(H,87,103)(H,88,106)(H,89,105)(H,90,101)(H,91,102)(H,92,100)(H,95,96)(H4,77,78,80)/t43-,52+,54+,55+,56+,57+,58-,59+,60+,61+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50321608

((3S,6S,9S,12S,15R,18S,21S,27R,30S)-18-((1H-imidazo...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C74H92N18O14/c1-3-4-24-54(67(100)92-61(38-63(95)96)73(106)88-56(64(76)97)33-44-17-8-5-9-18-44)86-71(104)59(36-48-39-81-53-25-15-14-23-51(48)53)90-68(101)55(26-16-31-80-74(77)78)87-70(103)58(35-46-21-12-7-13-22-46)89-72(105)60(37-49-40-79-42-83-49)91-69(102)57(34-45-19-10-6-11-20-45)85-62(94)41-82-65(98)43(2)84-66(99)52(75)32-47-27-29-50(93)30-28-47/h5-15,17-23,25,27-30,39-40,42-43,52,54-61,81,93H,3-4,16,24,26,31-38,41,75H2,1-2H3,(H2,76,97)(H,79,83)(H,82,98)(H,84,99)(H,85,94)(H,86,104)(H,87,103)(H,88,106)(H,89,105)(H,90,101)(H,91,102)(H,92,100)(H,95,96)(H4,77,78,80)/t43-,52+,54+,55+,56+,57+,58-,59+,60+,61+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50321596

((3S,6R,9S,12S,15S,18S)-3-((1H-imidazol-5-yl)methyl...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C76H90N16O12/c1-4-5-23-58(68(97)90-64(38-66(94)95)73(102)87-60(67(78)96)32-45-16-7-6-8-17-45)85-71(100)62(34-51-39-83-57-24-14-13-22-54(51)57)89-69(98)59(25-15-28-82-76(79)80)86-70(99)61(33-46-26-27-47-18-9-10-19-48(47)31-46)88-72(101)63(36-52-40-81-42-84-52)91-74(103)65-35-49-20-11-12-21-50(49)41-92(65)75(104)56(77)37-55-43(2)29-53(93)30-44(55)3/h6-14,16-22,24,26-27,29-31,39-40,42,56,58-65,83,93H,4-5,15,23,25,28,32-38,41,77H2,1-3H3,(H2,78,96)(H,81,84)(H,85,100)(H,86,99)(H,87,102)(H,88,101)(H,89,98)(H,90,97)(H,91,103)(H,94,95)(H4,79,80,82)/t56-,58-,59-,60-,61+,62-,63-,64-,65?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8(SO3) from human CCK1 receptor expressed in human HEK293 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50321596

((3S,6R,9S,12S,15S,18S)-3-((1H-imidazol-5-yl)methyl...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C76H90N16O12/c1-4-5-23-58(68(97)90-64(38-66(94)95)73(102)87-60(67(78)96)32-45-16-7-6-8-17-45)85-71(100)62(34-51-39-83-57-24-14-13-22-54(51)57)89-69(98)59(25-15-28-82-76(79)80)86-70(99)61(33-46-26-27-47-18-9-10-19-48(47)31-46)88-72(101)63(36-52-40-81-42-84-52)91-74(103)65-35-49-20-11-12-21-50(49)41-92(65)75(104)56(77)37-55-43(2)29-53(93)30-44(55)3/h6-14,16-22,24,26-27,29-31,39-40,42,56,58-65,83,93H,4-5,15,23,25,28,32-38,41,77H2,1-3H3,(H2,78,96)(H,81,84)(H,85,100)(H,86,99)(H,87,102)(H,88,101)(H,89,98)(H,90,97)(H,91,103)(H,94,95)(H4,79,80,82)/t56-,58-,59-,60-,61+,62-,63-,64-,65?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8(SO3) from human CCK1 receptor expressed in human HEK293 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50405713
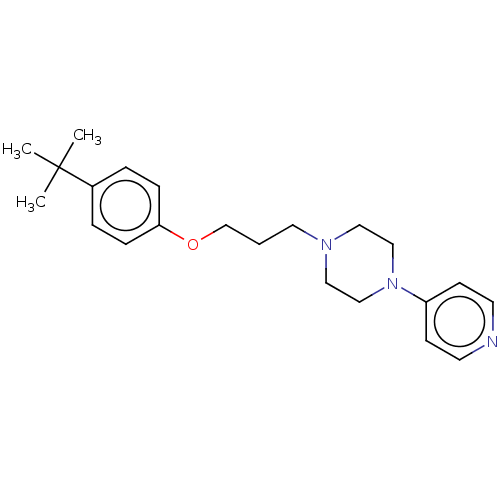
(CHEMBL4173854)Show InChI InChI=1S/C15H18ClNO3S2/c1-9(8-21)14(18)17-7-12(6-13(17)15(19)20)22-11-4-2-10(16)3-5-11/h2-5,9,12-13,21H,6-8H2,1H3,(H,19,20)/t9-,12+,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50552435
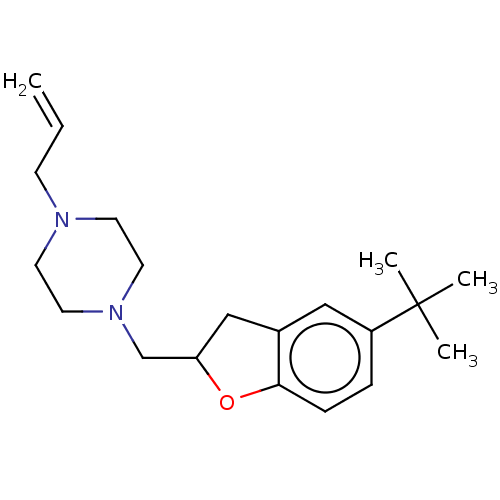
(CHEMBL4756432) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-Histamine from human histamine 3 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115924
BindingDB Entry DOI: 10.7270/Q2833WNJ |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50516759
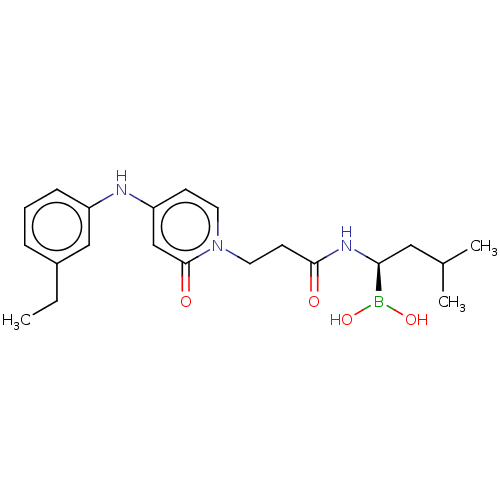
(CHEMBL4458610)Show SMILES CCc1cccc(Nc2ccn(CCC(=O)N[C@@H](CC(C)C)B(O)O)c(=O)c2)c1 |r| Show InChI InChI=1S/C21H30BN3O4/c1-4-16-6-5-7-17(13-16)23-18-8-10-25(21(27)14-18)11-9-20(26)24-19(22(28)29)12-15(2)3/h5-8,10,13-15,19,23,28-29H,4,9,11-12H2,1-3H3,(H,24,26)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S£o Paulo State University
Curated by ChEMBL
| Assay Description
Inhibition of human chymotrypsin-like activity of 20S proteasome (unknown origin) |
Eur J Med Chem 179: 791-804 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.092
BindingDB Entry DOI: 10.7270/Q28055ZM |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50321608

((3S,6S,9S,12S,15R,18S,21S,27R,30S)-18-((1H-imidazo...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C74H92N18O14/c1-3-4-24-54(67(100)92-61(38-63(95)96)73(106)88-56(64(76)97)33-44-17-8-5-9-18-44)86-71(104)59(36-48-39-81-53-25-15-14-23-51(48)53)90-68(101)55(26-16-31-80-74(77)78)87-70(103)58(35-46-21-12-7-13-22-46)89-72(105)60(37-49-40-79-42-83-49)91-69(102)57(34-45-19-10-6-11-20-45)85-62(94)41-82-65(98)43(2)84-66(99)52(75)32-47-27-29-50(93)30-28-47/h5-15,17-23,25,27-30,39-40,42-43,52,54-61,81,93H,3-4,16,24,26,31-38,41,75H2,1-2H3,(H2,76,97)(H,79,83)(H,82,98)(H,84,99)(H,85,94)(H,86,104)(H,87,103)(H,88,106)(H,89,105)(H,90,101)(H,91,102)(H,92,100)(H,95,96)(H4,77,78,80)/t43-,52+,54+,55+,56+,57+,58-,59+,60+,61+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8(SO3) from human CCK2 receptor expressed in human HEK293 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50321608

((3S,6S,9S,12S,15R,18S,21S,27R,30S)-18-((1H-imidazo...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C74H92N18O14/c1-3-4-24-54(67(100)92-61(38-63(95)96)73(106)88-56(64(76)97)33-44-17-8-5-9-18-44)86-71(104)59(36-48-39-81-53-25-15-14-23-51(48)53)90-68(101)55(26-16-31-80-74(77)78)87-70(103)58(35-46-21-12-7-13-22-46)89-72(105)60(37-49-40-79-42-83-49)91-69(102)57(34-45-19-10-6-11-20-45)85-62(94)41-82-65(98)43(2)84-66(99)52(75)32-47-27-29-50(93)30-28-47/h5-15,17-23,25,27-30,39-40,42-43,52,54-61,81,93H,3-4,16,24,26,31-38,41,75H2,1-2H3,(H2,76,97)(H,79,83)(H,82,98)(H,84,99)(H,85,94)(H,86,104)(H,87,103)(H,88,106)(H,89,105)(H,90,101)(H,91,102)(H,92,100)(H,95,96)(H4,77,78,80)/t43-,52+,54+,55+,56+,57+,58-,59+,60+,61+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8(SO3) from human CCK2 receptor expressed in human HEK293 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50321606

((3S,6S,9S,12S,15R,18S,21S,27R,30S)-18-((1H-imidazo...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,wU:8.20,58.71,93.99,78.84,wD:73.79,48.59,22.31,33.48,4.4,101.107,(24.59,-5.48,;25.93,-4.72,;25.93,-3.18,;24.59,-2.4,;24.59,-.86,;23.26,-.1,;21.93,-.87,;21.93,-2.41,;20.59,-.11,;20.59,1.44,;22,2.07,;23.25,1.19,;24.48,2.12,;23.98,3.58,;24.73,4.92,;23.94,6.24,;22.4,6.22,;21.65,4.87,;22.44,3.55,;19.25,-.87,;17.91,-.12,;17.91,1.43,;16.57,-.89,;16.57,-2.43,;17.91,-3.2,;17.91,-4.73,;19.24,-5.51,;19.24,-7.05,;17.93,-7.8,;20.61,-7.81,;15.25,-.1,;13.92,-.87,;13.92,-2.41,;12.59,-.1,;12.6,1.44,;13.92,2.21,;15.26,1.44,;16.59,2.21,;16.6,3.75,;17.93,4.53,;17.92,6.07,;16.58,6.84,;15.25,6.06,;15.26,4.52,;13.92,3.75,;11.26,-.86,;9.92,-.1,;9.92,1.45,;8.58,-.87,;8.58,-2.41,;9.92,-3.18,;11.32,-2.55,;12.36,-3.69,;11.59,-5.03,;10.08,-4.71,;7.25,-.09,;5.91,-.85,;5.91,-2.39,;4.58,-.08,;4.58,1.46,;5.91,2.23,;7.25,1.47,;8.58,2.24,;8.58,3.78,;7.24,4.55,;5.91,3.78,;3.25,-.85,;1.92,-.07,;1.92,1.47,;.58,-.85,;-.75,-.07,;-2.08,-.85,;-2.08,-2.38,;-3.41,-.07,;-3.41,1.47,;-4.75,-.84,;-6.09,-.08,;-6.09,1.47,;-7.42,-.86,;-7.4,-2.4,;-8.75,-.1,;-10.08,-.88,;-10.06,-2.42,;-8.72,-3.17,;-11.4,-3.2,;-12.73,-2.44,;-14.07,-3.22,;-12.76,-.9,;-11.41,-.13,;-11.43,1.41,;25.93,-.09,;25.93,1.45,;27.26,-.87,;28.59,-.09,;28.59,1.45,;29.93,2.21,;31.25,1.45,;29.93,3.76,;29.93,-.87,;29.93,-2.4,;31.26,-.1,;32.6,-.87,;32.6,-2.4,;33.93,-3.17,;35.28,-2.4,;36.61,-3.17,;36.61,-4.71,;35.27,-5.48,;33.94,-4.71,;33.93,-.1,;35.27,-.86,;33.93,1.45,)| Show InChI InChI=1S/C80H98N18O14/c1-5-6-25-60(73(106)98-67(40-69(101)102)79(112)94-62(70(82)103)34-48-18-9-7-10-19-48)92-77(110)65(37-53-41-87-59-26-16-15-24-56(53)59)96-74(107)61(27-17-30-86-80(83)84)93-76(109)64(36-50-28-29-51-22-13-14-23-52(51)33-50)95-78(111)66(38-54-42-85-44-89-54)97-75(108)63(35-49-20-11-8-12-21-49)91-68(100)43-88-71(104)47(4)90-72(105)58(81)39-57-45(2)31-55(99)32-46(57)3/h7-16,18-24,26,28-29,31-33,41-42,44,47,58,60-67,87,99H,5-6,17,25,27,30,34-40,43,81H2,1-4H3,(H2,82,103)(H,85,89)(H,88,104)(H,90,105)(H,91,100)(H,92,110)(H,93,109)(H,94,112)(H,95,111)(H,96,107)(H,97,108)(H,98,106)(H,101,102)(H4,83,84,86)/t47-,58+,60+,61+,62+,63+,64-,65+,66+,67+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8(SO3) from human CCK1 receptor expressed in human HEK293 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50321597
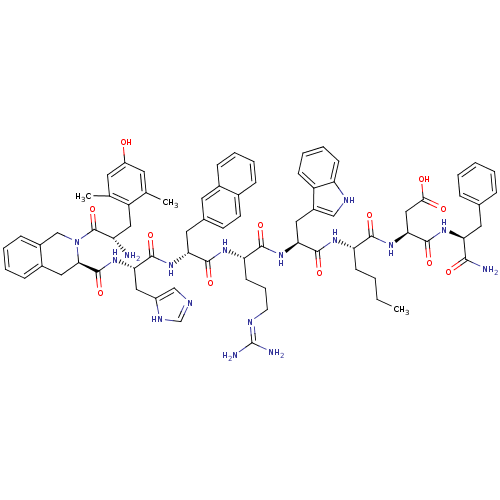
((3S,6R,9S,12S,15S,18S)-3-((1H-imidazol-5-yl)methyl...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H]1Cc2ccccc2CN1C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,wU:33.48,22.31,4.4,48.59,93.100,wD:70.77,58.62,8.20,85.92,(16.65,-3.98,;15.32,-4.75,;15.32,-6.29,;13.98,-7.06,;13.98,-8.6,;12.65,-9.36,;11.32,-8.59,;11.32,-7.05,;9.99,-9.35,;9.99,-10.9,;11.32,-11.67,;12.72,-11.05,;13.76,-12.19,;12.98,-13.52,;13.46,-14.99,;12.42,-16.14,;10.92,-15.81,;10.44,-14.35,;11.48,-13.21,;8.66,-8.59,;7.33,-9.38,;7.33,-10.92,;6,-8.61,;6,-7.07,;7.33,-6.3,;7.33,-4.76,;8.66,-3.97,;8.68,-2.43,;7.33,-1.66,;10,-1.66,;4.65,-9.35,;3.32,-8.59,;3.32,-7.05,;1.99,-9.36,;1.76,-10.89,;2.96,-11.84,;2.73,-13.37,;3.92,-14.33,;5.36,-13.77,;6.57,-14.74,;8.01,-14.17,;8.23,-12.64,;7.03,-11.68,;5.59,-12.24,;4.39,-11.29,;.66,-8.58,;-.66,-9.38,;-.66,-10.92,;-2,-8.61,;-2,-7.07,;-.66,-6.29,;.75,-6.93,;1.79,-5.78,;1.01,-4.45,;-.5,-4.76,;-3.33,-9.38,;-4.67,-8.61,;-4.67,-7.07,;-6,-9.38,;-7.33,-8.62,;-8.66,-9.39,;-9.99,-8.62,;-11.33,-9.39,;-11.33,-10.93,;-9.99,-11.69,;-8.66,-10.92,;-7.33,-11.7,;-6,-10.91,;-4.66,-11.68,;-3.33,-10.91,;-4.66,-13.22,;-5.99,-13.99,;-3.32,-13.99,;-3.32,-15.53,;-4.65,-16.3,;-5.98,-15.53,;-4.65,-17.84,;-3.31,-18.61,;-3.31,-20.15,;-1.98,-17.84,;-1.98,-16.29,;-.65,-15.52,;15.32,-9.37,;15.32,-10.91,;16.65,-8.59,;17.98,-9.36,;17.98,-10.91,;19.32,-11.67,;20.64,-10.9,;19.32,-13.21,;19.32,-8.59,;19.32,-7.05,;20.65,-9.36,;21.98,-8.59,;21.98,-7.05,;23.31,-6.28,;24.65,-7.06,;25.98,-6.29,;25.99,-4.75,;24.65,-3.98,;23.32,-4.75,;23.31,-9.36,;24.65,-8.59,;23.31,-10.91,)| Show InChI InChI=1S/C76H90N16O12/c1-4-5-23-58(68(97)90-64(38-66(94)95)73(102)87-60(67(78)96)32-45-16-7-6-8-17-45)85-71(100)62(34-51-39-83-57-24-14-13-22-54(51)57)89-69(98)59(25-15-28-82-76(79)80)86-70(99)61(33-46-26-27-47-18-9-10-19-48(47)31-46)88-72(101)63(36-52-40-81-42-84-52)91-74(103)65-35-49-20-11-12-21-50(49)41-92(65)75(104)56(77)37-55-43(2)29-53(93)30-44(55)3/h6-14,16-22,24,26-27,29-31,39-40,42,56,58-65,83,93H,4-5,15,23,25,28,32-38,41,77H2,1-3H3,(H2,78,96)(H,81,84)(H,85,100)(H,86,99)(H,87,102)(H,88,101)(H,89,98)(H,90,97)(H,91,103)(H,94,95)(H4,79,80,82)/t56-,58-,59-,60-,61+,62-,63-,64-,65+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50321606

((3S,6S,9S,12S,15R,18S,21S,27R,30S)-18-((1H-imidazo...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,wU:8.20,58.71,93.99,78.84,wD:73.79,48.59,22.31,33.48,4.4,101.107,(24.59,-5.48,;25.93,-4.72,;25.93,-3.18,;24.59,-2.4,;24.59,-.86,;23.26,-.1,;21.93,-.87,;21.93,-2.41,;20.59,-.11,;20.59,1.44,;22,2.07,;23.25,1.19,;24.48,2.12,;23.98,3.58,;24.73,4.92,;23.94,6.24,;22.4,6.22,;21.65,4.87,;22.44,3.55,;19.25,-.87,;17.91,-.12,;17.91,1.43,;16.57,-.89,;16.57,-2.43,;17.91,-3.2,;17.91,-4.73,;19.24,-5.51,;19.24,-7.05,;17.93,-7.8,;20.61,-7.81,;15.25,-.1,;13.92,-.87,;13.92,-2.41,;12.59,-.1,;12.6,1.44,;13.92,2.21,;15.26,1.44,;16.59,2.21,;16.6,3.75,;17.93,4.53,;17.92,6.07,;16.58,6.84,;15.25,6.06,;15.26,4.52,;13.92,3.75,;11.26,-.86,;9.92,-.1,;9.92,1.45,;8.58,-.87,;8.58,-2.41,;9.92,-3.18,;11.32,-2.55,;12.36,-3.69,;11.59,-5.03,;10.08,-4.71,;7.25,-.09,;5.91,-.85,;5.91,-2.39,;4.58,-.08,;4.58,1.46,;5.91,2.23,;7.25,1.47,;8.58,2.24,;8.58,3.78,;7.24,4.55,;5.91,3.78,;3.25,-.85,;1.92,-.07,;1.92,1.47,;.58,-.85,;-.75,-.07,;-2.08,-.85,;-2.08,-2.38,;-3.41,-.07,;-3.41,1.47,;-4.75,-.84,;-6.09,-.08,;-6.09,1.47,;-7.42,-.86,;-7.4,-2.4,;-8.75,-.1,;-10.08,-.88,;-10.06,-2.42,;-8.72,-3.17,;-11.4,-3.2,;-12.73,-2.44,;-14.07,-3.22,;-12.76,-.9,;-11.41,-.13,;-11.43,1.41,;25.93,-.09,;25.93,1.45,;27.26,-.87,;28.59,-.09,;28.59,1.45,;29.93,2.21,;31.25,1.45,;29.93,3.76,;29.93,-.87,;29.93,-2.4,;31.26,-.1,;32.6,-.87,;32.6,-2.4,;33.93,-3.17,;35.28,-2.4,;36.61,-3.17,;36.61,-4.71,;35.27,-5.48,;33.94,-4.71,;33.93,-.1,;35.27,-.86,;33.93,1.45,)| Show InChI InChI=1S/C80H98N18O14/c1-5-6-25-60(73(106)98-67(40-69(101)102)79(112)94-62(70(82)103)34-48-18-9-7-10-19-48)92-77(110)65(37-53-41-87-59-26-16-15-24-56(53)59)96-74(107)61(27-17-30-86-80(83)84)93-76(109)64(36-50-28-29-51-22-13-14-23-52(51)33-50)95-78(111)66(38-54-42-85-44-89-54)97-75(108)63(35-49-20-11-8-12-21-49)91-68(100)43-88-71(104)47(4)90-72(105)58(81)39-57-45(2)31-55(99)32-46(57)3/h7-16,18-24,26,28-29,31-33,41-42,44,47,58,60-67,87,99H,5-6,17,25,27,30,34-40,43,81H2,1-4H3,(H2,82,103)(H,85,89)(H,88,104)(H,90,105)(H,91,100)(H,92,110)(H,93,109)(H,94,112)(H,95,111)(H,96,107)(H,97,108)(H,98,106)(H,101,102)(H4,83,84,86)/t47-,58+,60+,61+,62+,63+,64-,65+,66+,67+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8(SO3) from human CCK1 receptor expressed in human HEK293 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50321597

((3S,6R,9S,12S,15S,18S)-3-((1H-imidazol-5-yl)methyl...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H]1Cc2ccccc2CN1C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,wU:33.48,22.31,4.4,48.59,93.100,wD:70.77,58.62,8.20,85.92,(16.65,-3.98,;15.32,-4.75,;15.32,-6.29,;13.98,-7.06,;13.98,-8.6,;12.65,-9.36,;11.32,-8.59,;11.32,-7.05,;9.99,-9.35,;9.99,-10.9,;11.32,-11.67,;12.72,-11.05,;13.76,-12.19,;12.98,-13.52,;13.46,-14.99,;12.42,-16.14,;10.92,-15.81,;10.44,-14.35,;11.48,-13.21,;8.66,-8.59,;7.33,-9.38,;7.33,-10.92,;6,-8.61,;6,-7.07,;7.33,-6.3,;7.33,-4.76,;8.66,-3.97,;8.68,-2.43,;7.33,-1.66,;10,-1.66,;4.65,-9.35,;3.32,-8.59,;3.32,-7.05,;1.99,-9.36,;1.76,-10.89,;2.96,-11.84,;2.73,-13.37,;3.92,-14.33,;5.36,-13.77,;6.57,-14.74,;8.01,-14.17,;8.23,-12.64,;7.03,-11.68,;5.59,-12.24,;4.39,-11.29,;.66,-8.58,;-.66,-9.38,;-.66,-10.92,;-2,-8.61,;-2,-7.07,;-.66,-6.29,;.75,-6.93,;1.79,-5.78,;1.01,-4.45,;-.5,-4.76,;-3.33,-9.38,;-4.67,-8.61,;-4.67,-7.07,;-6,-9.38,;-7.33,-8.62,;-8.66,-9.39,;-9.99,-8.62,;-11.33,-9.39,;-11.33,-10.93,;-9.99,-11.69,;-8.66,-10.92,;-7.33,-11.7,;-6,-10.91,;-4.66,-11.68,;-3.33,-10.91,;-4.66,-13.22,;-5.99,-13.99,;-3.32,-13.99,;-3.32,-15.53,;-4.65,-16.3,;-5.98,-15.53,;-4.65,-17.84,;-3.31,-18.61,;-3.31,-20.15,;-1.98,-17.84,;-1.98,-16.29,;-.65,-15.52,;15.32,-9.37,;15.32,-10.91,;16.65,-8.59,;17.98,-9.36,;17.98,-10.91,;19.32,-11.67,;20.64,-10.9,;19.32,-13.21,;19.32,-8.59,;19.32,-7.05,;20.65,-9.36,;21.98,-8.59,;21.98,-7.05,;23.31,-6.28,;24.65,-7.06,;25.98,-6.29,;25.99,-4.75,;24.65,-3.98,;23.32,-4.75,;23.31,-9.36,;24.65,-8.59,;23.31,-10.91,)| Show InChI InChI=1S/C76H90N16O12/c1-4-5-23-58(68(97)90-64(38-66(94)95)73(102)87-60(67(78)96)32-45-16-7-6-8-17-45)85-71(100)62(34-51-39-83-57-24-14-13-22-54(51)57)89-69(98)59(25-15-28-82-76(79)80)86-70(99)61(33-46-26-27-47-18-9-10-19-48(47)31-46)88-72(101)63(36-52-40-81-42-84-52)91-74(103)65-35-49-20-11-12-21-50(49)41-92(65)75(104)56(77)37-55-43(2)29-53(93)30-44(55)3/h6-14,16-22,24,26-27,29-31,39-40,42,56,58-65,83,93H,4-5,15,23,25,28,32-38,41,77H2,1-3H3,(H2,78,96)(H,81,84)(H,85,100)(H,86,99)(H,87,102)(H,88,101)(H,89,98)(H,90,97)(H,91,103)(H,94,95)(H4,79,80,82)/t56-,58-,59-,60-,61+,62-,63-,64-,65+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8(SO3) from human CCK1 receptor expressed in human HEK293 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50552430

(CHEMBL4798369) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-Histamine from human histamine 3 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115924
BindingDB Entry DOI: 10.7270/Q2833WNJ |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50516761
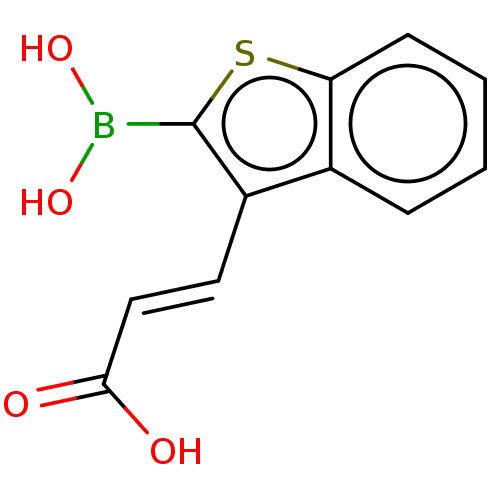
(CHEMBL3286879)Show InChI InChI=1S/C11H9BO4S/c13-10(14)6-5-8-7-3-1-2-4-9(7)17-11(8)12(15)16/h1-6,15-16H,(H,13,14)/b6-5+ | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S£o Paulo State University
Curated by ChEMBL
| Assay Description
Inhibition of bacterial beta lactamase TEM-1 |
Eur J Med Chem 179: 791-804 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.092
BindingDB Entry DOI: 10.7270/Q28055ZM |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50321604

((3S,6S,9S,12S,15R,18S,21S,24R,27S)-18-((1H-imidazo...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,wU:58.71,74.80,8.20,87.93,69.75,wD:48.59,22.31,4.4,33.48,95.101,(27.14,-11.6,;25.81,-10.83,;25.81,-9.29,;24.47,-8.52,;24.47,-6.97,;23.14,-6.2,;21.8,-6.97,;21.8,-8.5,;20.47,-6.2,;20.47,-4.66,;21.89,-4.07,;23.12,-5,;24.38,-4.1,;23.93,-2.63,;24.71,-1.31,;23.96,.04,;22.43,.05,;21.64,-1.26,;22.38,-2.61,;19.13,-6.97,;17.8,-6.2,;17.8,-4.66,;16.46,-6.97,;16.46,-8.51,;17.8,-9.28,;17.8,-10.82,;19.14,-11.58,;19.13,-13.12,;17.8,-13.89,;20.46,-13.9,;15.12,-6.21,;13.78,-6.97,;13.78,-8.51,;12.45,-6.2,;12.42,-4.66,;13.74,-3.87,;15.09,-4.61,;16.4,-3.81,;16.37,-2.28,;17.69,-1.49,;17.67,.05,;16.32,.8,;15,.01,;15.03,-1.52,;13.71,-2.32,;11.12,-6.97,;9.78,-6.21,;9.78,-4.66,;8.44,-6.98,;8.44,-8.51,;9.78,-9.29,;11.18,-8.65,;12.21,-9.8,;11.45,-11.14,;9.94,-10.82,;7.1,-6.21,;5.76,-6.97,;5.76,-8.5,;4.43,-6.2,;4.43,-4.66,;5.76,-3.89,;7.1,-4.65,;8.42,-3.88,;8.42,-2.34,;7.09,-1.57,;5.75,-2.34,;3.1,-6.99,;1.76,-6.22,;1.76,-4.68,;.42,-6.99,;.42,-8.53,;-.9,-6.21,;-2.25,-6.97,;-2.25,-8.51,;-3.58,-6.2,;-4.91,-6.98,;-3.58,-4.66,;-2.25,-3.89,;-.92,-4.66,;.41,-3.89,;.4,-2.35,;1.77,-1.59,;-.92,-1.58,;-2.25,-2.35,;25.81,-6.21,;25.81,-4.67,;27.15,-6.98,;28.48,-6.21,;28.48,-4.66,;29.82,-3.89,;31.15,-4.66,;29.82,-2.35,;29.82,-6.97,;29.82,-8.51,;31.15,-6.2,;32.49,-6.97,;32.49,-8.5,;33.83,-9.28,;35.16,-8.5,;36.5,-9.28,;36.5,-10.82,;35.16,-11.58,;33.83,-10.81,;33.83,-6.2,;35.15,-6.96,;33.83,-4.65,)| Show InChI InChI=1S/C76H91N17O13/c1-3-4-23-57(69(100)93-64(40-65(95)96)75(106)88-59(66(78)97)35-45-16-7-5-8-17-45)86-73(104)62(38-51-41-83-56-24-14-13-22-54(51)56)91-70(101)58(25-15-32-82-76(79)80)87-71(102)61(37-48-26-29-49-20-11-12-21-50(49)33-48)90-74(105)63(39-52-42-81-43-84-52)92-72(103)60(36-46-18-9-6-10-19-46)89-67(98)44(2)85-68(99)55(77)34-47-27-30-53(94)31-28-47/h5-14,16-22,24,26-31,33,41-44,55,57-64,83,94H,3-4,15,23,25,32,34-40,77H2,1-2H3,(H2,78,97)(H,81,84)(H,85,99)(H,86,104)(H,87,102)(H,88,106)(H,89,98)(H,90,105)(H,91,101)(H,92,103)(H,93,100)(H,95,96)(H4,79,80,82)/t44-,55+,57+,58+,59+,60+,61-,62+,63+,64+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human delta opioid receptor expressed in mouse HN9.10 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50321596

((3S,6R,9S,12S,15S,18S)-3-((1H-imidazol-5-yl)methyl...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C76H90N16O12/c1-4-5-23-58(68(97)90-64(38-66(94)95)73(102)87-60(67(78)96)32-45-16-7-6-8-17-45)85-71(100)62(34-51-39-83-57-24-14-13-22-54(51)57)89-69(98)59(25-15-28-82-76(79)80)86-70(99)61(33-46-26-27-47-18-9-10-19-48(47)31-46)88-72(101)63(36-52-40-81-42-84-52)91-74(103)65-35-49-20-11-12-21-50(49)41-92(65)75(104)56(77)37-55-43(2)29-53(93)30-44(55)3/h6-14,16-22,24,26-27,29-31,39-40,42,56,58-65,83,93H,4-5,15,23,25,28,32-38,41,77H2,1-3H3,(H2,78,96)(H,81,84)(H,85,100)(H,86,99)(H,87,102)(H,88,101)(H,89,98)(H,90,97)(H,91,103)(H,94,95)(H4,79,80,82)/t56-,58-,59-,60-,61+,62-,63-,64-,65?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50321609
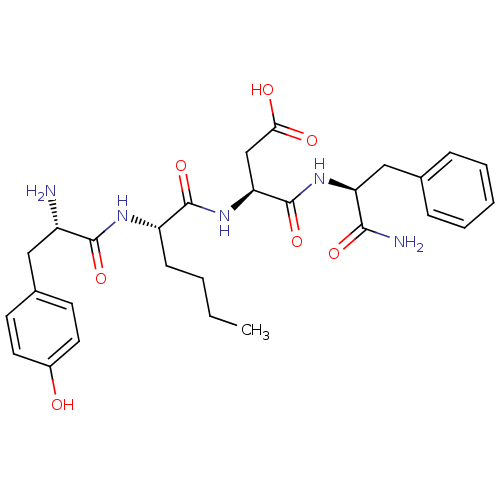
((S)-4-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)...)Show SMILES CCCC[C@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C28H37N5O7/c1-2-3-9-21(31-26(38)20(29)14-18-10-12-19(34)13-11-18)27(39)33-23(16-24(35)36)28(40)32-22(25(30)37)15-17-7-5-4-6-8-17/h4-8,10-13,20-23,34H,2-3,9,14-16,29H2,1H3,(H2,30,37)(H,31,38)(H,32,40)(H,33,39)(H,35,36)/t20-,21-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8(SO3) from human CCK2 receptor expressed in human HEK293 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data