 Found 418 hits with Last Name = 'ferrari' and Initial = 'v'
Found 418 hits with Last Name = 'ferrari' and Initial = 'v' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50246899

((S)-2-(3-((S)-1-carboxy-5-(4-iodobenzamido)pentyl)...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNC(=O)c1ccc(I)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H24IN3O8/c20-12-6-4-11(5-7-12)16(26)21-10-2-1-3-13(17(27)28)22-19(31)23-14(18(29)30)8-9-15(24)25/h4-7,13-14H,1-3,8-10H2,(H,21,26)(H,24,25)(H,27,28)(H,29,30)(H2,22,23,31)/t13-,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of GCP2 by top scintillation counter in presence of 30 nM NAA[3]G |
Bioorg Med Chem Lett 20: 7222-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.109
BindingDB Entry DOI: 10.7270/Q2GB24B9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional glutamate/proline--tRNA ligase
(Homo sapiens (Human)) | BDBM50614449

(CHEMBL1163086)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COS(=O)(=O)NC(=O)[C@@H]2CCCN2)[C@@H](O)[C@H]1O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PDB
UniChem
| | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50322367
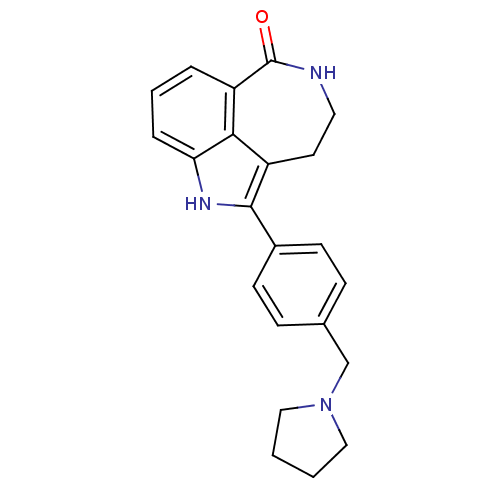
(5-(4-(pyrrolidin-1-ylmethyl)phenyl)-2,3,4,6-tetrah...)Show SMILES O=C1NCCc2c([nH]c3cccc1c23)-c1ccc(CN2CCCC2)cc1 Show InChI InChI=1S/C22H23N3O/c26-22-18-4-3-5-19-20(18)17(10-11-23-22)21(24-19)16-8-6-15(7-9-16)14-25-12-1-2-13-25/h3-9,24H,1-2,10-14H2,(H,23,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Brain Science Institute
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 |
J Med Chem 53: 4561-84 (2010)
Article DOI: 10.1021/jm100012m
BindingDB Entry DOI: 10.7270/Q2NV9JF0 |
More data for this
Ligand-Target Pair | |
Proline--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50614449

(CHEMBL1163086)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COS(=O)(=O)NC(=O)[C@@H]2CCCN2)[C@@H](O)[C@H]1O |r| | KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PDB
UniChem
| | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50154730
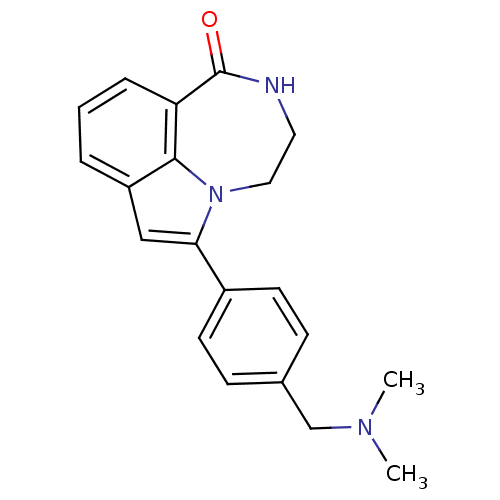
(6-(4-((dimethylamino)methyl)phenyl)-3,4-dihydro-[1...)Show InChI InChI=1S/C20H21N3O/c1-22(2)13-14-6-8-15(9-7-14)18-12-16-4-3-5-17-19(16)23(18)11-10-21-20(17)24/h3-9,12H,10-11,13H2,1-2H3,(H,21,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Brain Science Institute
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 |
J Med Chem 53: 4561-84 (2010)
Article DOI: 10.1021/jm100012m
BindingDB Entry DOI: 10.7270/Q2NV9JF0 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50401014
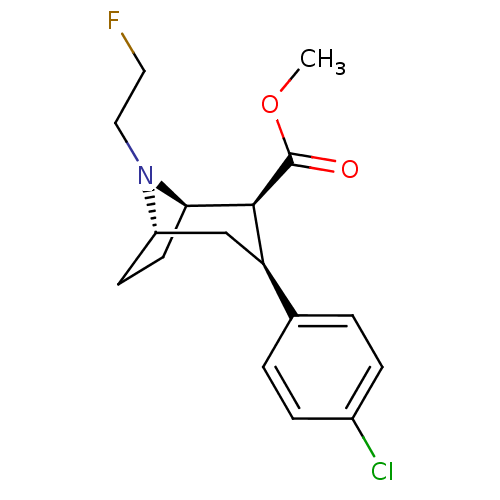
(CHEMBL2206307)Show SMILES COC(=O)[C@@H]1[C@H]2CC[C@@H](C[C@@H]1c1ccc(Cl)cc1)N2CCF |r,TLB:11:10:6.7:18,THB:2:4:6.7:18| Show InChI InChI=1S/C17H21ClFNO2/c1-22-17(21)16-14(11-2-4-12(18)5-3-11)10-13-6-7-15(16)20(13)9-8-19/h2-5,13-16H,6-10H2,1H3/t13-,14+,15+,16-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg University
Curated by ChEMBL
| Assay Description
Binding affinity to DAT |
Bioorg Med Chem Lett 22: 679-82 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.053
BindingDB Entry DOI: 10.7270/Q2ZW1N2D |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM8296
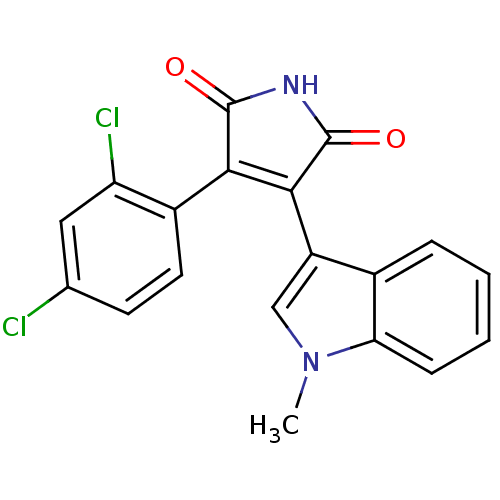
(3-(2,4-dichlorophenyl)-4-(1-methyl-1H-indol-3-yl)-...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2ccc(Cl)cc2Cl)c2ccccc12 |t:4| Show InChI InChI=1S/C19H12Cl2N2O2/c1-23-9-13(11-4-2-3-5-15(11)23)17-16(18(24)22-19(17)25)12-7-6-10(20)8-14(12)21/h2-9H,1H3,(H,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by ChEMBL
| Assay Description
Competitive inhibition of full length recombinant human GSK-3alpha expressed in baculovirus infected insect Sf9 cells using GS-2 peptide as substrate... |
ACS Med Chem Lett 6: 548-52 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00044
BindingDB Entry DOI: 10.7270/Q2348N35 |
More data for this
Ligand-Target Pair | |
Bifunctional glutamate/proline--tRNA ligase
(Homo sapiens (Human)) | BDBM50614450
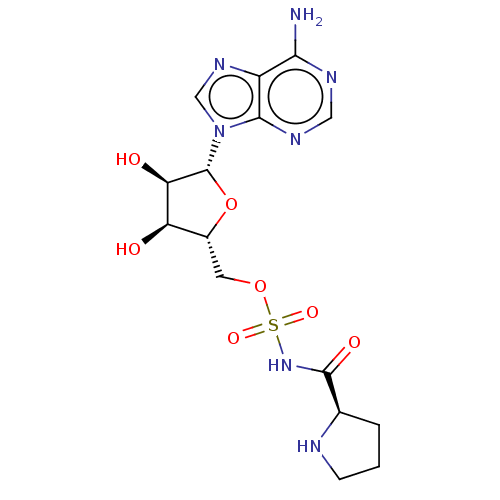
(CHEMBL5279127)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COS(=O)(=O)NC(=O)[C@H]2CCCN2)[C@@H](O)[C@H]1O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50614446

(CHEMBL5283680)Show SMILES C[C@@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@@H](Cn2cnc3c(N)ncnc23)[C@H](O)[C@@H]1O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 232 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glutamine--tRNA ligase
(Escherichia coli) | BDBM50614451
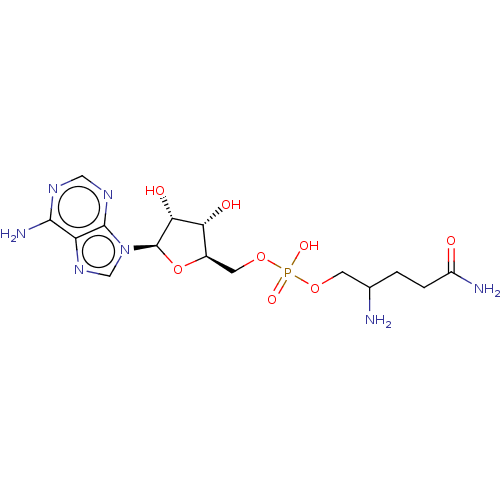
(CHEMBL5291357)Show SMILES NC(CCC(N)=O)COP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| UniChem
| | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Proline--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50614450

(CHEMBL5279127)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COS(=O)(=O)NC(=O)[C@H]2CCCN2)[C@@H](O)[C@H]1O |r| | KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50614428

(CHEMBL5283919)Show SMILES CCOC(=O)[C@H](Cc1ccccc1)NP(=O)(N[C@@H](Cc1ccccc1)C(=O)OCC)OCOCCN |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM108460

(CHEMBL2178393 | US11191732, Example 1 | US8604016,...)Show InChI InChI=1S/C16H18N6OS3/c17-15-21-19-13(25-15)6-8-24-9-7-14-20-22-16(26-14)18-12(23)10-11-4-2-1-3-5-11/h1-5H,6-10H2,(H2,17,21)(H,18,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of human kidney glutaminase (124 to 669) assessed as reduction of glutamine hydrolysis by double-reciprocal plot analysis |
J Med Chem 55: 10551-63 (2012)
Article DOI: 10.1021/jm301191p
BindingDB Entry DOI: 10.7270/Q2VD70M7 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Sus scrofa (pig)) | BDBM50031467

(5-HYDROXY-2-(HYDROXYMETHYL)-4H-PYRAN-4-ONE | 5-Hyd...)Show InChI InChI=1S/C6H6O4/c7-2-4-1-5(8)6(9)3-10-4/h1,3,7,9H,2H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University
Curated by ChEMBL
| Assay Description
Competitive inhibition of pig kidney DAAO using D-Alanine as substrate by Michaelis-Menten plot analysis |
Bioorg Med Chem Lett 23: 3910-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.062
BindingDB Entry DOI: 10.7270/Q2K35W2G |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50304738
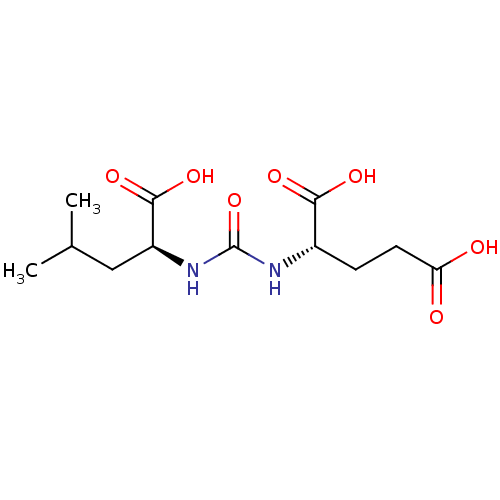
(2-(3-((S)-1-carboxy-3-methylbutyl)ureido)pentanedi...)Show SMILES CC(C)C[C@H](NC(=O)N[C@@H](CCC(O)=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C12H20N2O7/c1-6(2)5-8(11(19)20)14-12(21)13-7(10(17)18)3-4-9(15)16/h6-8H,3-5H2,1-2H3,(H,15,16)(H,17,18)(H,19,20)(H2,13,14,21)/t7-,8-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biotechnology of the Czech Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of N-terminally tagged human recombinant GCP2 (44 to 750 residues) extracellular domain expressed in Drosophila melanogaster S2 cells prei... |
Bioorg Med Chem 27: 255-264 (2019)
Article DOI: 10.1016/j.bmc.2018.11.022
BindingDB Entry DOI: 10.7270/Q2F47SC4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50503760
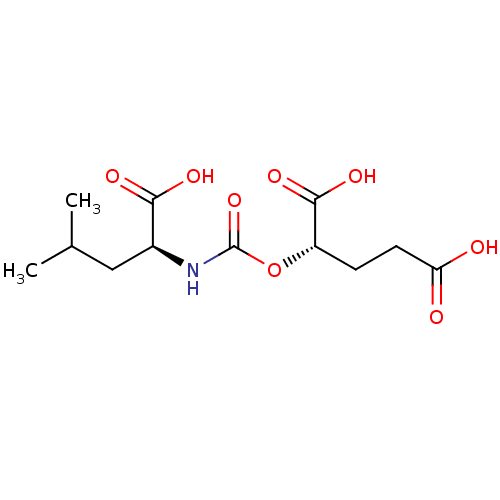
(CHEMBL4442450)Show SMILES CC(C)C[C@H](NC(=O)O[C@@H](CCC(O)=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C12H19NO8/c1-6(2)5-7(10(16)17)13-12(20)21-8(11(18)19)3-4-9(14)15/h6-8H,3-5H2,1-2H3,(H,13,20)(H,14,15)(H,16,17)(H,18,19)/t7-,8-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biotechnology of the Czech Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of N-terminally tagged human recombinant GCP2 (44 to 750 residues) extracellular domain expressed in Drosophila melanogaster S2 cells prei... |
Bioorg Med Chem 27: 255-264 (2019)
Article DOI: 10.1016/j.bmc.2018.11.022
BindingDB Entry DOI: 10.7270/Q2F47SC4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27525
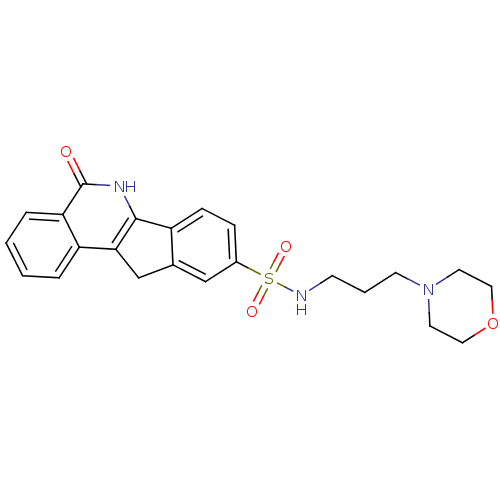
(N-[3-(morpholin-4-yl)propyl]-8-oxo-9-azatetracyclo...)Show SMILES O=c1[nH]c-2c(Cc3cc(ccc-23)S(=O)(=O)NCCCN2CCOCC2)c2ccccc12 Show InChI InChI=1S/C23H25N3O4S/c27-23-20-5-2-1-4-19(20)21-15-16-14-17(6-7-18(16)22(21)25-23)31(28,29)24-8-3-9-26-10-12-30-13-11-26/h1-2,4-7,14,24H,3,8-13,15H2,(H,25,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Brain Science Institute
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 |
J Med Chem 53: 4561-84 (2010)
Article DOI: 10.1021/jm100012m
BindingDB Entry DOI: 10.7270/Q2NV9JF0 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50296249
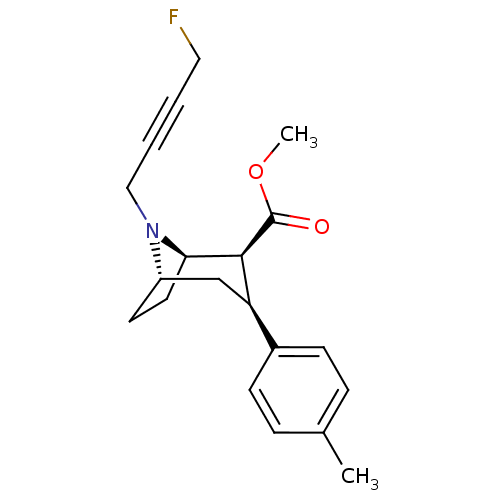
((1R,2S,3S,5S)-methyl 8-(4-fluorobut-2-ynyl)-3-p-to...)Show SMILES COC(=O)[C@@H]1[C@H]2CC[C@@H](C[C@@H]1c1ccc(C)cc1)N2CC#CCF |r,TLB:19:18:10.4.9:6.7| Show InChI InChI=1S/C20H24FNO2/c1-14-5-7-15(8-6-14)17-13-16-9-10-18(19(17)20(23)24-2)22(16)12-4-3-11-21/h5-8,16-19H,9-13H2,1-2H3/t16-,17+,18+,19-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.93 | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg University
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN35,428 from human DAT expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 679-82 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.053
BindingDB Entry DOI: 10.7270/Q2ZW1N2D |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50392040
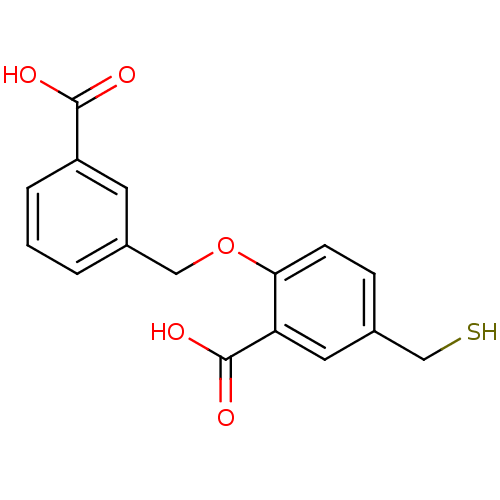
(CHEMBL2152556)Show InChI InChI=1S/C16H14O5S/c17-15(18)12-3-1-2-10(6-12)8-21-14-5-4-11(9-22)7-13(14)16(19)20/h1-7,22H,8-9H2,(H,17,18)(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay |
J Med Chem 55: 5922-32 (2012)
Article DOI: 10.1021/jm300488m
BindingDB Entry DOI: 10.7270/Q21J9BWN |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50392045
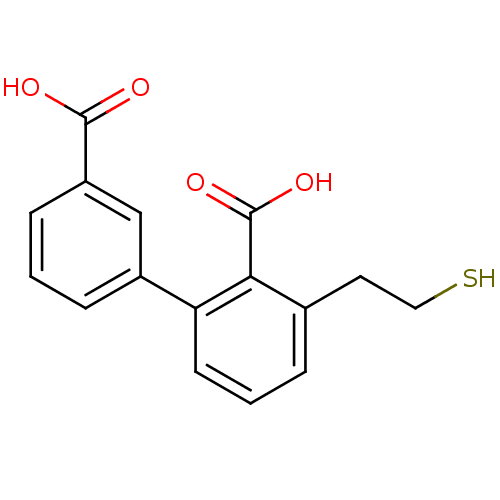
(CHEMBL2152561)Show InChI InChI=1S/C16H14O4S/c17-15(18)12-5-1-4-11(9-12)13-6-2-3-10(7-8-21)14(13)16(19)20/h1-6,9,21H,7-8H2,(H,17,18)(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay |
J Med Chem 55: 5922-32 (2012)
Article DOI: 10.1021/jm300488m
BindingDB Entry DOI: 10.7270/Q21J9BWN |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50296249

((1R,2S,3S,5S)-methyl 8-(4-fluorobut-2-ynyl)-3-p-to...)Show SMILES COC(=O)[C@@H]1[C@H]2CC[C@@H](C[C@@H]1c1ccc(C)cc1)N2CC#CCF |r,TLB:19:18:10.4.9:6.7| Show InChI InChI=1S/C20H24FNO2/c1-14-5-7-15(8-6-14)17-13-16-9-10-18(19(17)20(23)24-2)22(16)12-4-3-11-21/h5-8,16-19H,9-13H2,1-2H3/t16-,17+,18+,19-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.26 | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DA from human DAT expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 679-82 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.053
BindingDB Entry DOI: 10.7270/Q2ZW1N2D |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50322367

(5-(4-(pyrrolidin-1-ylmethyl)phenyl)-2,3,4,6-tetrah...)Show SMILES O=C1NCCc2c([nH]c3cccc1c23)-c1ccc(CN2CCCC2)cc1 Show InChI InChI=1S/C22H23N3O/c26-22-18-4-3-5-19-20(18)17(10-11-23-22)21(24-19)16-8-6-15(7-9-16)14-25-12-1-2-13-25/h3-9,24H,1-2,10-14H2,(H,23,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Brain Science Institute
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 |
J Med Chem 53: 4561-84 (2010)
Article DOI: 10.1021/jm100012m
BindingDB Entry DOI: 10.7270/Q2NV9JF0 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50392046
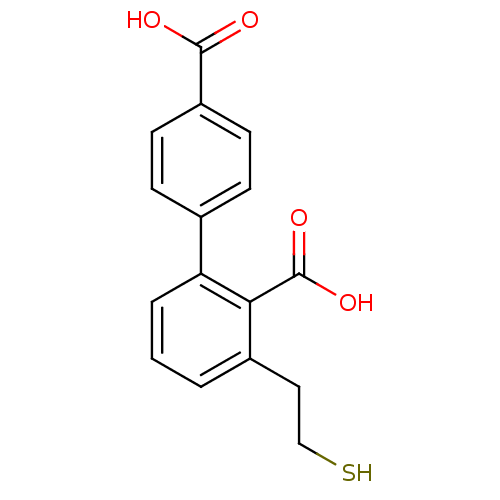
(CHEMBL2152562)Show InChI InChI=1S/C16H14O4S/c17-15(18)12-6-4-10(5-7-12)13-3-1-2-11(8-9-21)14(13)16(19)20/h1-7,21H,8-9H2,(H,17,18)(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay |
J Med Chem 55: 5922-32 (2012)
Article DOI: 10.1021/jm300488m
BindingDB Entry DOI: 10.7270/Q21J9BWN |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM27721
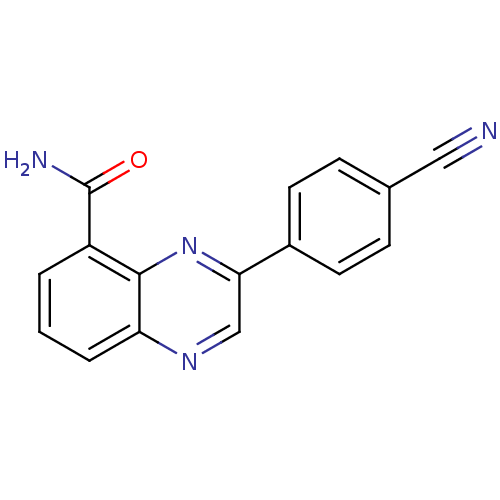
(3-(4-cyanophenyl)quinoxaline-5-carboxamide | CHEMB...)Show InChI InChI=1S/C16H10N4O/c17-8-10-4-6-11(7-5-10)14-9-19-13-3-1-2-12(16(18)21)15(13)20-14/h1-7,9H,(H2,18,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Brain Science Institute
Curated by ChEMBL
| Assay Description
Inhibition of PARP2 |
J Med Chem 53: 4561-84 (2010)
Article DOI: 10.1021/jm100012m
BindingDB Entry DOI: 10.7270/Q2NV9JF0 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50220858
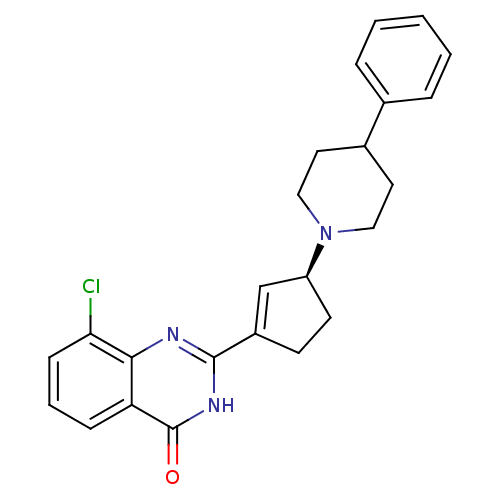
((S)-8-chloro-2-(3-(4-phenylpiperidin-1-yl)cyclopen...)Show SMILES Clc1cccc2c1nc([nH]c2=O)C1=C[C@H](CC1)N1CCC(CC1)c1ccccc1 |t:14| Show InChI InChI=1S/C24H24ClN3O/c25-21-8-4-7-20-22(21)26-23(27-24(20)29)18-9-10-19(15-18)28-13-11-17(12-14-28)16-5-2-1-3-6-16/h1-8,15,17,19H,9-14H2,(H,26,27,29)/t19-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Brain Science Institute
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 |
J Med Chem 53: 4561-84 (2010)
Article DOI: 10.1021/jm100012m
BindingDB Entry DOI: 10.7270/Q2NV9JF0 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50503756
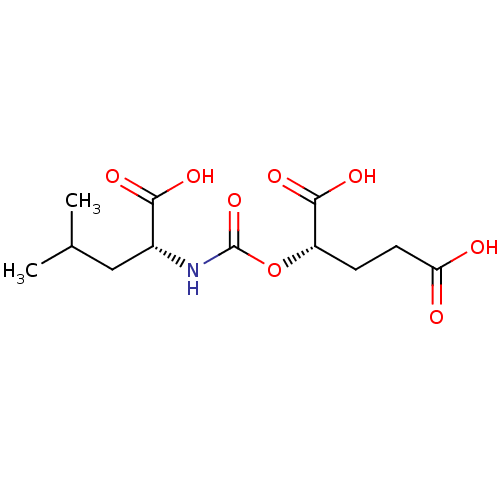
(CHEMBL4473741)Show SMILES CC(C)C[C@@H](NC(=O)O[C@@H](CCC(O)=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C12H19NO8/c1-6(2)5-7(10(16)17)13-12(20)21-8(11(18)19)3-4-9(14)15/h6-8H,3-5H2,1-2H3,(H,13,20)(H,14,15)(H,16,17)(H,18,19)/t7-,8+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biotechnology of the Czech Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of N-terminally tagged human recombinant GCP2 (44 to 750 residues) extracellular domain expressed in Drosophila melanogaster S2 cells prei... |
Bioorg Med Chem 27: 255-264 (2019)
Article DOI: 10.1016/j.bmc.2018.11.022
BindingDB Entry DOI: 10.7270/Q2F47SC4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50068775
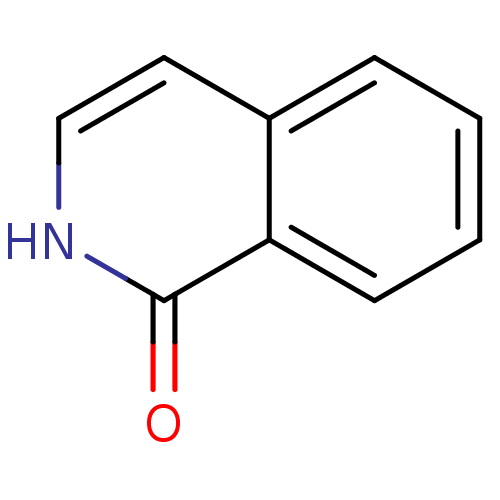
(2H-Isoquinolin-1-one | CHEMBL339695 | isoquinolin-...)Show InChI InChI=1S/C9H7NO/c11-9-8-4-2-1-3-7(8)5-6-10-9/h1-6H,(H,10,11) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Brain Science Institute
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 |
J Med Chem 53: 4561-84 (2010)
Article DOI: 10.1021/jm100012m
BindingDB Entry DOI: 10.7270/Q2NV9JF0 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50416360
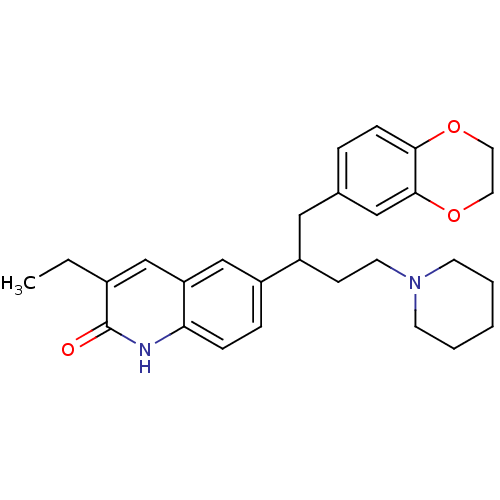
(CHEMBL1171298)Show SMILES CCc1cc2cc(ccc2[nH]c1=O)C(CCN1CCCCC1)Cc1ccc2OCCOc2c1 Show InChI InChI=1S/C28H34N2O3/c1-2-21-18-24-19-22(7-8-25(24)29-28(21)31)23(10-13-30-11-4-3-5-12-30)16-20-6-9-26-27(17-20)33-15-14-32-26/h6-9,17-19,23H,2-5,10-16H2,1H3,(H,29,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Brain Science Institute
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 |
J Med Chem 53: 4561-84 (2010)
Article DOI: 10.1021/jm100012m
BindingDB Entry DOI: 10.7270/Q2NV9JF0 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50416361
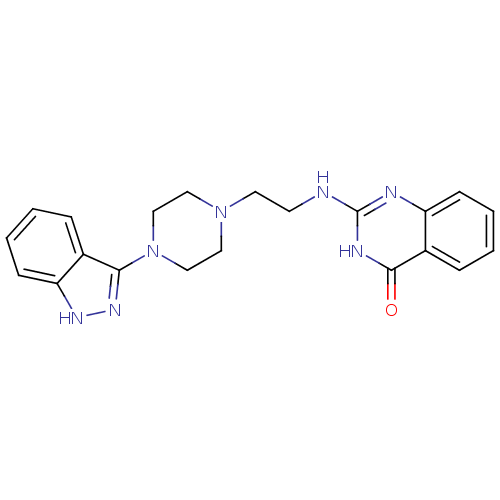
(CHEMBL1171304)Show SMILES O=c1[nH]c(NCCN2CCN(CC2)c2n[nH]c3ccccc23)nc2ccccc12 Show InChI InChI=1S/C21H23N7O/c29-20-16-6-2-3-7-17(16)23-21(24-20)22-9-10-27-11-13-28(14-12-27)19-15-5-1-4-8-18(15)25-26-19/h1-8H,9-14H2,(H,25,26)(H2,22,23,24,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.93 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Brain Science Institute
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 |
J Med Chem 53: 4561-84 (2010)
Article DOI: 10.1021/jm100012m
BindingDB Entry DOI: 10.7270/Q2NV9JF0 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50101114
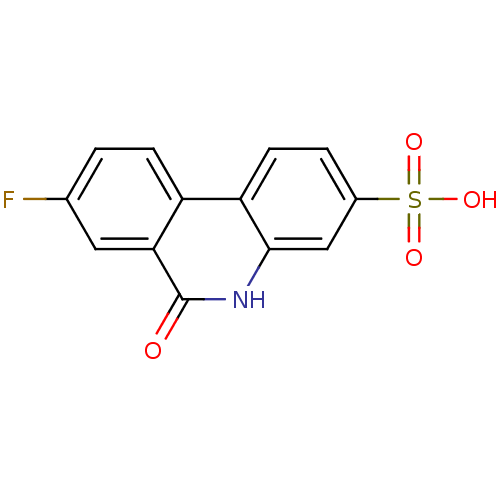
(8-Fluoro-6-oxo-5,6-dihydro-phenanthridine-3-sulfon...)Show InChI InChI=1S/C13H8FNO4S/c14-7-1-3-9-10-4-2-8(20(17,18)19)6-12(10)15-13(16)11(9)5-7/h1-6H,(H,15,16)(H,17,18,19) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against purified recombinant human Poly (ADP-ribose) polymerase 1 |
Bioorg Med Chem Lett 11: 1687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2348KWN |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50614445
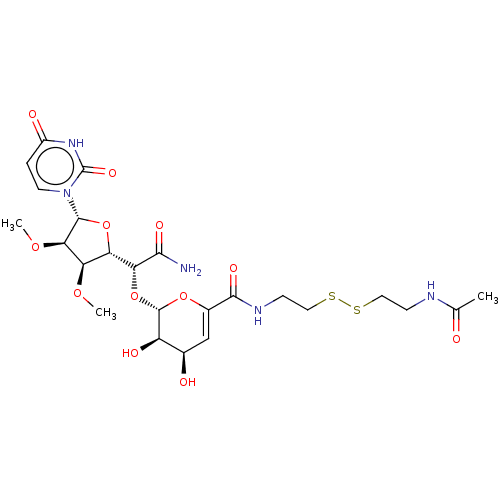
(CHEMBL5282738)Show SMILES [H][C@@]1(O[C@H]([C@H](OC)[C@@H]1OC)n1ccc(=O)[nH]c1=O)[C@@H](O[C@@H]1OC(=C[C@@H](O)[C@H]1O)C(=O)NCCSSCCNC(C)=O)C(N)=O |r,c:24| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50614431
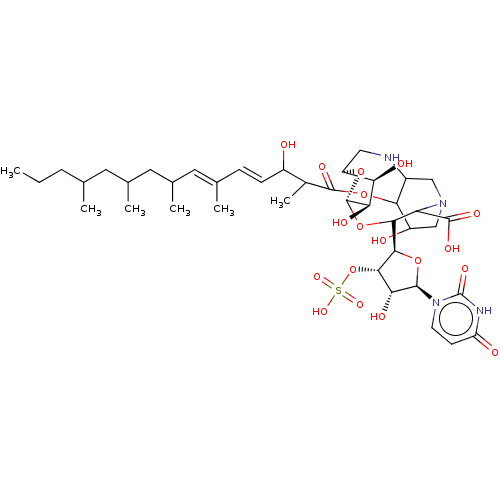
(CHEMBL5289236)Show SMILES CCCC(C)CC(C)CC(C)\C=C(/C)\C=C\C(O)C(C)C(=O)OC1C(O)CN2CC1NC[C@H]1O[C@@H](OC([C@H]3O[C@H]([C@H](O)[C@@H]3OS(O)(=O)=O)n3ccc(=O)[nH]c3=O)C2C(O)=O)[C@H](O)[C@@H]1O |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27708
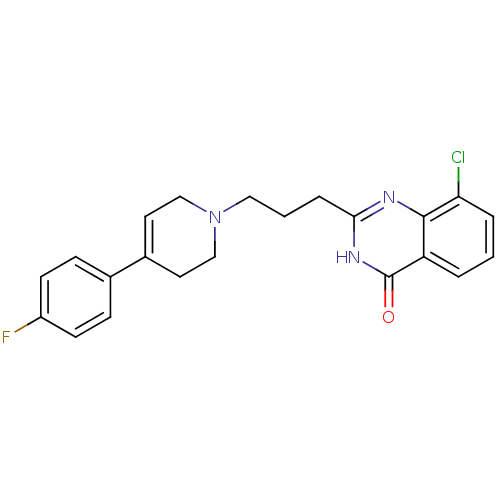
(8-chloro-2-{3-[4-(4-fluorophenyl)-1,2,3,6-tetrahyd...)Show SMILES Fc1ccc(cc1)C1=CCN(CCCc2nc3c(Cl)cccc3c(=O)[nH]2)CC1 |t:8| Show InChI InChI=1S/C22H21ClFN3O/c23-19-4-1-3-18-21(19)25-20(26-22(18)28)5-2-12-27-13-10-16(11-14-27)15-6-8-17(24)9-7-15/h1,3-4,6-10H,2,5,11-14H2,(H,25,26,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Brain Science Institute
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 |
J Med Chem 53: 4561-84 (2010)
Article DOI: 10.1021/jm100012m
BindingDB Entry DOI: 10.7270/Q2NV9JF0 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50101129
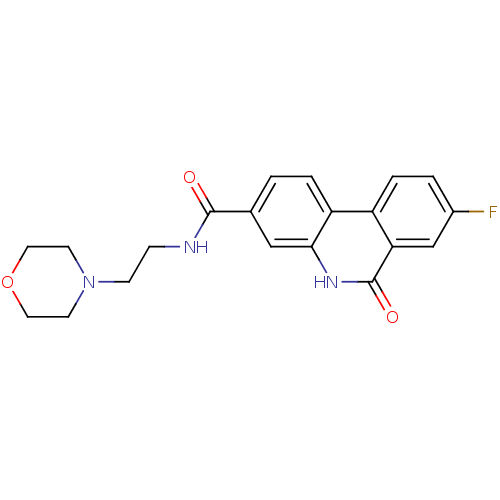
(8-Fluoro-6-oxo-5,6-dihydro-phenanthridine-3-carbox...)Show SMILES Fc1ccc2c3ccc(cc3[nH]c(=O)c2c1)C(=O)NCCN1CCOCC1 Show InChI InChI=1S/C20H20FN3O3/c21-14-2-4-15-16-3-1-13(11-18(16)23-20(26)17(15)12-14)19(25)22-5-6-24-7-9-27-10-8-24/h1-4,11-12H,5-10H2,(H,22,25)(H,23,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against purified recombinant human Poly (ADP-ribose) polymerase 1 |
Bioorg Med Chem Lett 11: 1687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2348KWN |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50614447

(CHEMBL5287030)Show SMILES CCC(C)C(N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)nc(I)nc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50101129

(8-Fluoro-6-oxo-5,6-dihydro-phenanthridine-3-carbox...)Show SMILES Fc1ccc2c3ccc(cc3[nH]c(=O)c2c1)C(=O)NCCN1CCOCC1 Show InChI InChI=1S/C20H20FN3O3/c21-14-2-4-15-16-3-1-13(11-18(16)23-20(26)17(15)12-14)19(25)22-5-6-24-7-9-27-10-8-24/h1-4,11-12H,5-10H2,(H,22,25)(H,23,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Brain Science Institute
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 |
J Med Chem 53: 4561-84 (2010)
Article DOI: 10.1021/jm100012m
BindingDB Entry DOI: 10.7270/Q2NV9JF0 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM17762

(3-[2-carboxy-2-(3-sulfanylpropyl)ethyl]benzoic aci...)Show InChI InChI=1S/C13H16O4S/c14-12(15)10-4-1-3-9(7-10)8-11(13(16)17)5-2-6-18/h1,3-4,7,11,18H,2,5-6,8H2,(H,14,15)(H,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay |
J Med Chem 55: 5922-32 (2012)
Article DOI: 10.1021/jm300488m
BindingDB Entry DOI: 10.7270/Q21J9BWN |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM17762

(3-[2-carboxy-2-(3-sulfanylpropyl)ethyl]benzoic aci...)Show InChI InChI=1S/C13H16O4S/c14-12(15)10-4-1-3-9(7-10)8-11(13(16)17)5-2-6-18/h1,3-4,7,11,18H,2,5-6,8H2,(H,14,15)(H,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of GCP2 by top scintillation counter in presence of 30 nM NAA[3]G |
Bioorg Med Chem Lett 20: 7222-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.109
BindingDB Entry DOI: 10.7270/Q2GB24B9 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50503754
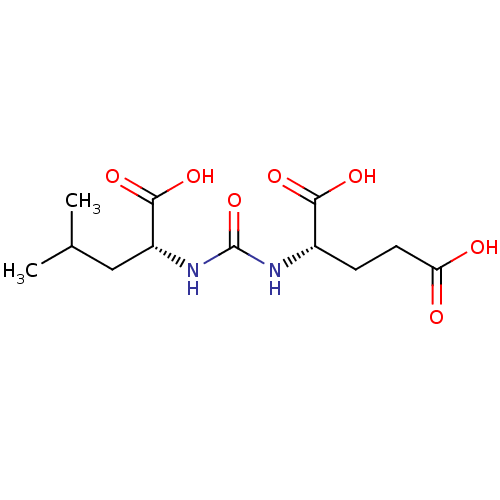
(CHEMBL4458733)Show SMILES CC(C)C[C@@H](NC(=O)N[C@@H](CCC(O)=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C12H20N2O7/c1-6(2)5-8(11(19)20)14-12(21)13-7(10(17)18)3-4-9(15)16/h6-8H,3-5H2,1-2H3,(H,15,16)(H,17,18)(H,19,20)(H2,13,14,21)/t7-,8+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biotechnology of the Czech Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of N-terminally tagged human recombinant GCP2 (44 to 750 residues) extracellular domain expressed in Drosophila melanogaster S2 cells prei... |
Bioorg Med Chem 27: 255-264 (2019)
Article DOI: 10.1016/j.bmc.2018.11.022
BindingDB Entry DOI: 10.7270/Q2F47SC4 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50392041
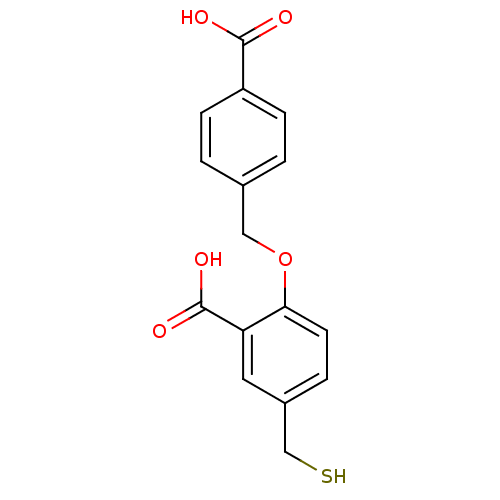
(CHEMBL2152557)Show InChI InChI=1S/C16H14O5S/c17-15(18)12-4-1-10(2-5-12)8-21-14-6-3-11(9-22)7-13(14)16(19)20/h1-7,22H,8-9H2,(H,17,18)(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay |
J Med Chem 55: 5922-32 (2012)
Article DOI: 10.1021/jm300488m
BindingDB Entry DOI: 10.7270/Q21J9BWN |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Staphylococcus aureus (strain MRSA252)) | BDBM50614429

(CHEMBL5265940)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1OC)n1ccc(=O)[nH]c1=O)[C@@H](O[C@@H]1OC(=C[C@@H](O)[C@H]1O)C(=O)N[C@H]1CCC[C@@H](C)NC1=O)C(N)=O |r,c:23| | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50503757

(CHEMBL4541841)Show SMILES CC(C)C[C@H](OC(=O)N[C@@H](CCC(O)=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C12H19NO8/c1-6(2)5-8(11(18)19)21-12(20)13-7(10(16)17)3-4-9(14)15/h6-8H,3-5H2,1-2H3,(H,13,20)(H,14,15)(H,16,17)(H,18,19)/t7-,8-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biotechnology of the Czech Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of N-terminally tagged human recombinant GCP2 (44 to 750 residues) extracellular domain expressed in Drosophila melanogaster S2 cells prei... |
Bioorg Med Chem 27: 255-264 (2019)
Article DOI: 10.1016/j.bmc.2018.11.022
BindingDB Entry DOI: 10.7270/Q2F47SC4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50120267
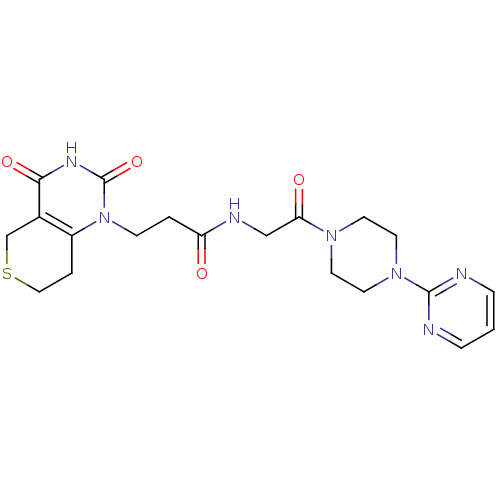
(3-(2,4-Dioxo-3,4,7,8-tetrahydro-2H,5H-thiopyrano[4...)Show SMILES O=C(CCn1c2CCSCc2c(=O)[nH]c1=O)NCC(=O)N1CCN(CC1)c1ncccn1 Show InChI InChI=1S/C20H25N7O4S/c28-16(2-6-27-15-3-11-32-13-14(15)18(30)24-20(27)31)23-12-17(29)25-7-9-26(10-8-25)19-21-4-1-5-22-19/h1,4-5H,2-3,6-13H2,(H,23,28)(H,24,30,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Brain Science Institute
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 |
J Med Chem 53: 4561-84 (2010)
Article DOI: 10.1021/jm100012m
BindingDB Entry DOI: 10.7270/Q2NV9JF0 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27497
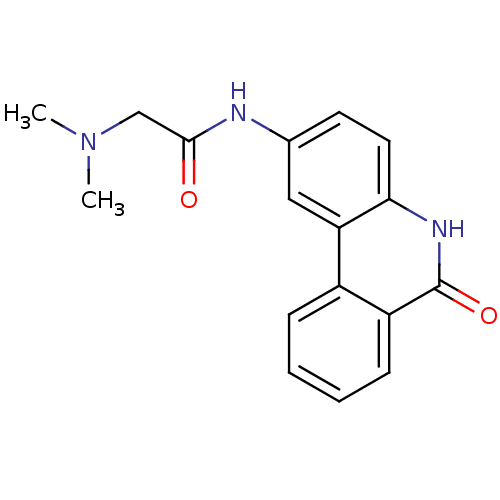
(2-(dimethylamino)-N-(6-oxo-5,6-dihydrophenanthridi...)Show InChI InChI=1S/C17H17N3O2/c1-20(2)10-16(21)18-11-7-8-15-14(9-11)12-5-3-4-6-13(12)17(22)19-15/h3-9H,10H2,1-2H3,(H,18,21)(H,19,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Brain Science Institute
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 |
J Med Chem 53: 4561-84 (2010)
Article DOI: 10.1021/jm100012m
BindingDB Entry DOI: 10.7270/Q2NV9JF0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50101124
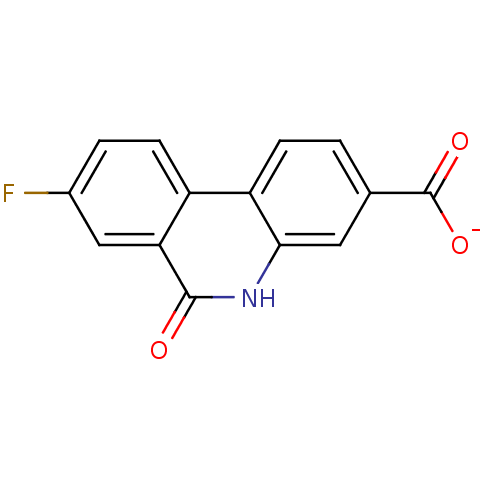
(CHEMBL298053 | sodium 8-fluoro-6-oxo-5,6-dihydroph...)Show InChI InChI=1S/C14H8FNO3/c15-8-2-4-9-10-3-1-7(14(18)19)5-12(10)16-13(17)11(9)6-8/h1-6H,(H,16,17)(H,18,19)/p-1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against purified recombinant human Poly (ADP-ribose) polymerase 1 |
Bioorg Med Chem Lett 11: 1687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2348KWN |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50332228
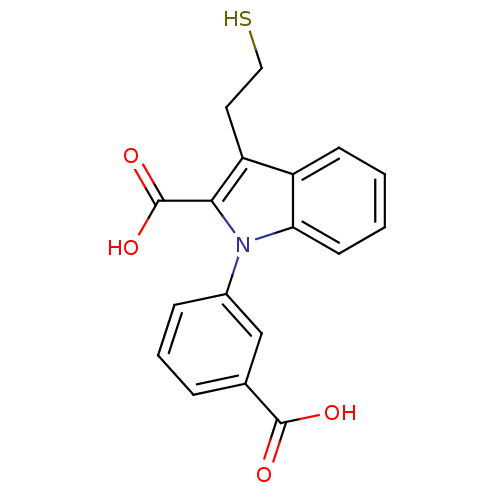
(1-(3-Carboxyphenyl)-3-(2-mercapto-ethyl)-1H-indole...)Show InChI InChI=1S/C18H15NO4S/c20-17(21)11-4-3-5-12(10-11)19-15-7-2-1-6-13(15)14(8-9-24)16(19)18(22)23/h1-7,10,24H,8-9H2,(H,20,21)(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of GCP2 by top scintillation counter in presence of 30 nM NAA[3]G |
Bioorg Med Chem Lett 20: 7222-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.109
BindingDB Entry DOI: 10.7270/Q2GB24B9 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50332218
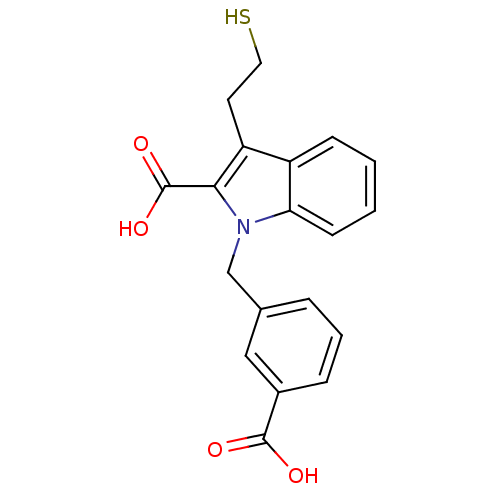
(1-[(3-carboxyphenyl)methyl]-3-(2-mercaptoethyl)-1H...)Show InChI InChI=1S/C19H17NO4S/c21-18(22)13-5-3-4-12(10-13)11-20-16-7-2-1-6-14(16)15(8-9-25)17(20)19(23)24/h1-7,10,25H,8-9,11H2,(H,21,22)(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of GCP2 by top scintillation counter in presence of 30 nM NAA[3]G |
Bioorg Med Chem Lett 20: 7222-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.109
BindingDB Entry DOI: 10.7270/Q2GB24B9 |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Bacillus subtilis) | BDBM50614433

(CHEMBL5272467)Show SMILES C[C@@H](NC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O)C(=O)N[C@@H]([C@H](C)N(C)C(=O)[C@@H](N)CN)C(=O)N\C=C1\C[C@@H](O)[C@@H](O1)n1ccc(=O)[nH]c1=O |r| | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50332217
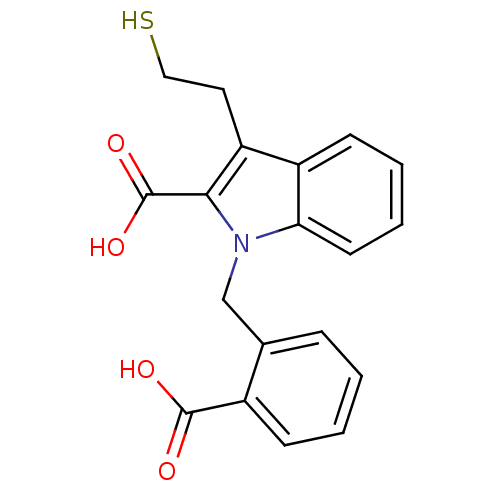
(1-[(2-carboxyphenyl)methyl]-3-(2-mercaptoethyl)-1H...)Show InChI InChI=1S/C19H17NO4S/c21-18(22)13-6-2-1-5-12(13)11-20-16-8-4-3-7-14(16)15(9-10-25)17(20)19(23)24/h1-8,25H,9-11H2,(H,21,22)(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of GCP2 by top scintillation counter in presence of 30 nM NAA[3]G |
Bioorg Med Chem Lett 20: 7222-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.109
BindingDB Entry DOI: 10.7270/Q2GB24B9 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50332228

(1-(3-Carboxyphenyl)-3-(2-mercapto-ethyl)-1H-indole...)Show InChI InChI=1S/C18H15NO4S/c20-17(21)11-4-3-5-12(10-11)19-15-7-2-1-6-13(15)14(8-9-24)16(19)18(22)23/h1-7,10,24H,8-9H2,(H,20,21)(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay |
J Med Chem 55: 5922-32 (2012)
Article DOI: 10.1021/jm300488m
BindingDB Entry DOI: 10.7270/Q21J9BWN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data