 Found 101 hits with Last Name = 'finck' and Initial = 'bn'
Found 101 hits with Last Name = 'finck' and Initial = 'bn' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50391056
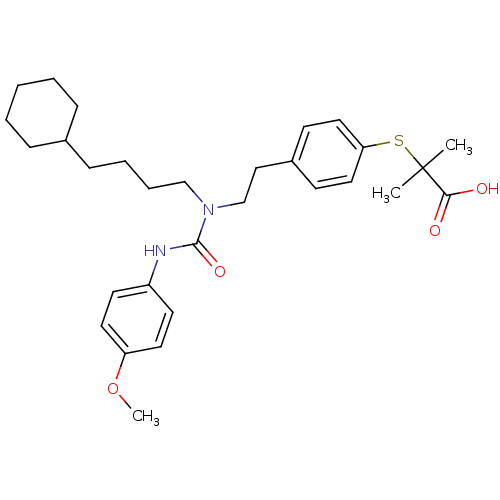
(CHEMBL2088421)Show SMILES COc1ccc(NC(=O)N(CCCCC2CCCCC2)CCc2ccc(SC(C)(C)C(O)=O)cc2)cc1 Show InChI InChI=1S/C30H42N2O4S/c1-30(2,28(33)34)37-27-18-12-24(13-19-27)20-22-32(21-8-7-11-23-9-5-4-6-10-23)29(35)31-25-14-16-26(36-3)17-15-25/h12-19,23H,4-11,20-22H2,1-3H3,(H,31,35)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Agonist activity at PPARalpha-LBD expressed in HEK293 cells co-expressing GAL4-DBD after 16 to 19 hrs by beta lactamase reporter gene assay |
Bioorg Med Chem Lett 22: 6233-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.010
BindingDB Entry DOI: 10.7270/Q25M66SF |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50099491
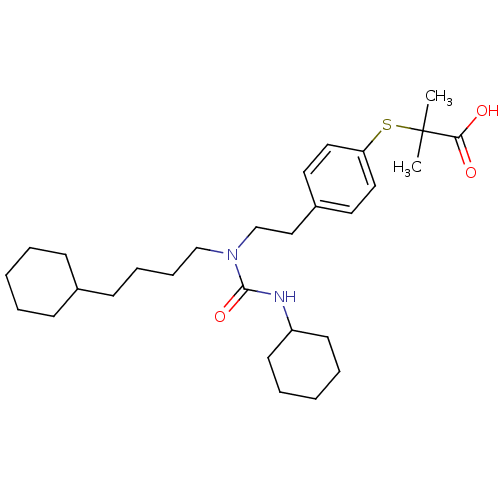
(2-(4-(2-(3-cyclohexyl-1-(4-cyclohexylbutyl)ureido)...)Show SMILES CC(C)(Sc1ccc(CCN(CCCCC2CCCCC2)C(=O)NC2CCCCC2)cc1)C(O)=O Show InChI InChI=1S/C29H46N2O3S/c1-29(2,27(32)33)35-26-18-16-24(17-19-26)20-22-31(28(34)30-25-14-7-4-8-15-25)21-10-9-13-23-11-5-3-6-12-23/h16-19,23,25H,3-15,20-22H2,1-2H3,(H,30,34)(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Agonist activity at PPARalpha-LBD expressed in HEK293 cells co-expressing GAL4-DBD after 16 to 19 hrs by beta lactamase reporter gene assay |
Bioorg Med Chem Lett 22: 6233-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.010
BindingDB Entry DOI: 10.7270/Q25M66SF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50391057
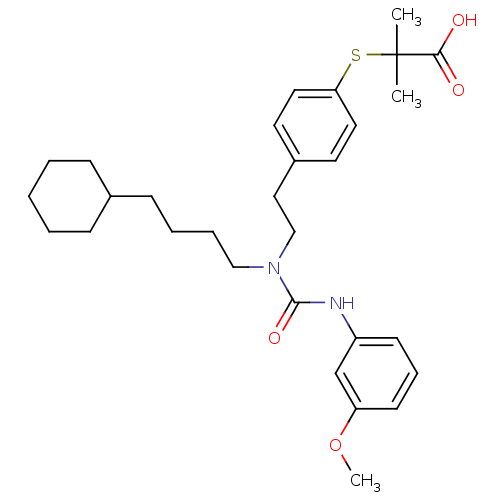
(CHEMBL2088422)Show SMILES COc1cccc(NC(=O)N(CCCCC2CCCCC2)CCc2ccc(SC(C)(C)C(O)=O)cc2)c1 Show InChI InChI=1S/C30H42N2O4S/c1-30(2,28(33)34)37-27-17-15-24(16-18-27)19-21-32(20-8-7-12-23-10-5-4-6-11-23)29(35)31-25-13-9-14-26(22-25)36-3/h9,13-18,22-23H,4-8,10-12,19-21H2,1-3H3,(H,31,35)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Agonist activity at PPARalpha-LBD expressed in HEK293 cells co-expressing GAL4-DBD after 16 to 19 hrs by beta lactamase reporter gene assay |
Bioorg Med Chem Lett 22: 6233-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.010
BindingDB Entry DOI: 10.7270/Q25M66SF |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50099489

(2-(4-{2-[1-(4-Cyclohexyl-butyl)-3-(2-methoxy-pheny...)Show SMILES COc1ccccc1NC(=O)N(CCCCC1CCCCC1)CCc1ccc(SC(C)(C)C(O)=O)cc1 Show InChI InChI=1S/C30H42N2O4S/c1-30(2,28(33)34)37-25-18-16-24(17-19-25)20-22-32(21-10-9-13-23-11-5-4-6-12-23)29(35)31-26-14-7-8-15-27(26)36-3/h7-8,14-19,23H,4-6,9-13,20-22H2,1-3H3,(H,31,35)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Agonist activity at PPARalpha-LBD expressed in HEK293 cells co-expressing GAL4-DBD after 16 to 19 hrs by beta lactamase reporter gene assay |
Bioorg Med Chem Lett 22: 6233-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.010
BindingDB Entry DOI: 10.7270/Q25M66SF |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50241116

(CHEMBL4066332)Show SMILES CC(C)(C)c1ccc(cc1)C(=O)NCc1ccc(NS(C)(=O)=O)cc1Cl Show InChI InChI=1S/C19H23ClN2O3S/c1-19(2,3)15-8-5-13(6-9-15)18(23)21-12-14-7-10-16(11-17(14)20)22-26(4,24)25/h5-11,22H,12H2,1-4H3,(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50609069

(CHEMBL5281274)Show SMILES COc1cc(ccc1Cn1ccc2ccc(NC(=O)c3ccc(cc3)C(C)(C)C)cc12)C(O)=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50009073

(4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1C Show InChI InChI=1S/C31H33N3O6S/c1-20-8-4-7-11-29(20)41(37,38)33-30(35)22-13-12-21(28(17-22)39-3)16-23-19-34(2)27-15-14-24(18-26(23)27)32-31(36)40-25-9-5-6-10-25/h4,7-8,11-15,17-19,25H,5-6,9-10,16H2,1-3H3,(H,32,36)(H,33,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50609070
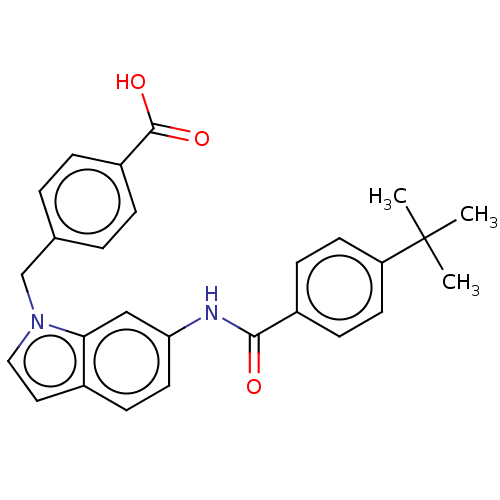
(CHEMBL5273108)Show SMILES CC(C)(C)c1ccc(cc1)C(=O)Nc1ccc2ccn(Cc3ccc(cc3)C(O)=O)c2c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50609025
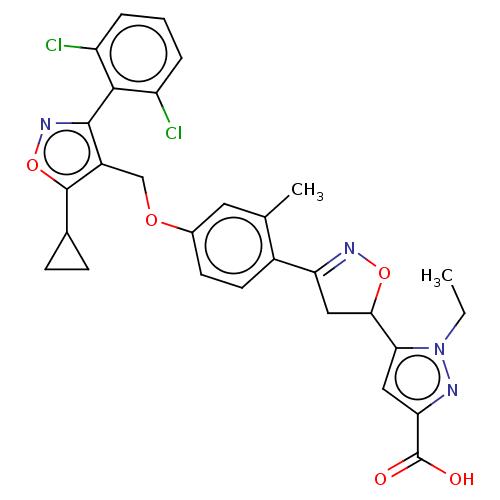
(CHEMBL5270645)Show SMILES CCn1nc(cc1C1CC(=NO1)c1ccc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)cc1C)C(O)=O |c:10,(3.94,5.97,;5.42,5.57,;5.82,4.08,;7.29,3.6,;7.29,2.07,;5.82,1.59,;4.92,2.84,;3.38,2.84,;2.35,1.69,;.94,2.32,;1.1,3.85,;2.61,4.17,;-.4,1.55,;-.4,.01,;-1.72,-.76,;-3.06,-0,;-4.39,-.77,;-5.73,-0,;-7.06,-.77,;-8.47,-.14,;-9.5,-1.29,;-8.73,-2.62,;-7.22,-2.3,;-6.13,-3.39,;-4.64,-2.99,;-4.25,-1.51,;-3.56,-4.08,;-3.96,-5.57,;-5.44,-5.97,;-6.53,-4.88,;-8.02,-5.28,;-8.86,1.34,;-8.47,2.83,;-9.95,2.43,;-3.06,1.55,;-1.72,2.32,;-1.72,3.86,;8.62,1.3,;8.62,-.24,;9.95,2.07,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50609024
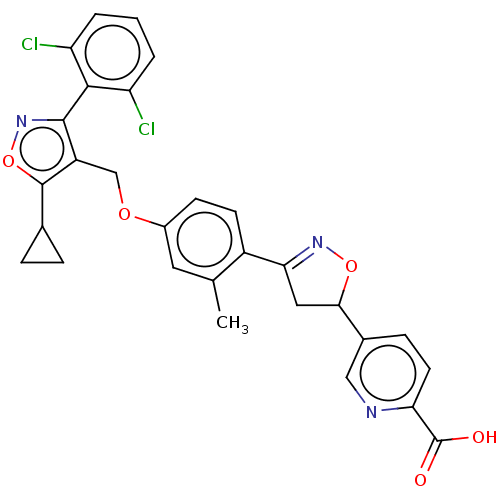
(CHEMBL5289009)Show SMILES Cc1cc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)ccc1C1=NOC(C1)c1ccc(nc1)C(O)=O |t:29,(-1.9,4.75,;-1.9,3.21,;-3.23,2.44,;-3.23,.9,;-4.57,.13,;-5.9,.9,;-7.24,.13,;-8.64,.75,;-9.67,-.39,;-8.9,-1.72,;-7.4,-1.4,;-6.31,-2.49,;-4.82,-2.09,;-4.42,-.61,;-3.73,-3.18,;-4.13,-4.67,;-5.61,-5.07,;-6.71,-3.99,;-8.19,-4.38,;-9.04,2.24,;-8.64,3.73,;-10.13,3.33,;-1.9,.14,;-.57,.9,;-.57,2.45,;.76,3.22,;.92,4.75,;2.43,5.07,;3.2,3.73,;2.17,2.59,;4.74,3.73,;5.51,5.07,;7.05,5.07,;7.82,3.73,;7.05,2.39,;5.51,2.39,;9.36,3.73,;10.13,2.4,;10.13,5.07,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50609023
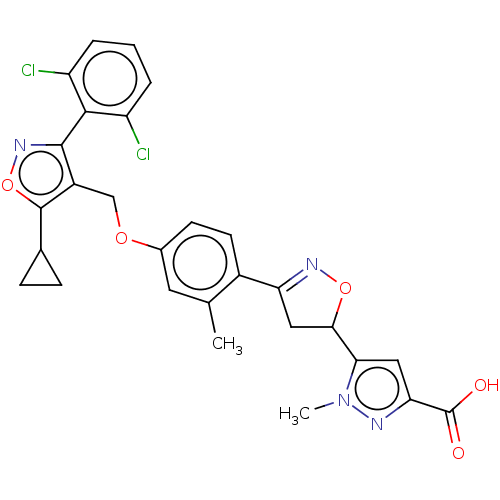
(CHEMBL5268548)Show SMILES Cc1cc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)ccc1C1=NOC(C1)c1cc(nn1C)C(O)=O |t:29,(-1.72,4.06,;-1.72,2.52,;-3.06,1.75,;-3.06,.2,;-4.39,-.57,;-5.73,.2,;-7.06,-.57,;-8.47,.05,;-9.5,-1.09,;-8.73,-2.42,;-7.22,-2.1,;-6.13,-3.19,;-4.64,-2.79,;-4.25,-1.31,;-3.56,-3.88,;-3.96,-5.37,;-5.44,-5.77,;-6.53,-4.69,;-8.02,-5.08,;-8.87,1.54,;-8.47,3.03,;-9.95,2.63,;-1.72,-.56,;-.4,.21,;-.4,1.75,;.94,2.52,;1.1,4.05,;2.61,4.37,;3.38,3.03,;2.35,1.89,;4.92,3.03,;5.82,1.79,;7.29,2.27,;7.29,3.8,;5.82,4.28,;5.42,5.77,;8.62,1.5,;9.95,2.27,;8.62,-.04,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50609021
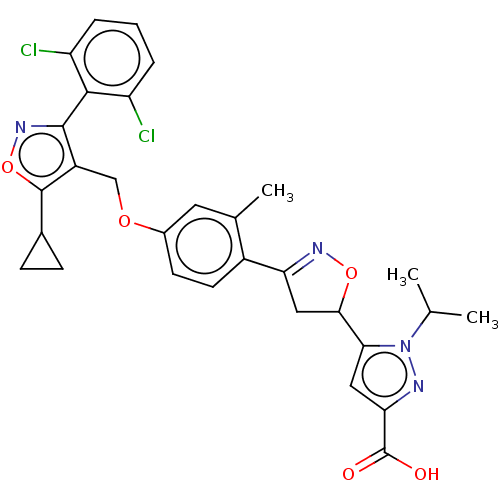
(CHEMBL5275000)Show SMILES CC(C)n1nc(cc1C1CC(=NO1)c1ccc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)cc1C)C(O)=O |c:11,(3.94,5.62,;5.42,5.22,;6.51,6.31,;5.82,3.74,;7.29,3.26,;7.29,1.72,;5.82,1.24,;4.92,2.49,;3.38,2.49,;2.35,1.35,;.94,1.97,;1.1,3.5,;2.61,3.82,;-.4,1.2,;-.4,-.34,;-1.72,-1.11,;-3.06,-.35,;-4.39,-1.12,;-5.73,-.35,;-7.06,-1.12,;-8.47,-.49,;-9.5,-1.63,;-8.73,-2.97,;-7.22,-2.65,;-6.13,-3.74,;-4.64,-3.34,;-4.25,-1.85,;-3.56,-4.42,;-3.96,-5.91,;-5.44,-6.31,;-6.53,-5.23,;-8.02,-5.63,;-8.86,1,;-8.47,2.49,;-9.95,2.09,;-3.06,1.2,;-1.72,1.97,;-1.72,3.51,;8.62,.95,;9.95,1.72,;8.62,-.59,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50609020
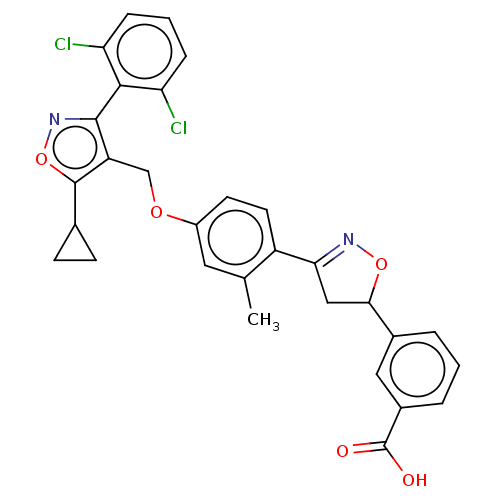
(CHEMBL5266567)Show SMILES Cc1cc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)ccc1C1=NOC(C1)c1cccc(c1)C(O)=O |t:29,(-1.52,4.75,;-1.52,3.21,;-2.85,2.44,;-2.85,.9,;-4.19,.13,;-5.52,.9,;-6.85,.13,;-8.26,.75,;-9.29,-.39,;-8.52,-1.72,;-7.01,-1.4,;-5.93,-2.49,;-4.44,-2.09,;-4.04,-.61,;-3.35,-3.18,;-3.75,-4.67,;-5.23,-5.07,;-6.33,-3.99,;-7.81,-4.38,;-8.66,2.24,;-8.26,3.73,;-9.75,3.33,;-1.51,.14,;-.19,.9,;-.19,2.45,;1.15,3.22,;1.31,4.75,;2.81,5.07,;3.58,3.73,;2.55,2.59,;5.12,3.73,;5.89,5.07,;7.43,5.07,;8.2,3.73,;7.44,2.4,;5.9,2.4,;8.21,1.07,;9.75,1.07,;7.44,-.26,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50609022

(CHEMBL5267052)Show SMILES Cc1cc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)ccc1C1=NOC(C1)c1cc(ccn1)C(O)=O |t:29,(-1.52,4.75,;-1.52,3.21,;-2.85,2.44,;-2.85,.9,;-4.19,.13,;-5.52,.9,;-6.85,.13,;-8.26,.75,;-9.29,-.39,;-8.52,-1.72,;-7.01,-1.4,;-5.93,-2.49,;-4.44,-2.09,;-4.04,-.61,;-3.35,-3.18,;-3.75,-4.67,;-5.23,-5.07,;-6.33,-3.99,;-7.81,-4.38,;-8.66,2.24,;-8.26,3.73,;-9.75,3.33,;-1.51,.14,;-.19,.9,;-.19,2.45,;1.15,3.22,;1.31,4.75,;2.81,5.07,;3.58,3.73,;2.55,2.59,;5.12,3.73,;5.9,2.4,;7.44,2.4,;8.2,3.73,;7.43,5.07,;5.89,5.07,;8.21,1.07,;9.75,1.07,;7.44,-.26,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50609012

(CHEMBL5271527)Show SMILES [H][C@]12OC[C@@H](Oc3nc4c(OC(C)C)cc(cc4s3)C(=O)NS(=O)(=O)N3CCCCC3)[C@]1([H])OC[C@H]2OCc1c(onc1-c1c(Cl)cccc1Cl)C1CC1 |r,wU:35.40,31.35,4.4,wD:1.0,(-4.12,-1.06,;-2.82,-1.9,;-2.43,-.41,;-.89,-.33,;-.34,-1.77,;1.15,-2.17,;2.24,-1.08,;3.76,-1.32,;4.46,.05,;5.95,.45,;7.04,-.64,;8.53,-.24,;9.62,-1.33,;8.93,1.25,;6.34,1.94,;5.26,3.02,;3.77,2.62,;3.37,1.14,;2,.44,;5.65,4.51,;4.57,5.6,;7.14,4.91,;7.54,6.4,;7.14,7.89,;6.05,6.8,;9.03,6.8,;9.43,8.28,;10.92,8.68,;12,7.59,;11.61,6.1,;10.12,5.71,;-1.53,-2.74,;-.24,-3.58,;-1.93,-4.22,;-3.47,-4.3,;-4.02,-2.87,;-5.51,-2.47,;-6.6,-3.56,;-8.08,-3.16,;-8.56,-1.69,;-10.1,-1.69,;-10.58,-3.16,;-9.33,-4.06,;-9.33,-5.6,;-8,-6.38,;-6.66,-5.61,;-8,-7.91,;-9.33,-8.68,;-10.66,-7.92,;-10.67,-6.38,;-12,-5.61,;-7.79,-.36,;-6.45,.41,;-7.79,1.18,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | <30 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50609013

(CHEMBL5279682)Show SMILES [H][C@]12OC[C@@H](Oc3nc4c(OC(C)C)cc(cc4s3)C(=O)NS(=O)(=O)N3CCCC3)[C@]1([H])OC[C@H]2OCc1c(onc1-c1c(Cl)cccc1Cl)C1CC1 |r,wU:34.39,30.34,4.4,wD:1.0,(-3.87,-.84,;-2.58,-1.67,;-2.18,-.19,;-.64,-.11,;-.09,-1.54,;1.39,-1.94,;2.48,-.85,;4,-1.09,;4.7,.28,;6.2,.68,;7.29,-.41,;8.77,-.01,;9.86,-1.1,;9.17,1.48,;6.59,2.17,;5.5,3.25,;4.01,2.85,;3.61,1.37,;2.24,.67,;5.9,4.73,;4.81,5.82,;7.39,5.13,;7.79,6.62,;7.39,8.11,;6.3,7.02,;9.27,7.02,;9.82,8.46,;11.36,8.38,;11.76,6.89,;10.47,6.05,;-1.29,-2.51,;0,-3.35,;-1.69,-4,;-3.23,-4.08,;-3.78,-2.64,;-5.26,-2.24,;-6.35,-3.33,;-7.84,-2.93,;-8.32,-1.47,;-9.86,-1.47,;-10.33,-2.93,;-9.09,-3.84,;-9.09,-5.38,;-7.75,-6.15,;-6.42,-5.38,;-7.75,-7.69,;-9.09,-8.46,;-10.42,-7.69,;-10.43,-6.15,;-11.76,-5.38,;-7.55,-.14,;-6.21,.63,;-7.55,1.4,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | <30 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50609014

(CHEMBL5272555)Show SMILES [H][C@]12OC[C@@H](Oc3nc4c(OC(C)C)cc(cc4s3)C(O)=O)[C@]1([H])OC[C@H]2OCc1c(onc1-c1c(Cl)cccc1Cl)C1CC1 |r,wU:26.30,22.25,4.4,wD:1.0,(-2.92,.48,;-1.63,-.36,;-1.23,1.13,;.3,1.21,;.86,-.23,;2.34,-.63,;3.43,.46,;4.95,.22,;5.65,1.59,;7.15,1.99,;8.23,.91,;9.72,1.3,;10.81,.21,;10.12,2.79,;7.53,3.48,;6.45,4.56,;4.96,4.16,;4.56,2.68,;3.19,1.98,;6.85,6.05,;8.34,6.45,;5.76,7.14,;-.34,-1.2,;.95,-2.04,;-.74,-2.68,;-2.28,-2.76,;-2.83,-1.33,;-4.32,-.93,;-5.4,-2.02,;-6.89,-1.62,;-7.37,-.15,;-8.91,-.15,;-9.38,-1.62,;-8.14,-2.52,;-8.14,-4.06,;-6.8,-4.83,;-5.47,-4.06,;-6.81,-6.37,;-8.14,-7.14,;-9.47,-6.38,;-9.48,-4.84,;-10.81,-4.07,;-6.6,1.18,;-5.26,1.95,;-6.6,2.72,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | <30 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50609015
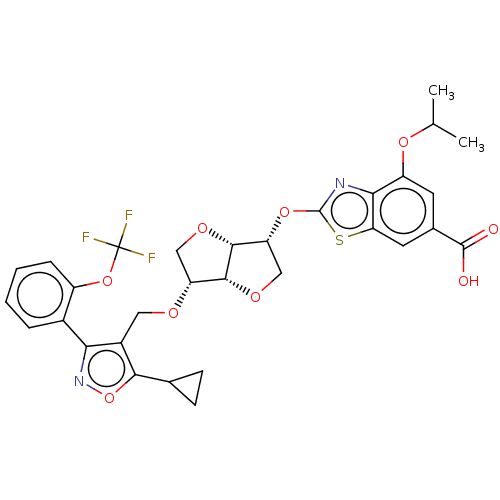
(CHEMBL5283322)Show SMILES [H][C@]12OC[C@@H](Oc3nc4c(OC(C)C)cc(cc4s3)C(O)=O)[C@@]1([H])OC[C@H]2OCc1c(onc1-c1ccccc1OC(F)(F)F)C1CC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | <30 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50609016
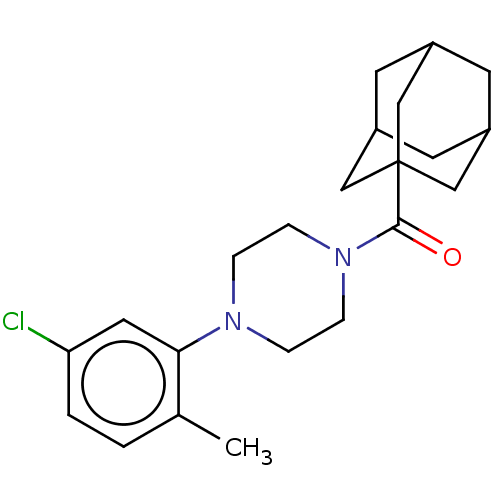
(CHEMBL5287066)Show SMILES Cc1ccc(Cl)cc1N1CCN(CC1)C(=O)C12CC3CC(CC(C3)C1)C2 |TLB:19:20:17.18.23:24,23:18:25:22.24.21,23:22:25:17.18.19,THB:19:18:24:20.25.21| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PDB
UniChem
| PDB
| n/a | n/a | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50609017
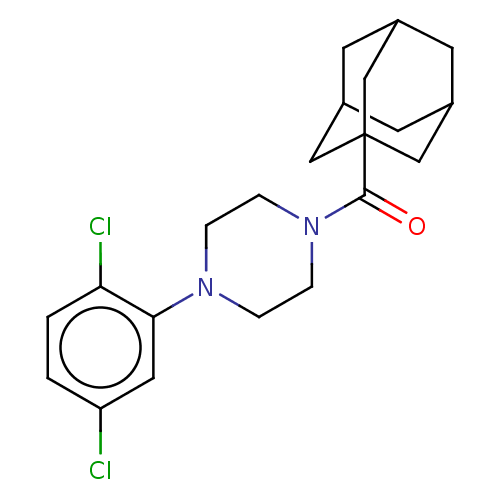
(CHEMBL5284954)Show SMILES Clc1ccc(Cl)c(c1)N1CCN(CC1)C(=O)C12CC3CC(CC(C3)C1)C2 |TLB:19:20:18.17.23:24,THB:19:18:24:25.20.21,21:20:17:23.22.24,21:22:25.20.19:17| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50609018

(CHEMBL5269689)Show SMILES Clc1cccc(c1)N1CCN(CC1)C(=O)C12CC3CC(CC(C3)C1)C2 |TLB:18:19:17.16.22:23,THB:18:17:23:24.19.20,20:19:16:22.21.23,20:21:24.19.18:16| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
RNA-binding protein FXR2
(Homo sapiens) | BDBM50185708

(CHEMBL3822464)Show SMILES OC(=O)c1ccc(cc1)[C@@H]1C[C@H]1c1ccc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)cc1Cl |r,wU:11.13,wD:9.9,(10.44,1.17,;10,.02,;10.78,-.94,;8.48,-.23,;7.51,.96,;5.99,.71,;5.45,-.71,;6.42,-1.92,;7.94,-1.67,;4.13,-1.5,;3.35,-2.83,;2.67,-1.54,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;-2.67,1.54,;-4,.77,;-5.34,1.53,;-5.48,3.05,;-6.99,3.37,;-7.76,2.04,;-6.73,.9,;-7.04,-.61,;-8.54,-.98,;-9.39,-.09,;-8.97,-2.45,;-7.9,-3.57,;-6.41,-3.2,;-5.98,-1.72,;-4.78,-1.43,;-4.33,4.08,;-2.93,4.46,;-4.05,5.51,;,1.54,;1.33,.77,;2.4,1.39,)| Show InChI InChI=1S/C29H22Cl3NO4/c30-23-2-1-3-24(31)26(23)27-22(28(37-33-27)16-6-7-16)14-36-18-10-11-19(25(32)12-18)21-13-20(21)15-4-8-17(9-5-15)29(34)35/h1-5,8-12,16,20-21H,6-7,13-14H2,(H,34,35)/t20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
RNA-binding protein FXR2
(Homo sapiens) | BDBM50609019
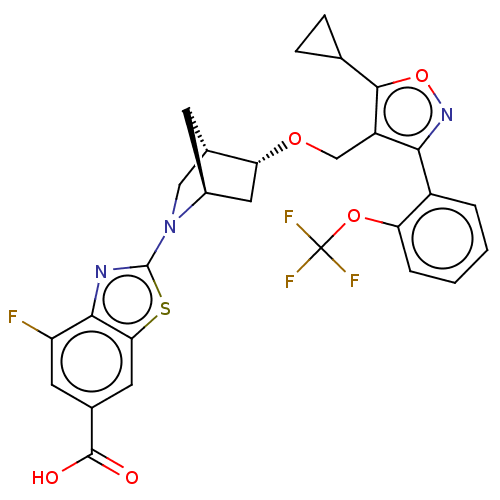
(CHEMBL5267458)Show SMILES [H][C@]12CN(c3nc4c(F)cc(cc4s3)C(O)=O)[C@]([H])(C[C@H]1OCc1c(onc1-c1ccccc1OC(F)(F)F)C1CC1)C2 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50609026
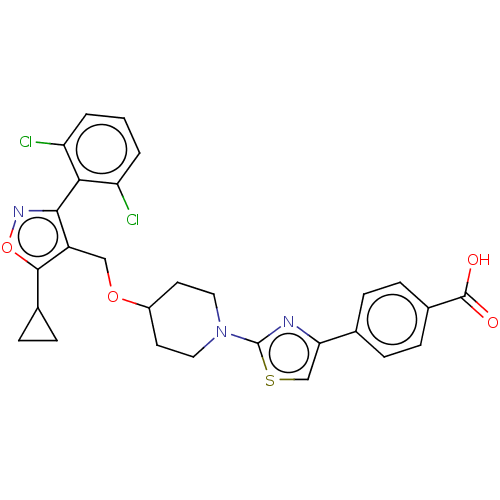
(CHEMBL5266939)Show SMILES OC(=O)c1ccc(cc1)-c1csc(n1)N1CCC(CC1)OCc1c(onc1-c1c(Cl)cccc1Cl)C1CC1 |(10.13,2.4,;9.36,3.73,;10.13,5.07,;7.82,3.73,;7.05,5.07,;5.51,5.07,;4.74,3.73,;5.51,2.39,;7.05,2.39,;3.2,3.73,;2.43,5.07,;.92,4.75,;.76,3.22,;2.17,2.59,;-.57,2.45,;-.57,.9,;-1.9,.14,;-3.23,.9,;-3.23,2.44,;-1.9,3.21,;-4.57,.13,;-5.9,.9,;-7.24,.13,;-8.64,.75,;-9.67,-.39,;-8.9,-1.72,;-7.4,-1.4,;-6.31,-2.49,;-4.82,-2.09,;-4.42,-.61,;-3.73,-3.18,;-4.13,-4.67,;-5.61,-5.07,;-6.71,-3.99,;-8.2,-4.38,;-9.04,2.24,;-8.64,3.73,;-10.13,3.33,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50609020

(CHEMBL5266567)Show SMILES Cc1cc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)ccc1C1=NOC(C1)c1cccc(c1)C(O)=O |t:29,(-1.52,4.75,;-1.52,3.21,;-2.85,2.44,;-2.85,.9,;-4.19,.13,;-5.52,.9,;-6.85,.13,;-8.26,.75,;-9.29,-.39,;-8.52,-1.72,;-7.01,-1.4,;-5.93,-2.49,;-4.44,-2.09,;-4.04,-.61,;-3.35,-3.18,;-3.75,-4.67,;-5.23,-5.07,;-6.33,-3.99,;-7.81,-4.38,;-8.66,2.24,;-8.26,3.73,;-9.75,3.33,;-1.51,.14,;-.19,.9,;-.19,2.45,;1.15,3.22,;1.31,4.75,;2.81,5.07,;3.58,3.73,;2.55,2.59,;5.12,3.73,;5.89,5.07,;7.43,5.07,;8.2,3.73,;7.44,2.4,;5.9,2.4,;8.21,1.07,;9.75,1.07,;7.44,-.26,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | <30 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50609021

(CHEMBL5275000)Show SMILES CC(C)n1nc(cc1C1CC(=NO1)c1ccc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)cc1C)C(O)=O |c:11,(3.94,5.62,;5.42,5.22,;6.51,6.31,;5.82,3.74,;7.29,3.26,;7.29,1.72,;5.82,1.24,;4.92,2.49,;3.38,2.49,;2.35,1.35,;.94,1.97,;1.1,3.5,;2.61,3.82,;-.4,1.2,;-.4,-.34,;-1.72,-1.11,;-3.06,-.35,;-4.39,-1.12,;-5.73,-.35,;-7.06,-1.12,;-8.47,-.49,;-9.5,-1.63,;-8.73,-2.97,;-7.22,-2.65,;-6.13,-3.74,;-4.64,-3.34,;-4.25,-1.85,;-3.56,-4.42,;-3.96,-5.91,;-5.44,-6.31,;-6.53,-5.23,;-8.02,-5.63,;-8.86,1,;-8.47,2.49,;-9.95,2.09,;-3.06,1.2,;-1.72,1.97,;-1.72,3.51,;8.62,.95,;9.95,1.72,;8.62,-.59,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | 34 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50609022

(CHEMBL5267052)Show SMILES Cc1cc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)ccc1C1=NOC(C1)c1cc(ccn1)C(O)=O |t:29,(-1.52,4.75,;-1.52,3.21,;-2.85,2.44,;-2.85,.9,;-4.19,.13,;-5.52,.9,;-6.85,.13,;-8.26,.75,;-9.29,-.39,;-8.52,-1.72,;-7.01,-1.4,;-5.93,-2.49,;-4.44,-2.09,;-4.04,-.61,;-3.35,-3.18,;-3.75,-4.67,;-5.23,-5.07,;-6.33,-3.99,;-7.81,-4.38,;-8.66,2.24,;-8.26,3.73,;-9.75,3.33,;-1.51,.14,;-.19,.9,;-.19,2.45,;1.15,3.22,;1.31,4.75,;2.81,5.07,;3.58,3.73,;2.55,2.59,;5.12,3.73,;5.9,2.4,;7.44,2.4,;8.2,3.73,;7.43,5.07,;5.89,5.07,;8.21,1.07,;9.75,1.07,;7.44,-.26,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50609023

(CHEMBL5268548)Show SMILES Cc1cc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)ccc1C1=NOC(C1)c1cc(nn1C)C(O)=O |t:29,(-1.72,4.06,;-1.72,2.52,;-3.06,1.75,;-3.06,.2,;-4.39,-.57,;-5.73,.2,;-7.06,-.57,;-8.47,.05,;-9.5,-1.09,;-8.73,-2.42,;-7.22,-2.1,;-6.13,-3.19,;-4.64,-2.79,;-4.25,-1.31,;-3.56,-3.88,;-3.96,-5.37,;-5.44,-5.77,;-6.53,-4.69,;-8.02,-5.08,;-8.87,1.54,;-8.47,3.03,;-9.95,2.63,;-1.72,-.56,;-.4,.21,;-.4,1.75,;.94,2.52,;1.1,4.05,;2.61,4.37,;3.38,3.03,;2.35,1.89,;4.92,3.03,;5.82,1.79,;7.29,2.27,;7.29,3.8,;5.82,4.28,;5.42,5.77,;8.62,1.5,;9.95,2.27,;8.62,-.04,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50609024

(CHEMBL5289009)Show SMILES Cc1cc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)ccc1C1=NOC(C1)c1ccc(nc1)C(O)=O |t:29,(-1.9,4.75,;-1.9,3.21,;-3.23,2.44,;-3.23,.9,;-4.57,.13,;-5.9,.9,;-7.24,.13,;-8.64,.75,;-9.67,-.39,;-8.9,-1.72,;-7.4,-1.4,;-6.31,-2.49,;-4.82,-2.09,;-4.42,-.61,;-3.73,-3.18,;-4.13,-4.67,;-5.61,-5.07,;-6.71,-3.99,;-8.19,-4.38,;-9.04,2.24,;-8.64,3.73,;-10.13,3.33,;-1.9,.14,;-.57,.9,;-.57,2.45,;.76,3.22,;.92,4.75,;2.43,5.07,;3.2,3.73,;2.17,2.59,;4.74,3.73,;5.51,5.07,;7.05,5.07,;7.82,3.73,;7.05,2.39,;5.51,2.39,;9.36,3.73,;10.13,2.4,;10.13,5.07,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50609025

(CHEMBL5270645)Show SMILES CCn1nc(cc1C1CC(=NO1)c1ccc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)cc1C)C(O)=O |c:10,(3.94,5.97,;5.42,5.57,;5.82,4.08,;7.29,3.6,;7.29,2.07,;5.82,1.59,;4.92,2.84,;3.38,2.84,;2.35,1.69,;.94,2.32,;1.1,3.85,;2.61,4.17,;-.4,1.55,;-.4,.01,;-1.72,-.76,;-3.06,-0,;-4.39,-.77,;-5.73,-0,;-7.06,-.77,;-8.47,-.14,;-9.5,-1.29,;-8.73,-2.62,;-7.22,-2.3,;-6.13,-3.39,;-4.64,-2.99,;-4.25,-1.51,;-3.56,-4.08,;-3.96,-5.57,;-5.44,-5.97,;-6.53,-4.88,;-8.02,-5.28,;-8.86,1.34,;-8.47,2.83,;-9.95,2.43,;-3.06,1.55,;-1.72,2.32,;-1.72,3.86,;8.62,1.3,;8.62,-.24,;9.95,2.07,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50609027

(CHEMBL5270341)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OCc1c(onc1-c1ccccc1OC(F)(F)F)C1CC1)N2c1nc(cs1)-c1ccc(C(O)=O)c(c1)C(F)(F)F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50609028
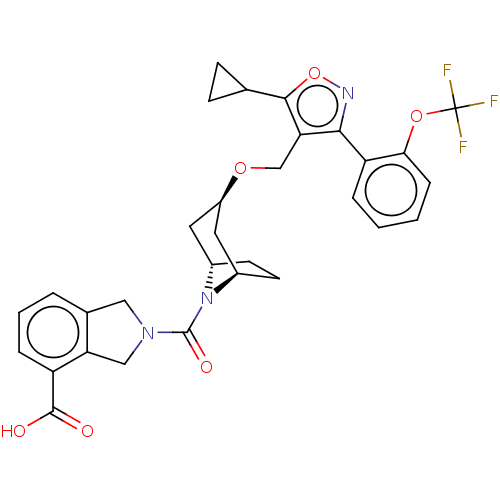
(CHEMBL5279195)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OCc1c(onc1-c1ccccc1OC(F)(F)F)C1CC1)N2C(=O)N1Cc2cccc(C(O)=O)c2C1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50609029

(CHEMBL5276532)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OCc1c(onc1-c1c(Cl)cccc1Cl)C1CC1)N2C(=O)N1Cc2cccc(C(O)=O)c2C1 |r,wD:7.9,1.0,4.4,(-1.09,4.85,;-1.09,3.31,;-1.76,2.18,;-1.09,1,;.24,1,;.24,-.54,;-1.09,.23,;-2.42,1,;-2.42,2.54,;-3.76,.23,;-5.09,1,;-6.43,.23,;-7.81,.85,;-8.84,-.28,;-8.07,-1.62,;-6.58,-1.31,;-5.45,-2.34,;-5.76,-3.82,;-7.2,-4.29,;-4.63,-4.85,;-3.14,-4.39,;-2.83,-2.9,;-3.96,-1.87,;-3.56,-.39,;-8.12,2.39,;-7.66,3.82,;-9.2,3.51,;.24,2.54,;1.58,3.31,;1.58,4.85,;2.91,2.54,;3.07,1.01,;4.58,.69,;5.35,-.65,;6.89,-.65,;7.66,.69,;6.89,2.02,;7.66,3.35,;9.2,3.35,;6.89,4.69,;5.35,2.02,;4.32,3.16,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50609030

(CHEMBL5281263)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OCc1c(onc1-c1c(Cl)cccc1Cl)C1CC1)N2C(=O)N1Cc2ccc(cc2C1)C(O)=O |r,wD:7.9,4.4,1.0,(-1.47,4.85,;-1.47,3.31,;-2.14,2.18,;-1.47,1,;-.14,1,;-.14,-.54,;-1.47,.23,;-2.81,1,;-2.81,2.54,;-4.14,.23,;-5.48,1,;-6.81,.23,;-8.2,.85,;-9.22,-.28,;-8.45,-1.62,;-6.96,-1.31,;-5.84,-2.34,;-6.14,-3.82,;-7.58,-4.28,;-5.01,-4.85,;-3.53,-4.39,;-3.22,-2.9,;-4.35,-1.87,;-3.95,-.39,;-8.5,2.39,;-8.04,3.82,;-9.58,3.51,;-.14,2.54,;1.19,3.31,;1.19,4.85,;2.53,2.54,;2.69,1.01,;4.19,.69,;4.97,-.65,;6.51,-.65,;7.27,.69,;6.5,2.02,;4.96,2.02,;3.93,3.16,;8.81,.69,;9.58,-.65,;9.58,2.02,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50609031

(CHEMBL5268294)Show SMILES [Na;v0+].[H][C@]12[#6]-[#6][C@]([H])([#6]-[#6@@H](-[#6]1)-[#8]-[#6]-c1c(onc1-c1ccccc1-[#8]C(F)(F)F)-[#6]-1-[#6]-[#6]-1)[#7]2-c1nc2c(F)cc(cc2s1)S([#8-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | <500 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50609032

(CHEMBL5271645)Show SMILES [Na;v0+].[H][C@]12[#6]-[#6][C@]([H])([#6]-[#6@@H](-[#6]1)-[#8]-[#6]-c1c(onc1-c1ccccc1-[#8]C(F)(F)F)-[#6]-1-[#6]-[#6]-1)[#7]2-c1nc2c(-[#6])cc(cc2s1)S([#8-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | <500 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50609026

(CHEMBL5266939)Show SMILES OC(=O)c1ccc(cc1)-c1csc(n1)N1CCC(CC1)OCc1c(onc1-c1c(Cl)cccc1Cl)C1CC1 |(10.13,2.4,;9.36,3.73,;10.13,5.07,;7.82,3.73,;7.05,5.07,;5.51,5.07,;4.74,3.73,;5.51,2.39,;7.05,2.39,;3.2,3.73,;2.43,5.07,;.92,4.75,;.76,3.22,;2.17,2.59,;-.57,2.45,;-.57,.9,;-1.9,.14,;-3.23,.9,;-3.23,2.44,;-1.9,3.21,;-4.57,.13,;-5.9,.9,;-7.24,.13,;-8.64,.75,;-9.67,-.39,;-8.9,-1.72,;-7.4,-1.4,;-6.31,-2.49,;-4.82,-2.09,;-4.42,-.61,;-3.73,-3.18,;-4.13,-4.67,;-5.61,-5.07,;-6.71,-3.99,;-8.2,-4.38,;-9.04,2.24,;-8.64,3.73,;-10.13,3.33,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50609033

(CHEMBL5285848)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OCc1c(onc1-c1c(Cl)cccc1Cl)C1CC1)N2S(=O)(=O)Nc1ccc(C(O)=O)c(C)c1 |r,wD:7.9,4.4,1.0,(-1.28,3.7,;-1.28,2.16,;-1.95,1.03,;-1.28,-.15,;.05,-.15,;.05,-1.69,;-1.28,-.92,;-2.62,-.15,;-2.62,1.39,;-3.95,-.92,;-5.28,-.15,;-6.62,-.92,;-8,-.31,;-9.03,-1.44,;-8.26,-2.77,;-6.77,-2.46,;-5.64,-3.49,;-5.95,-4.98,;-7.39,-5.44,;-4.82,-6,;-3.33,-5.54,;-3.03,-4.05,;-4.16,-3.03,;-3.76,-1.54,;-8.31,1.23,;-7.85,2.67,;-9.39,2.36,;.05,1.39,;1.39,2.16,;2.16,3.49,;.62,3.49,;2.72,1.38,;4.05,2.16,;4.05,3.7,;5.39,4.46,;6.72,3.69,;8.05,4.46,;9.39,3.69,;8.05,6,;6.72,2.16,;8.06,1.39,;5.39,1.38,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | <50 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50609034

(CHEMBL5282568)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OCc1c(onc1-c1c(Cl)cccc1Cl)C1CC1)N2S(=O)(=O)Nc1ccc(cc1F)C(O)=O |r,wD:7.9,1.0,4.4,(-1.28,3.7,;-1.28,2.16,;-1.95,1.03,;-1.28,-.15,;.05,-.15,;.05,-1.69,;-1.28,-.92,;-2.62,-.15,;-2.62,1.39,;-3.95,-.92,;-5.28,-.15,;-6.62,-.92,;-8,-.31,;-9.03,-1.44,;-8.26,-2.77,;-6.77,-2.46,;-5.64,-3.49,;-4.16,-3.03,;-3.76,-1.54,;-3.03,-4.05,;-3.33,-5.54,;-4.82,-6,;-5.95,-4.98,;-7.39,-5.44,;-8.31,1.23,;-9.39,2.36,;-7.85,2.67,;.05,1.39,;1.39,2.16,;.62,3.49,;2.16,3.49,;2.72,1.38,;4.05,2.16,;4.05,3.7,;5.39,4.46,;6.72,3.69,;6.72,2.16,;5.39,1.38,;5.39,-.16,;8.05,4.46,;9.39,3.69,;8.05,6,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | <50 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50609035

(CHEMBL5279380)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OCc1c(onc1-c1c(Cl)cccc1Cl)C1CC1)N2c1noc(n1)-c1ccc(cc1)C(O)=O |r,wD:7.9,4.4,1.0,(-.43,1.36,;-.43,-.18,;-1.09,-1.31,;-.43,-2.49,;.91,-2.49,;.91,-4.03,;-.43,-3.26,;-1.76,-2.49,;-1.76,-.95,;-3.09,-3.26,;-4.43,-2.49,;-5.76,-3.26,;-7.15,-2.64,;-8.17,-3.77,;-7.4,-5.11,;-5.91,-4.8,;-4.79,-5.82,;-5.09,-7.31,;-6.53,-7.77,;-3.97,-8.34,;-2.48,-7.88,;-2.17,-6.39,;-3.3,-5.36,;-2.9,-3.87,;-7.45,-1.1,;-6.99,.33,;-8.53,.03,;.91,-.95,;2.24,-.18,;3.65,-.81,;4.68,.34,;3.91,1.67,;2.4,1.35,;4.68,3.01,;6.23,3.01,;6.99,4.34,;6.22,5.67,;4.68,5.67,;3.91,4.34,;6.99,7,;8.53,7,;6.22,8.34,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | <50 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50609036
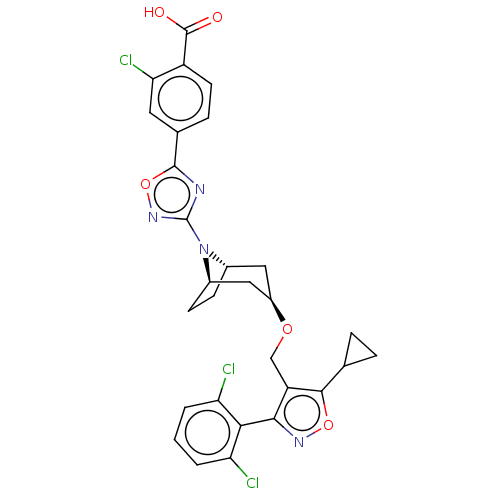
(CHEMBL5267810)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OCc1c(onc1-c1c(Cl)cccc1Cl)C1CC1)N2c1noc(n1)-c1ccc(C(O)=O)c(Cl)c1 |r,wD:7.9,1.0,4.4,(-.43,1.36,;-.43,-.18,;-1.09,-1.31,;-.43,-2.49,;.91,-2.49,;.91,-4.03,;-.43,-3.26,;-1.76,-2.49,;-1.76,-.95,;-3.09,-3.26,;-4.43,-2.49,;-5.76,-3.26,;-7.15,-2.64,;-8.17,-3.77,;-7.4,-5.11,;-5.92,-4.8,;-4.79,-5.82,;-5.09,-7.31,;-6.53,-7.77,;-3.97,-8.34,;-2.48,-7.88,;-2.17,-6.39,;-3.3,-5.36,;-2.9,-3.87,;-7.45,-1.1,;-6.99,.33,;-8.53,.03,;.91,-.95,;2.24,-.18,;3.65,-.81,;4.68,.34,;3.91,1.67,;2.4,1.35,;4.68,3.01,;3.91,4.34,;4.68,5.67,;6.22,5.67,;6.99,7,;8.53,7,;6.22,8.34,;6.99,4.34,;8.53,4.34,;6.23,3.01,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | <50 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50609037
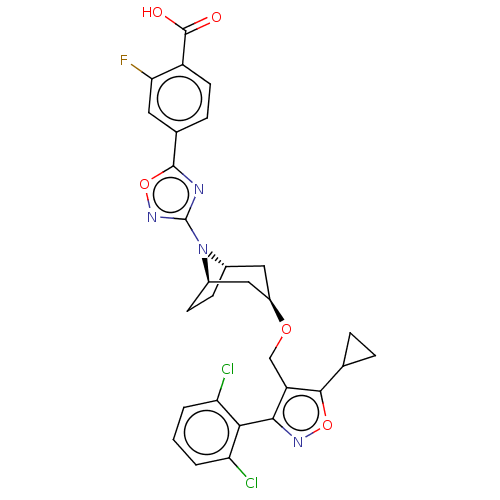
(CHEMBL5266846)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OCc1c(onc1-c1c(Cl)cccc1Cl)C1CC1)N2c1noc(n1)-c1ccc(C(O)=O)c(F)c1 |r,wD:7.9,4.4,1.0,(-.43,1.36,;-.43,-.18,;-1.09,-1.31,;-.43,-2.49,;.91,-2.49,;.91,-4.03,;-.43,-3.26,;-1.76,-2.49,;-1.76,-.95,;-3.09,-3.26,;-4.43,-2.49,;-5.76,-3.26,;-7.15,-2.64,;-8.17,-3.77,;-7.4,-5.11,;-5.92,-4.8,;-4.79,-5.82,;-5.09,-7.31,;-6.53,-7.77,;-3.97,-8.34,;-2.48,-7.88,;-2.17,-6.39,;-3.3,-5.36,;-2.9,-3.87,;-7.45,-1.1,;-6.99,.33,;-8.53,.03,;.91,-.95,;2.24,-.18,;3.65,-.81,;4.68,.34,;3.91,1.67,;2.4,1.35,;4.68,3.01,;3.91,4.34,;4.68,5.67,;6.22,5.67,;6.99,7,;8.53,7,;6.22,8.34,;6.99,4.34,;8.53,4.34,;6.23,3.01,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | <50 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50609038

(CHEMBL5277834)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OCc1c(onc1-c1c(Cl)cccc1Cl)C1CC1)N2c1noc(n1)-c1ccc(C(O)=O)c(OC)c1 |r,wD:7.9,4.4,1.0,(-.81,1.36,;-.81,-.18,;-1.48,-1.31,;-.81,-2.49,;.52,-2.49,;.52,-4.03,;-.81,-3.26,;-2.14,-2.49,;-2.14,-.95,;-3.48,-3.26,;-4.81,-2.49,;-6.15,-3.26,;-7.53,-2.64,;-8.56,-3.77,;-7.79,-5.11,;-6.3,-4.8,;-5.17,-5.82,;-5.48,-7.31,;-6.92,-7.77,;-4.35,-8.34,;-2.86,-7.88,;-2.55,-6.39,;-3.68,-5.36,;-3.28,-3.87,;-7.84,-1.1,;-7.38,.33,;-8.92,.03,;.52,-.95,;1.86,-.18,;3.26,-.81,;4.3,.34,;3.53,1.67,;2.02,1.35,;4.3,3.01,;3.53,4.34,;4.3,5.67,;5.84,5.67,;6.61,7,;8.15,7,;5.84,8.34,;6.61,4.34,;8.15,4.34,;8.92,5.68,;5.84,3.01,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | <50 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50609039

(CHEMBL5282580)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OCc1c(onc1-c1c(Cl)cccc1Cl)C1CC1)N2c1noc(n1)-c1ccc(C(O)=O)c(C)c1 |r,wD:7.9,4.4,1.0,(-.43,1.36,;-.43,-.18,;-1.09,-1.31,;-.43,-2.49,;.91,-2.49,;.91,-4.03,;-.43,-3.26,;-1.76,-2.49,;-1.76,-.95,;-3.09,-3.26,;-4.43,-2.49,;-5.76,-3.26,;-7.15,-2.64,;-8.17,-3.77,;-7.4,-5.11,;-5.92,-4.8,;-4.79,-5.82,;-5.09,-7.31,;-6.53,-7.77,;-3.97,-8.34,;-2.48,-7.88,;-2.17,-6.39,;-3.3,-5.36,;-2.9,-3.87,;-7.45,-1.1,;-6.99,.33,;-8.53,.03,;.91,-.95,;2.24,-.18,;3.65,-.81,;4.68,.34,;3.91,1.67,;2.4,1.35,;4.68,3.01,;3.91,4.34,;4.68,5.67,;6.22,5.67,;6.99,7,;8.53,7,;6.22,8.34,;6.99,4.34,;8.53,4.34,;6.23,3.01,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | <50 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50609040
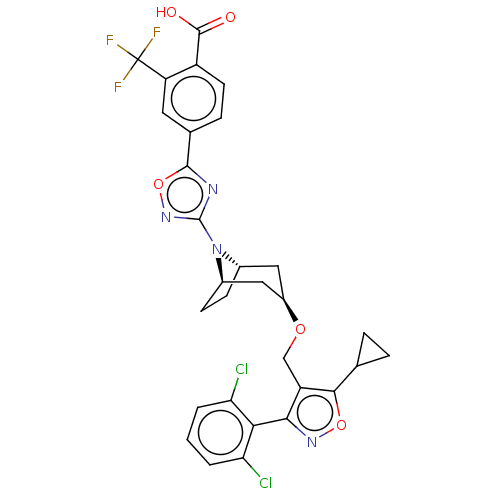
(CHEMBL5288343)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OCc1c(onc1-c1c(Cl)cccc1Cl)C1CC1)N2c1noc(n1)-c1ccc(C(O)=O)c(c1)C(F)(F)F |r,wD:7.9,4.4,1.0,(-1.19,1.36,;-1.19,-.18,;-1.86,-1.31,;-1.19,-2.49,;.14,-2.49,;.14,-4.03,;-1.19,-3.26,;-2.53,-2.49,;-2.53,-.95,;-3.86,-3.26,;-5.2,-2.49,;-6.53,-3.26,;-7.92,-2.64,;-8.94,-3.77,;-8.17,-5.11,;-6.68,-4.8,;-5.56,-5.82,;-5.86,-7.31,;-7.3,-7.77,;-4.73,-8.34,;-3.25,-7.88,;-2.94,-6.39,;-4.07,-5.36,;-3.67,-3.87,;-8.22,-1.1,;-7.76,.33,;-9.3,.03,;.14,-.95,;1.47,-.18,;2.88,-.81,;3.91,.34,;3.14,1.67,;1.63,1.35,;3.91,3.01,;3.14,4.34,;3.91,5.67,;5.45,5.67,;6.22,7,;7.76,7,;5.45,8.34,;6.22,4.34,;5.46,3.01,;7.76,4.34,;8.53,5.68,;8.53,3.01,;9.3,4.34,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | <50 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50609041

(CHEMBL5268282)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OCc1c(onc1-c1c(Cl)cccc1Cl)C1CC1)N2c1noc(n1)-c1cccc(c1)C(O)=O |r,wD:7.9,4.4,1.0,(.35,1.36,;.35,-.18,;-.32,-1.31,;.35,-2.49,;1.68,-2.49,;1.68,-4.03,;.35,-3.26,;-.99,-2.49,;-.99,-.95,;-2.32,-3.26,;-3.66,-2.49,;-4.99,-3.26,;-6.38,-2.64,;-7.4,-3.77,;-6.63,-5.11,;-5.14,-4.8,;-4.02,-5.82,;-4.32,-7.31,;-5.76,-7.77,;-3.19,-8.34,;-1.71,-7.88,;-1.4,-6.39,;-2.53,-5.36,;-2.13,-3.87,;-6.68,-1.1,;-6.22,.33,;-7.76,.03,;1.68,-.95,;3.01,-.18,;4.42,-.81,;5.45,.34,;4.68,1.67,;3.18,1.35,;5.45,3.01,;7,3.01,;7.76,4.34,;6.99,5.67,;5.45,5.67,;4.68,4.34,;4.68,7,;5.45,8.34,;3.14,7,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | <50 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50609042

(CHEMBL5266200)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OCc1c(onc1-c1c(Cl)cccc1Cl)C1CC1)N2c1noc(n1)-c1cccc(n1)C(O)=O |r,wD:7.9,1.0,4.4,(.35,1.36,;.35,-.18,;-.32,-1.31,;.35,-2.49,;1.68,-2.49,;1.68,-4.03,;.35,-3.26,;-.99,-2.49,;-.99,-.95,;-2.32,-3.26,;-3.66,-2.49,;-4.99,-3.26,;-6.38,-2.64,;-7.4,-3.77,;-6.63,-5.11,;-5.14,-4.8,;-4.02,-5.82,;-4.32,-7.31,;-5.76,-7.77,;-3.19,-8.34,;-1.71,-7.88,;-1.4,-6.39,;-2.53,-5.36,;-2.13,-3.87,;-6.68,-1.1,;-6.22,.33,;-7.76,.03,;1.68,-.95,;3.01,-.18,;4.42,-.81,;5.45,.34,;4.68,1.67,;3.18,1.35,;5.45,3.01,;7,3.01,;7.76,4.34,;6.99,5.67,;5.45,5.67,;4.68,4.34,;4.68,7,;5.45,8.34,;3.14,7,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | <50 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50609043
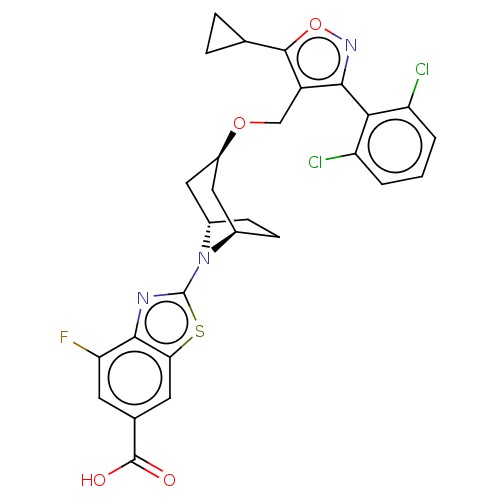
(CHEMBL5290473)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OCc1c(onc1-c1c(Cl)cccc1Cl)C1CC1)N2c1nc2c(F)cc(cc2s1)C(O)=O |r,wD:7.9,4.4,1.0,(-.42,2.7,;-.42,1.16,;-1.09,.03,;-.42,-1.15,;.91,-1.15,;.91,-2.69,;-.42,-1.92,;-1.76,-1.15,;-1.76,.39,;-3.09,-1.92,;-4.43,-1.15,;-5.76,-1.92,;-7.15,-1.31,;-8.17,-2.44,;-7.4,-3.77,;-5.92,-3.46,;-4.79,-4.49,;-5.09,-5.98,;-6.53,-6.44,;-3.97,-7,;-2.48,-6.54,;-2.17,-5.05,;-3.3,-4.03,;-2.9,-2.54,;-7.45,.23,;-6.99,1.67,;-8.53,1.36,;.91,.39,;2.24,1.16,;3.65,.53,;4.68,1.67,;6.23,1.67,;7,.34,;6.99,3.01,;6.22,4.34,;4.68,4.34,;3.91,3.01,;2.41,2.69,;6.99,5.67,;8.53,5.67,;6.22,7,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM16111

(2-{[2-fluoro-4-(3-methoxyphenyl)phenyl]carbamoyl}c...)Show SMILES COc1cccc(c1)-c1ccc(NC(=O)C2=C(CCC2)C(O)=O)c(F)c1 |t:16| Show InChI InChI=1S/C20H18FNO4/c1-26-14-5-2-4-12(10-14)13-8-9-18(17(21)11-13)22-19(23)15-6-3-7-16(15)20(24)25/h2,4-5,8-11H,3,6-7H2,1H3,(H,22,23)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| n/a | n/a | n/a | n/a | 450 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50241116

(CHEMBL4066332)Show SMILES CC(C)(C)c1ccc(cc1)C(=O)NCc1ccc(NS(C)(=O)=O)cc1Cl Show InChI InChI=1S/C19H23ClN2O3S/c1-19(2,3)15-8-5-13(6-9-15)18(23)21-12-14-7-10-16(11-17(14)20)22-26(4,24)25/h5-11,22H,12H2,1-4H3,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data