 Found 650 hits with Last Name = 'fink' and Initial = 'a'
Found 650 hits with Last Name = 'fink' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50587810
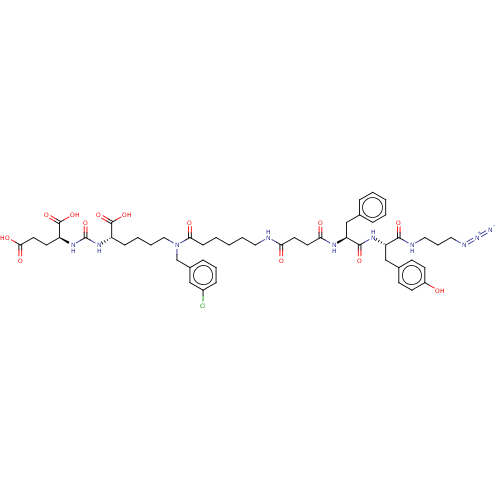
(CHEMBL5193353)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCN(Cc1cccc(Cl)c1)C(=O)CCCCCNC(=O)CCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)NCCCN=[N+]=[N-])C(O)=O)C(O)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01935
BindingDB Entry DOI: 10.7270/Q2NS0ZWP |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50587817
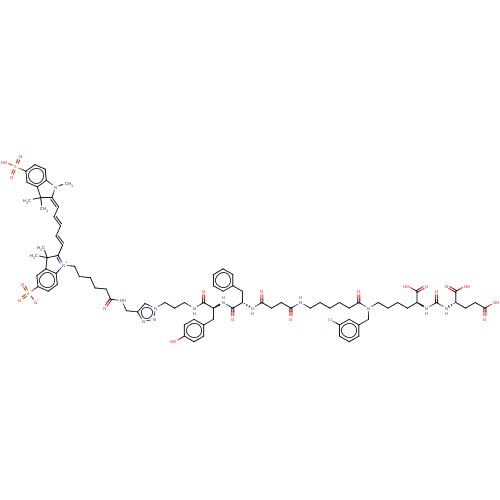
(CHEMBL5186540)Show SMILES CN1\C(=C\C=C\C=C\C2=[N+](CCCCCC(=O)NCc3cn(CCCNC(=O)[C@H](Cc4ccc(O)cc4)NC(=O)[C@H](Cc4ccccc4)NC(=O)CCC(=O)NCCCCCC(=O)N(CCCC[C@H](NC(=O)N[C@@H](CCC(O)=O)C(O)=O)C(O)=O)Cc4cccc(Cl)c4)nn3)c3ccc(cc3C2(C)C)S([O-])(=O)=O)C(C)(C)c2cc(ccc12)S(O)(=O)=O |r,c:8| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01935
BindingDB Entry DOI: 10.7270/Q2NS0ZWP |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50194815
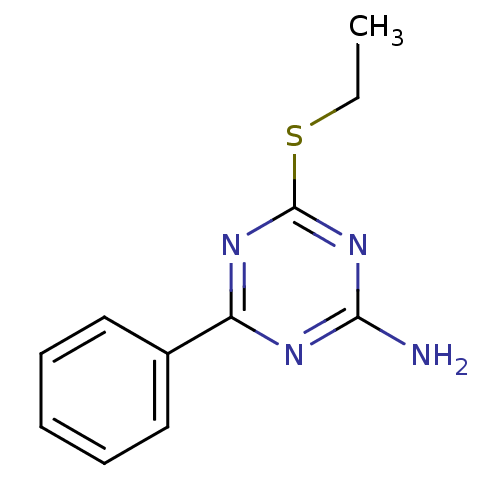
(4-(ethylthio)-6-phenyl-1,3,5-triazin-2-amine | CHE...)Show InChI InChI=1S/C11H12N4S/c1-2-16-11-14-9(13-10(12)15-11)8-6-4-3-5-7-8/h3-7H,2H2,1H3,(H2,12,13,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from human recombinant adenosine A2A receptor |
Bioorg Med Chem Lett 16: 5993-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.116
BindingDB Entry DOI: 10.7270/Q2J102TH |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50587818

(CHEMBL5206903)Show SMILES CN1\C(=C\C=C2/CCCC(\C=C\C3=[N+](CCCCCC(=O)NCc4cn(CCCNC(=O)[C@H](Cc5ccc(O)cc5)NC(=O)[C@H](Cc5ccccc5)NC(=O)CCC(=O)NCCCCCC(=O)N(CCCC[C@H](NC(=O)N[C@@H](CCC(O)=O)C(O)=O)C(O)=O)Cc5cccc(Cl)c5)nn4)c4ccc(cc4C3(C)C)S([O-])(=O)=O)=C2)C(C)(C)c2cc(ccc12)S(O)(=O)=O |r,c:12,117| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01935
BindingDB Entry DOI: 10.7270/Q2NS0ZWP |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50194818
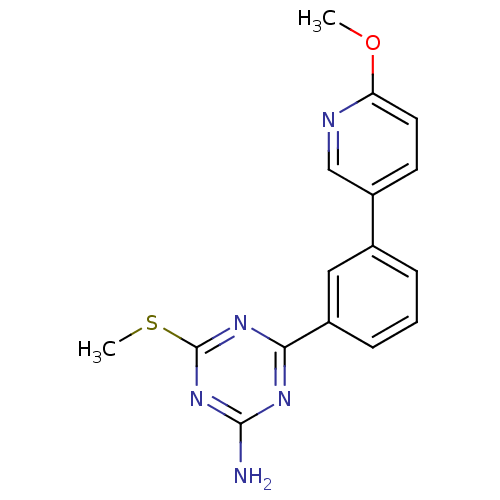
(4-(3-(6-methoxypyridin-3-yl)phenyl)-6-(methylthio)...)Show InChI InChI=1S/C16H15N5OS/c1-22-13-7-6-12(9-18-13)10-4-3-5-11(8-10)14-19-15(17)21-16(20-14)23-2/h3-9H,1-2H3,(H2,17,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from human recombinant adenosine A2A receptor |
Bioorg Med Chem Lett 16: 5993-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.116
BindingDB Entry DOI: 10.7270/Q2J102TH |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50283607
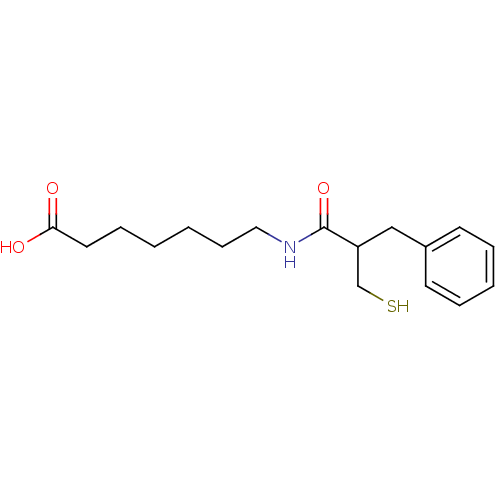
(7-(2-Mercaptomethyl-3-phenyl-propionylamino)-hepta...)Show InChI InChI=1S/C17H25NO3S/c19-16(20)10-6-1-2-7-11-18-17(21)15(13-22)12-14-8-4-3-5-9-14/h3-5,8-9,15,22H,1-2,6-7,10-13H2,(H,18,21)(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition against neutral endopeptidase 24.11 (NEP) in rat kidney cortex membrane |
Bioorg Med Chem Lett 4: 2673-2676 (1994)
Article DOI: 10.1016/S0960-894X(01)80694-6
BindingDB Entry DOI: 10.7270/Q2X34XXT |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50194824
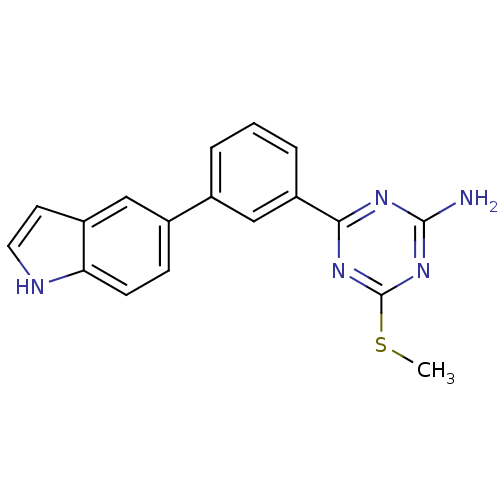
(4-(3-(1H-indol-5-yl)phenyl)-6-(methylthio)-1,3,5-t...)Show SMILES CSc1nc(N)nc(n1)-c1cccc(c1)-c1ccc2[nH]ccc2c1 Show InChI InChI=1S/C18H15N5S/c1-24-18-22-16(21-17(19)23-18)14-4-2-3-11(10-14)12-5-6-15-13(9-12)7-8-20-15/h2-10,20H,1H3,(H2,19,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from human recombinant adenosine A2A receptor |
Bioorg Med Chem Lett 16: 5993-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.116
BindingDB Entry DOI: 10.7270/Q2J102TH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50194821
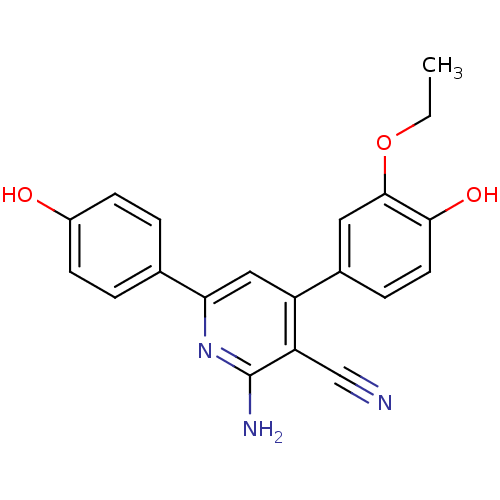
(2-amino-4-(3-ethoxy-4-hydroxyphenyl)-6-(4-hydroxyp...)Show SMILES CCOc1cc(ccc1O)-c1cc(nc(N)c1C#N)-c1ccc(O)cc1 Show InChI InChI=1S/C20H17N3O3/c1-2-26-19-9-13(5-8-18(19)25)15-10-17(23-20(22)16(15)11-21)12-3-6-14(24)7-4-12/h3-10,24-25H,2H2,1H3,(H2,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from recombinant adenosine A1 receptor |
Bioorg Med Chem Lett 16: 5993-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.116
BindingDB Entry DOI: 10.7270/Q2J102TH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50194814
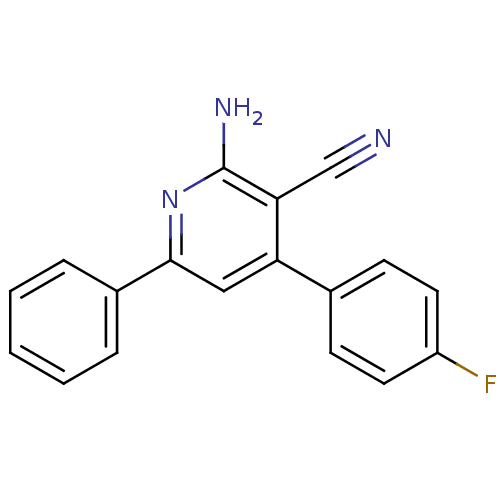
(2-amino-4-(4-fluorophenyl)-6-phenylnicotinonitrile...)Show InChI InChI=1S/C18H12FN3/c19-14-8-6-12(7-9-14)15-10-17(13-4-2-1-3-5-13)22-18(21)16(15)11-20/h1-10H,(H2,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from recombinant adenosine A1 receptor |
Bioorg Med Chem Lett 16: 5993-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.116
BindingDB Entry DOI: 10.7270/Q2J102TH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50194815

(4-(ethylthio)-6-phenyl-1,3,5-triazin-2-amine | CHE...)Show InChI InChI=1S/C11H12N4S/c1-2-16-11-14-9(13-10(12)15-11)8-6-4-3-5-7-8/h3-7H,2H2,1H3,(H2,12,13,14,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from recombinant adenosine A1 receptor |
Bioorg Med Chem Lett 16: 5993-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.116
BindingDB Entry DOI: 10.7270/Q2J102TH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50194814

(2-amino-4-(4-fluorophenyl)-6-phenylnicotinonitrile...)Show InChI InChI=1S/C18H12FN3/c19-14-8-6-12(7-9-14)15-10-17(13-4-2-1-3-5-13)22-18(21)16(15)11-20/h1-10H,(H2,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from human recombinant adenosine A2A receptor |
Bioorg Med Chem Lett 16: 5993-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.116
BindingDB Entry DOI: 10.7270/Q2J102TH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50194817
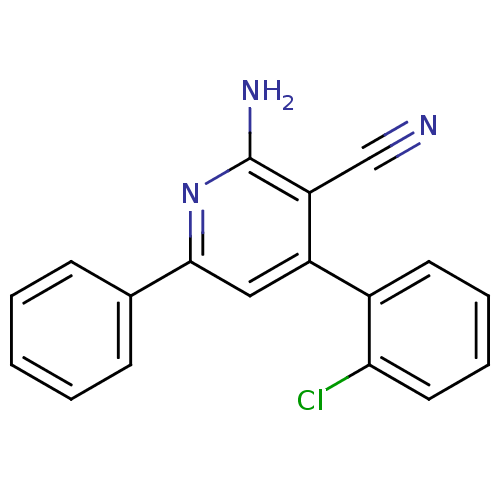
(2-amino-4-(2-chlorophenyl)-6-phenylnicotinonitrile...)Show InChI InChI=1S/C18H12ClN3/c19-16-9-5-4-8-13(16)14-10-17(12-6-2-1-3-7-12)22-18(21)15(14)11-20/h1-10H,(H2,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from human recombinant adenosine A2A receptor |
Bioorg Med Chem Lett 16: 5993-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.116
BindingDB Entry DOI: 10.7270/Q2J102TH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50194822
(2-amino-4-(4-hydroxy-3-methoxyphenyl)-6-(4-hydroxy...)Show InChI InChI=1S/C19H15N3O3/c1-25-18-8-12(4-7-17(18)24)14-9-16(22-19(21)15(14)10-20)11-2-5-13(23)6-3-11/h2-9,23-24H,1H3,(H2,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from recombinant adenosine A1 receptor |
Bioorg Med Chem Lett 16: 5993-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.116
BindingDB Entry DOI: 10.7270/Q2J102TH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50194825
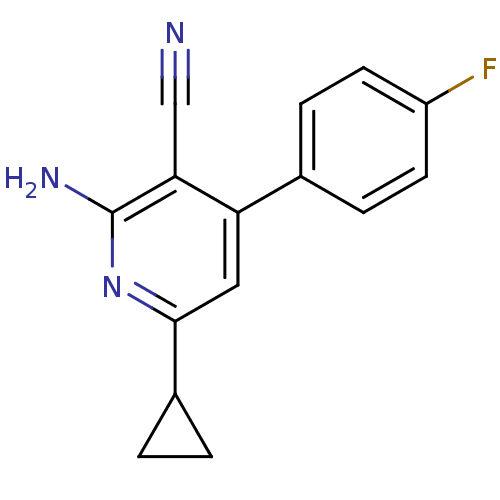
(2-amino-6-cyclopropyl-4-(4-fluorophenyl)nicotinoni...)Show InChI InChI=1S/C15H12FN3/c16-11-5-3-9(4-6-11)12-7-14(10-1-2-10)19-15(18)13(12)8-17/h3-7,10H,1-2H2,(H2,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from human recombinant adenosine A2A receptor |
Bioorg Med Chem Lett 16: 5993-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.116
BindingDB Entry DOI: 10.7270/Q2J102TH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50194811
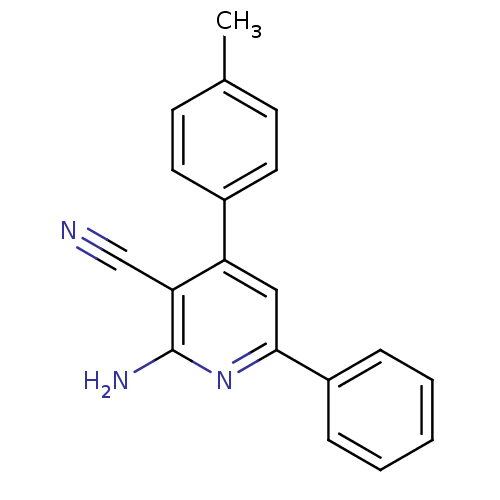
(2-amino-6-phenyl-4-p-tolylnicotinonitrile | CHEMBL...)Show InChI InChI=1S/C19H15N3/c1-13-7-9-14(10-8-13)16-11-18(15-5-3-2-4-6-15)22-19(21)17(16)12-20/h2-11H,1H3,(H2,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 118 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from human recombinant adenosine A2A receptor |
Bioorg Med Chem Lett 16: 5993-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.116
BindingDB Entry DOI: 10.7270/Q2J102TH |
More data for this
Ligand-Target Pair | |
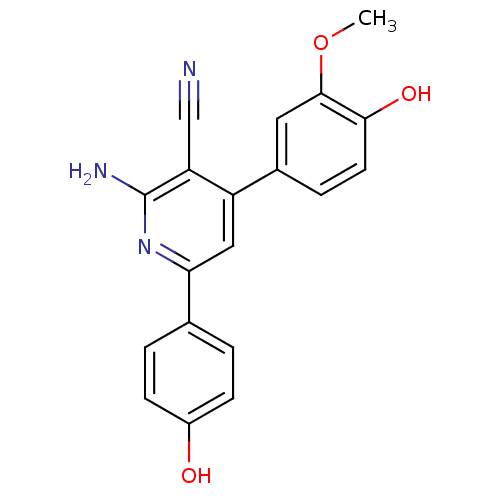
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50194822

(2-amino-4-(4-hydroxy-3-methoxyphenyl)-6-(4-hydroxy...)Show InChI InChI=1S/C19H15N3O3/c1-25-18-8-12(4-7-17(18)24)14-9-16(22-19(21)15(14)10-20)11-2-5-13(23)6-3-11/h2-9,23-24H,1H3,(H2,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from human recombinant adenosine A2A receptor |
Bioorg Med Chem Lett 16: 5993-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.116
BindingDB Entry DOI: 10.7270/Q2J102TH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50194823
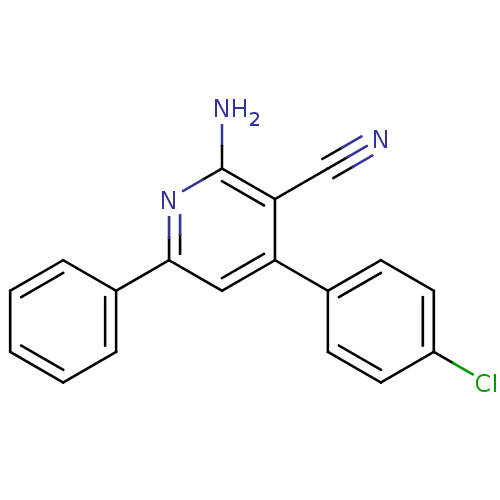
(2-amino-4-(4-chlorophenyl)-6-phenylnicotinonitrile...)Show InChI InChI=1S/C18H12ClN3/c19-14-8-6-12(7-9-14)15-10-17(13-4-2-1-3-5-13)22-18(21)16(15)11-20/h1-10H,(H2,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 192 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from recombinant adenosine A1 receptor |
Bioorg Med Chem Lett 16: 5993-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.116
BindingDB Entry DOI: 10.7270/Q2J102TH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50194823

(2-amino-4-(4-chlorophenyl)-6-phenylnicotinonitrile...)Show InChI InChI=1S/C18H12ClN3/c19-14-8-6-12(7-9-14)15-10-17(13-4-2-1-3-5-13)22-18(21)16(15)11-20/h1-10H,(H2,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 201 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from human recombinant adenosine A2A receptor |
Bioorg Med Chem Lett 16: 5993-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.116
BindingDB Entry DOI: 10.7270/Q2J102TH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50194820
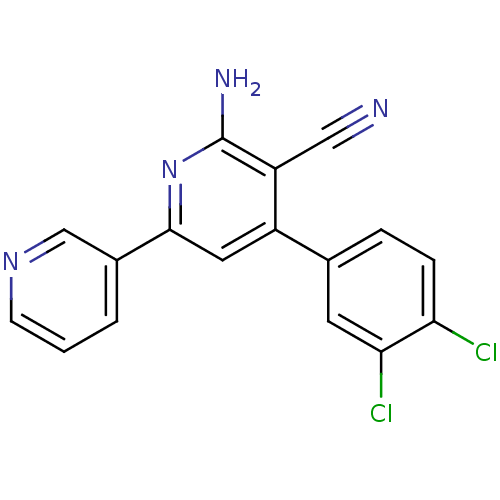
(2-amino-4-(3,4-dichlorophenyl)-6-(pyridin-3-yl)nic...)Show InChI InChI=1S/C17H10Cl2N4/c18-14-4-3-10(6-15(14)19)12-7-16(11-2-1-5-22-9-11)23-17(21)13(12)8-20/h1-7,9H,(H2,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from human recombinant adenosine A2A receptor |
Bioorg Med Chem Lett 16: 5993-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.116
BindingDB Entry DOI: 10.7270/Q2J102TH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50194824

(4-(3-(1H-indol-5-yl)phenyl)-6-(methylthio)-1,3,5-t...)Show SMILES CSc1nc(N)nc(n1)-c1cccc(c1)-c1ccc2[nH]ccc2c1 Show InChI InChI=1S/C18H15N5S/c1-24-18-22-16(21-17(19)23-18)14-4-2-3-11(10-14)12-5-6-15-13(9-12)7-8-20-15/h2-10,20H,1H3,(H2,19,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 218 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from recombinant adenosine A1 receptor |
Bioorg Med Chem Lett 16: 5993-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.116
BindingDB Entry DOI: 10.7270/Q2J102TH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50194826
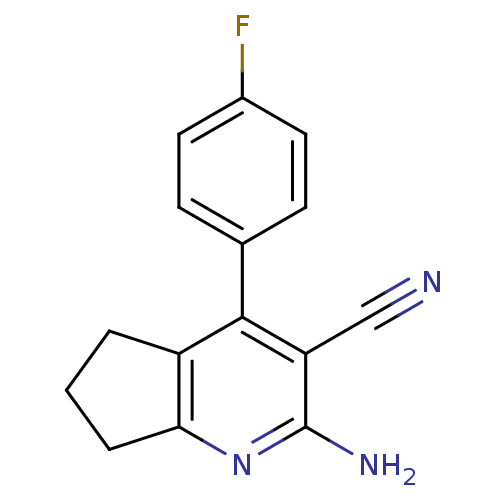
(2-amino-4-(4-fluorophenyl)-6,7-dihydro-5H-cyclopen...)Show InChI InChI=1S/C15H12FN3/c16-10-6-4-9(5-7-10)14-11-2-1-3-13(11)19-15(18)12(14)8-17/h4-7H,1-3H2,(H2,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 232 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from human recombinant adenosine A2A receptor |
Bioorg Med Chem Lett 16: 5993-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.116
BindingDB Entry DOI: 10.7270/Q2J102TH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50194817

(2-amino-4-(2-chlorophenyl)-6-phenylnicotinonitrile...)Show InChI InChI=1S/C18H12ClN3/c19-16-9-5-4-8-13(16)14-10-17(12-6-2-1-3-7-12)22-18(21)15(14)11-20/h1-10H,(H2,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 239 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from recombinant adenosine A1 receptor |
Bioorg Med Chem Lett 16: 5993-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.116
BindingDB Entry DOI: 10.7270/Q2J102TH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50194821

(2-amino-4-(3-ethoxy-4-hydroxyphenyl)-6-(4-hydroxyp...)Show SMILES CCOc1cc(ccc1O)-c1cc(nc(N)c1C#N)-c1ccc(O)cc1 Show InChI InChI=1S/C20H17N3O3/c1-2-26-19-9-13(5-8-18(19)25)15-10-17(23-20(22)16(15)11-21)12-3-6-14(24)7-4-12/h3-10,24-25H,2H2,1H3,(H2,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 267 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from human recombinant adenosine A2A receptor |
Bioorg Med Chem Lett 16: 5993-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.116
BindingDB Entry DOI: 10.7270/Q2J102TH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50194811

(2-amino-6-phenyl-4-p-tolylnicotinonitrile | CHEMBL...)Show InChI InChI=1S/C19H15N3/c1-13-7-9-14(10-8-13)16-11-18(15-5-3-2-4-6-15)22-19(21)17(16)12-20/h2-11H,1H3,(H2,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 274 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from recombinant adenosine A1 receptor |
Bioorg Med Chem Lett 16: 5993-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.116
BindingDB Entry DOI: 10.7270/Q2J102TH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50194816
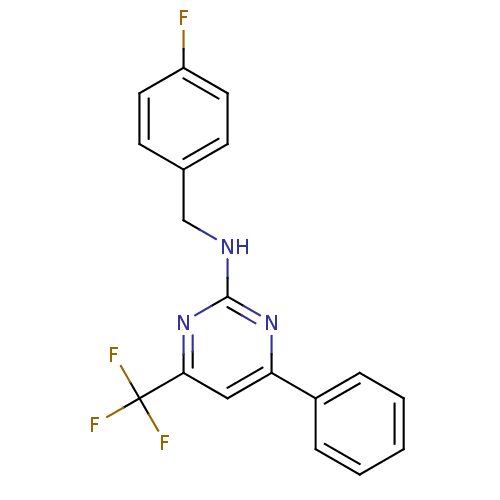
(CHEMBL221219 | N-(4-fluorobenzyl)-4-phenyl-6-(trif...)Show InChI InChI=1S/C18H13F4N3/c19-14-8-6-12(7-9-14)11-23-17-24-15(13-4-2-1-3-5-13)10-16(25-17)18(20,21)22/h1-10H,11H2,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 287 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from human recombinant adenosine A2A receptor |
Bioorg Med Chem Lett 16: 5993-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.116
BindingDB Entry DOI: 10.7270/Q2J102TH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50194813
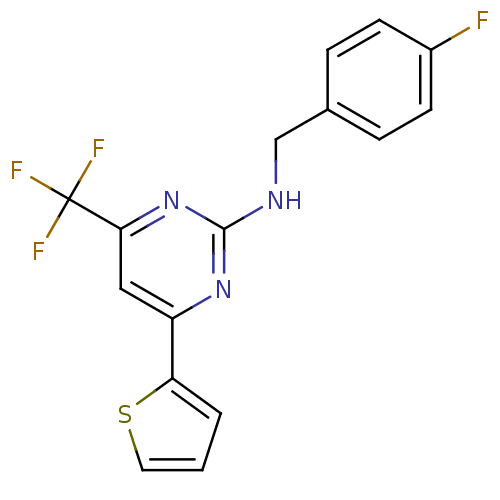
(CHEMBL221220 | N-(4-fluorobenzyl)-4-(thiophen-2-yl...)Show InChI InChI=1S/C16H11F4N3S/c17-11-5-3-10(4-6-11)9-21-15-22-12(13-2-1-7-24-13)8-14(23-15)16(18,19)20/h1-8H,9H2,(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 287 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from human recombinant adenosine A2A receptor |
Bioorg Med Chem Lett 16: 5993-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.116
BindingDB Entry DOI: 10.7270/Q2J102TH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50194812
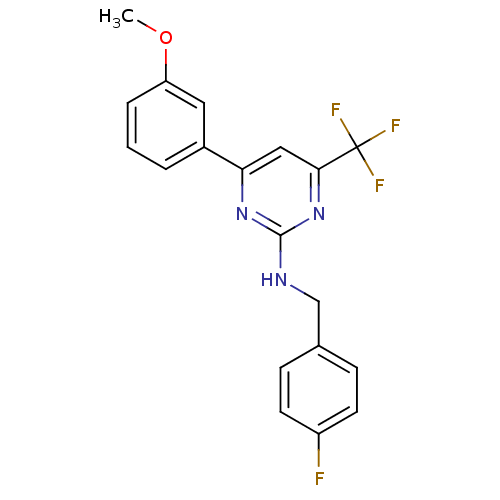
(CHEMBL220287 | N-(4-fluorobenzyl)-4-(3-methoxyphen...)Show SMILES COc1cccc(c1)-c1cc(nc(NCc2ccc(F)cc2)n1)C(F)(F)F Show InChI InChI=1S/C19H15F4N3O/c1-27-15-4-2-3-13(9-15)16-10-17(19(21,22)23)26-18(25-16)24-11-12-5-7-14(20)8-6-12/h2-10H,11H2,1H3,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from human recombinant adenosine A2A receptor |
Bioorg Med Chem Lett 16: 5993-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.116
BindingDB Entry DOI: 10.7270/Q2J102TH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50194819
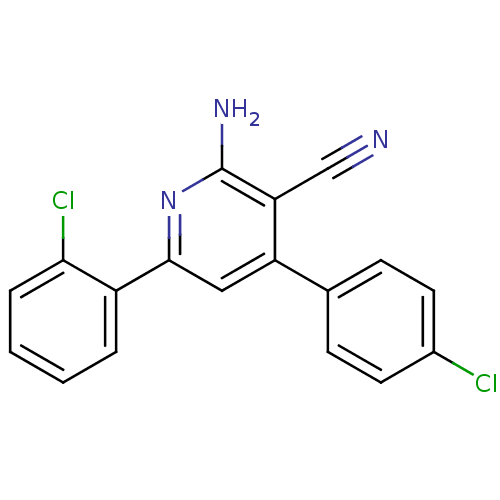
(2-amino-6-(2-chlorophenyl)-4-(4-chlorophenyl)nicot...)Show InChI InChI=1S/C18H11Cl2N3/c19-12-7-5-11(6-8-12)14-9-17(23-18(22)15(14)10-21)13-3-1-2-4-16(13)20/h1-9H,(H2,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 403 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from human recombinant adenosine A2A receptor |
Bioorg Med Chem Lett 16: 5993-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.116
BindingDB Entry DOI: 10.7270/Q2J102TH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50194827
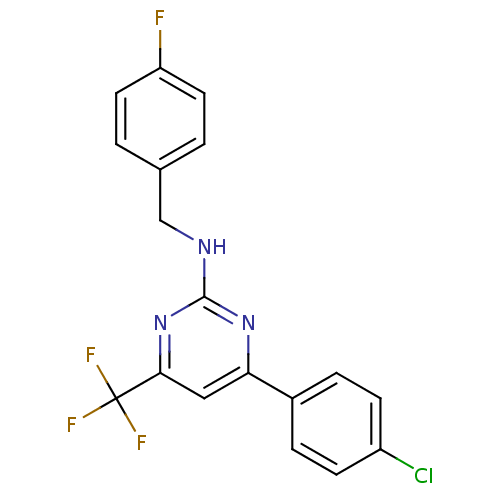
(CHEMBL374236 | N-(4-fluorobenzyl)-4-(4-chloropheny...)Show SMILES Fc1ccc(CNc2nc(cc(n2)C(F)(F)F)-c2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C18H12ClF4N3/c19-13-5-3-12(4-6-13)15-9-16(18(21,22)23)26-17(25-15)24-10-11-1-7-14(20)8-2-11/h1-9H,10H2,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 454 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from human recombinant adenosine A2A receptor |
Bioorg Med Chem Lett 16: 5993-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.116
BindingDB Entry DOI: 10.7270/Q2J102TH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50194818

(4-(3-(6-methoxypyridin-3-yl)phenyl)-6-(methylthio)...)Show InChI InChI=1S/C16H15N5OS/c1-22-13-7-6-12(9-18-13)10-4-3-5-11(8-10)14-19-15(17)21-16(20-14)23-2/h3-9H,1-2H3,(H2,17,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from recombinant adenosine A1 receptor |
Bioorg Med Chem Lett 16: 5993-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.116
BindingDB Entry DOI: 10.7270/Q2J102TH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50194825

(2-amino-6-cyclopropyl-4-(4-fluorophenyl)nicotinoni...)Show InChI InChI=1S/C15H12FN3/c16-11-5-3-9(4-6-11)12-7-14(10-1-2-10)19-15(18)13(12)8-17/h3-7,10H,1-2H2,(H2,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 865 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from recombinant adenosine A1 receptor |
Bioorg Med Chem Lett 16: 5993-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.116
BindingDB Entry DOI: 10.7270/Q2J102TH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50194819

(2-amino-6-(2-chlorophenyl)-4-(4-chlorophenyl)nicot...)Show InChI InChI=1S/C18H11Cl2N3/c19-12-7-5-11(6-8-12)14-9-17(23-18(22)15(14)10-21)13-3-1-2-4-16(13)20/h1-9H,(H2,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from recombinant adenosine A1 receptor |
Bioorg Med Chem Lett 16: 5993-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.116
BindingDB Entry DOI: 10.7270/Q2J102TH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50194820

(2-amino-4-(3,4-dichlorophenyl)-6-(pyridin-3-yl)nic...)Show InChI InChI=1S/C17H10Cl2N4/c18-14-4-3-10(6-15(14)19)12-7-16(11-2-1-5-22-9-11)23-17(21)13(12)8-20/h1-7,9H,(H2,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from recombinant adenosine A1 receptor |
Bioorg Med Chem Lett 16: 5993-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.116
BindingDB Entry DOI: 10.7270/Q2J102TH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50194813

(CHEMBL221220 | N-(4-fluorobenzyl)-4-(thiophen-2-yl...)Show InChI InChI=1S/C16H11F4N3S/c17-11-5-3-10(4-6-11)9-21-15-22-12(13-2-1-7-24-13)8-14(23-15)16(18,19)20/h1-8H,9H2,(H,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from recombinant adenosine A1 receptor |
Bioorg Med Chem Lett 16: 5993-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.116
BindingDB Entry DOI: 10.7270/Q2J102TH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50194812

(CHEMBL220287 | N-(4-fluorobenzyl)-4-(3-methoxyphen...)Show SMILES COc1cccc(c1)-c1cc(nc(NCc2ccc(F)cc2)n1)C(F)(F)F Show InChI InChI=1S/C19H15F4N3O/c1-27-15-4-2-3-13(9-15)16-10-17(19(21,22)23)26-18(25-16)24-11-12-5-7-14(20)8-6-12/h2-10H,11H2,1H3,(H,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from recombinant adenosine A1 receptor |
Bioorg Med Chem Lett 16: 5993-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.116
BindingDB Entry DOI: 10.7270/Q2J102TH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50194827

(CHEMBL374236 | N-(4-fluorobenzyl)-4-(4-chloropheny...)Show SMILES Fc1ccc(CNc2nc(cc(n2)C(F)(F)F)-c2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C18H12ClF4N3/c19-13-5-3-12(4-6-13)15-9-16(18(21,22)23)26-17(25-15)24-10-11-1-7-14(20)8-2-11/h1-9H,10H2,(H,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from recombinant adenosine A1 receptor |
Bioorg Med Chem Lett 16: 5993-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.116
BindingDB Entry DOI: 10.7270/Q2J102TH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50194816

(CHEMBL221219 | N-(4-fluorobenzyl)-4-phenyl-6-(trif...)Show InChI InChI=1S/C18H13F4N3/c19-14-8-6-12(7-9-14)11-23-17-24-15(13-4-2-1-3-5-13)10-16(25-17)18(20,21)22/h1-10H,11H2,(H,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from recombinant adenosine A1 receptor |
Bioorg Med Chem Lett 16: 5993-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.116
BindingDB Entry DOI: 10.7270/Q2J102TH |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281205
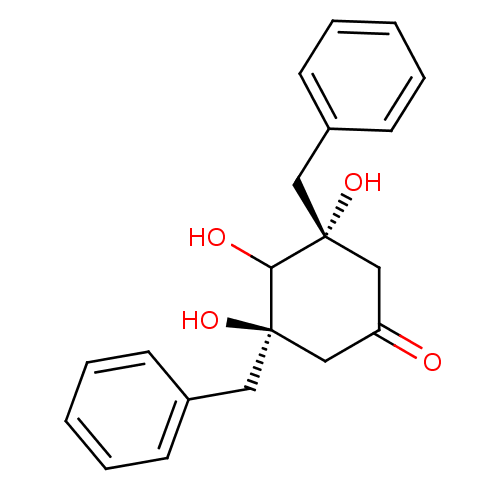
((3R,5R)-3,5-Dibenzyl-3,4,5-trihydroxy-cyclohexanon...)Show SMILES OC1[C@@](O)(Cc2ccccc2)CC(=O)C[C@]1(O)Cc1ccccc1 Show InChI InChI=1S/C20H22O4/c21-17-13-19(23,11-15-7-3-1-4-8-15)18(22)20(24,14-17)12-16-9-5-2-6-10-16/h1-10,18,22-24H,11-14H2/t19-,20-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of recombinant HIV-1 protease at 37 degrees C and pH of 6. |
Bioorg Med Chem Lett 3: 2717-2722 (1993)
Article DOI: 10.1016/S0960-894X(01)80749-6
BindingDB Entry DOI: 10.7270/Q2ZP4623 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281202
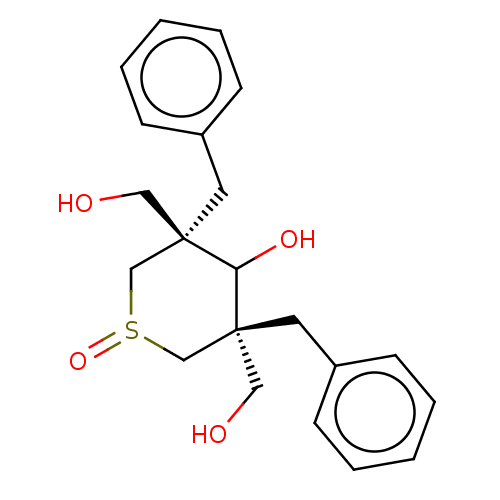
((3S,5S)-3,5-Dibenzyl-3,5-bis-hydroxymethyl-1-oxo-t...)Show SMILES OC[C@]1(Cc2ccccc2)C[S+]([O-])C[C@](CO)(Cc2ccccc2)C1O |r,wU:2.2,14.15,wD:14.17,2.1,(4.21,-7.86,;5.54,-7.08,;6.87,-7.85,;6.89,-9.39,;5.81,-10.49,;6.22,-11.96,;5.14,-13.06,;3.63,-12.69,;3.22,-11.2,;4.3,-10.1,;6.87,-6.31,;8.2,-5.56,;8.19,-4,;9.53,-6.3,;9.53,-7.84,;10.31,-9.17,;9.55,-10.51,;10.86,-7.07,;12.19,-7.84,;12.18,-9.39,;13.51,-10.16,;14.84,-9.39,;14.85,-7.85,;13.52,-7.08,;8.21,-8.62,;8.21,-10.16,)| Show InChI InChI=1S/C21H26O4S/c22-13-20(11-17-7-3-1-4-8-17)15-26(25)16-21(14-23,19(20)24)12-18-9-5-2-6-10-18/h1-10,19,22-24H,11-16H2/t19?,20-,21-,26?/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of recombinant HIV-1 protease at 37 degrees C and pH of 6. |
Bioorg Med Chem Lett 3: 2717-2722 (1993)
Article DOI: 10.1016/S0960-894X(01)80749-6
BindingDB Entry DOI: 10.7270/Q2ZP4623 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281202

((3S,5S)-3,5-Dibenzyl-3,5-bis-hydroxymethyl-1-oxo-t...)Show SMILES OC[C@]1(Cc2ccccc2)C[S+]([O-])C[C@](CO)(Cc2ccccc2)C1O |r,wU:2.2,14.15,wD:14.17,2.1,(4.21,-7.86,;5.54,-7.08,;6.87,-7.85,;6.89,-9.39,;5.81,-10.49,;6.22,-11.96,;5.14,-13.06,;3.63,-12.69,;3.22,-11.2,;4.3,-10.1,;6.87,-6.31,;8.2,-5.56,;8.19,-4,;9.53,-6.3,;9.53,-7.84,;10.31,-9.17,;9.55,-10.51,;10.86,-7.07,;12.19,-7.84,;12.18,-9.39,;13.51,-10.16,;14.84,-9.39,;14.85,-7.85,;13.52,-7.08,;8.21,-8.62,;8.21,-10.16,)| Show InChI InChI=1S/C21H26O4S/c22-13-20(11-17-7-3-1-4-8-17)15-26(25)16-21(14-23,19(20)24)12-18-9-5-2-6-10-18/h1-10,19,22-24H,11-16H2/t19?,20-,21-,26?/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of recombinant HIV-1 protease at 37 degrees C and pH of 6. |
Bioorg Med Chem Lett 3: 2717-2722 (1993)
Article DOI: 10.1016/S0960-894X(01)80749-6
BindingDB Entry DOI: 10.7270/Q2ZP4623 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281207
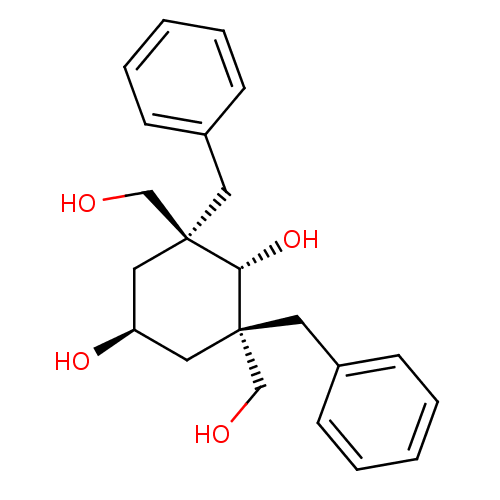
((2S,6S)-2,6-Dibenzyl-2,6-bis-hydroxymethyl-cyclohe...)Show SMILES OC[C@]1(Cc2ccccc2)C[C@H](O)C[C@](CO)(Cc2ccccc2)[C@H]1O |wU:2.2,14.15,24.27,wD:14.17,2.1,11.12,(15.23,-12.28,;13.9,-13.04,;12.57,-12.28,;12.56,-10.74,;13.64,-9.64,;13.23,-8.15,;14.32,-7.05,;15.82,-7.45,;16.23,-8.94,;15.14,-10.04,;12.57,-13.81,;11.24,-14.58,;11.24,-16.12,;9.91,-13.81,;9.91,-12.28,;9.14,-10.94,;9.9,-9.59,;8.58,-13.04,;7.25,-12.25,;7.26,-10.71,;5.93,-9.94,;4.6,-10.69,;4.59,-12.24,;5.92,-13.01,;11.24,-11.5,;11.24,-9.96,)| Show InChI InChI=1S/C22H28O4/c23-15-21(11-17-7-3-1-4-8-17)13-19(25)14-22(16-24,20(21)26)12-18-9-5-2-6-10-18/h1-10,19-20,23-26H,11-16H2/t19-,20-,21-,22-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of recombinant HIV-1 protease at 37 degrees C and pH of 6. |
Bioorg Med Chem Lett 3: 2717-2722 (1993)
Article DOI: 10.1016/S0960-894X(01)80749-6
BindingDB Entry DOI: 10.7270/Q2ZP4623 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281204

((3S,5S)-3,5-Dibenzyl-4-hydroxy-3,5-bis-hydroxymeth...)Show SMILES OC[C@]1(Cc2ccccc2)CC(=O)C[C@](CO)(Cc2ccccc2)C1O Show InChI InChI=1S/C22H26O4/c23-15-21(11-17-7-3-1-4-8-17)13-19(25)14-22(16-24,20(21)26)12-18-9-5-2-6-10-18/h1-10,20,23-24,26H,11-16H2/t21-,22-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
| 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of recombinant HIV-1 protease at 37 degrees C and pH of 6. |
Bioorg Med Chem Lett 3: 2717-2722 (1993)
Article DOI: 10.1016/S0960-894X(01)80749-6
BindingDB Entry DOI: 10.7270/Q2ZP4623 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281206
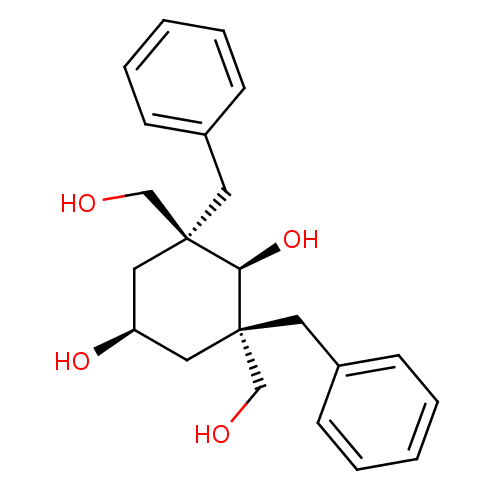
((2S,6S)-2,6-Dibenzyl-2,6-bis-hydroxymethyl-cyclohe...)Show SMILES OC[C@]1(Cc2ccccc2)C[C@H](O)C[C@](CO)(Cc2ccccc2)[C@@H]1O |wU:14.17,2.1,24.27,11.12,wD:2.2,14.15,(9.9,-9.59,;9.14,-10.94,;9.91,-12.28,;8.58,-13.04,;7.25,-12.25,;7.26,-10.71,;5.93,-9.94,;4.6,-10.69,;4.59,-12.24,;5.92,-13.01,;9.91,-13.81,;11.24,-14.58,;11.24,-16.12,;12.57,-13.81,;12.57,-12.28,;13.9,-13.04,;15.23,-12.28,;12.56,-10.74,;13.64,-9.64,;13.23,-8.15,;14.32,-7.05,;15.82,-7.45,;16.23,-8.94,;15.14,-10.04,;11.24,-11.5,;11.24,-9.96,)| Show InChI InChI=1S/C22H28O4/c23-15-21(11-17-7-3-1-4-8-17)13-19(25)14-22(16-24,20(21)26)12-18-9-5-2-6-10-18/h1-10,19-20,23-26H,11-16H2/t19-,20+,21-,22-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 8.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of recombinant HIV-1 protease at 37 degrees C and pH of 6. |
Bioorg Med Chem Lett 3: 2717-2722 (1993)
Article DOI: 10.1016/S0960-894X(01)80749-6
BindingDB Entry DOI: 10.7270/Q2ZP4623 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281208
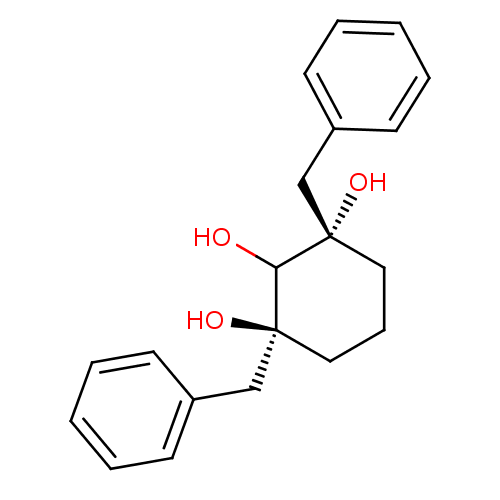
((1S,3S)-1,3-Dibenzyl-cyclohexane-1,2,3-triol | CHE...)Show InChI InChI=1S/C20H24O3/c21-18-19(22,14-16-8-3-1-4-9-16)12-7-13-20(18,23)15-17-10-5-2-6-11-17/h1-6,8-11,18,21-23H,7,12-15H2/t19-,20-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of recombinant HIV-1 protease at 37 degrees C and pH of 6. |
Bioorg Med Chem Lett 3: 2717-2722 (1993)
Article DOI: 10.1016/S0960-894X(01)80749-6
BindingDB Entry DOI: 10.7270/Q2ZP4623 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281203
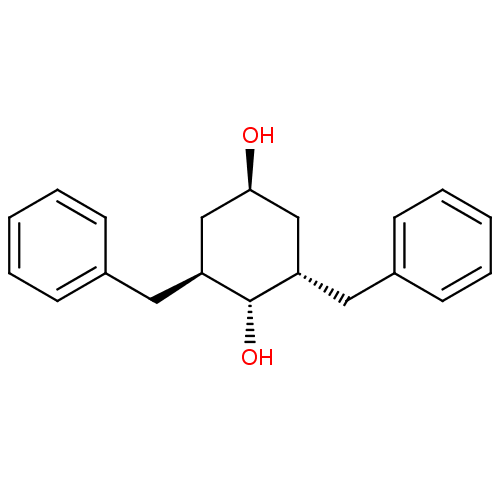
((2S,6S)-2,6-Dibenzyl-cyclohexane-1,4-diol | CHEMBL...)Show SMILES O[C@H]1C[C@H](Cc2ccccc2)[C@H](O)[C@@H](Cc2ccccc2)C1 |wU:3.3,11.12,wD:13.14,1.0,(11.67,-10.37,;11.67,-8.83,;13,-8.06,;13,-6.53,;13,-4.99,;14.08,-3.9,;13.67,-2.41,;14.75,-1.31,;16.26,-1.7,;16.67,-3.2,;15.57,-4.29,;11.67,-5.75,;11.67,-4.21,;10.34,-6.53,;9.01,-7.29,;7.68,-6.52,;7.68,-4.97,;6.37,-4.18,;5.02,-4.95,;5.02,-6.49,;6.35,-7.26,;10.34,-8.06,)| Show InChI InChI=1S/C20H24O2/c21-19-13-17(11-15-7-3-1-4-8-15)20(22)18(14-19)12-16-9-5-2-6-10-16/h1-10,17-22H,11-14H2/t17-,18-,19-,20-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of recombinant HIV-1 protease at 37 degrees C and pH of 6. |
Bioorg Med Chem Lett 3: 2717-2722 (1993)
Article DOI: 10.1016/S0960-894X(01)80749-6
BindingDB Entry DOI: 10.7270/Q2ZP4623 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM50011972

(CHEMBL385189 | Sar-Arg-Val-Tyr-Ile-His-Pro-Thi-OH)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1cccs1)C(O)=O Show InChI InChI=1S/C47H69N13O10S/c1-6-27(4)39(44(67)56-34(21-29-23-51-25-53-29)45(68)60-18-8-12-36(60)42(65)57-35(46(69)70)22-31-10-9-19-71-31)59-41(64)33(20-28-13-15-30(61)16-14-28)55-43(66)38(26(2)3)58-40(63)32(54-37(62)24-50-5)11-7-17-52-47(48)49/h9-10,13-16,19,23,25-27,32-36,38-39,50,61H,6-8,11-12,17-18,20-22,24H2,1-5H3,(H,51,53)(H,54,62)(H,55,66)(H,56,67)(H,57,65)(H,58,63)(H,59,64)(H,69,70)(H4,48,49,52)/t27-,32-,33-,34-,35-,36-,38-,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition of [125I]-AII specific binding towards angiotensin II receptor in rat mesenteric membranes. |
J Med Chem 34: 1514-7 (1991)
BindingDB Entry DOI: 10.7270/Q2TD9WBV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230790

(CHEMBL292892)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1[N+]([O-])=O)C(O)=O Show InChI InChI=1S/C23H23N3O6S/c1-2-3-6-21-24-13-18(10-17(23(29)30)11-19-5-4-9-33-19)25(21)14-16-8-7-15(22(27)28)12-20(16)26(31)32/h4-5,7-10,12-13H,2-3,6,11,14H2,1H3,(H,27,28)(H,29,30)/b17-10+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230779

(CHEMBL294686)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)c2nn[nH]n2)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24N6O2S/c1-2-3-6-21-24-14-19(29(21)15-16-7-9-17(10-8-16)23(30)31)12-18(22-25-27-28-26-22)13-20-5-4-11-32-20/h4-5,7-12,14H,2-3,6,13,15H2,1H3,(H,30,31)(H,25,26,27,28)/b18-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230811
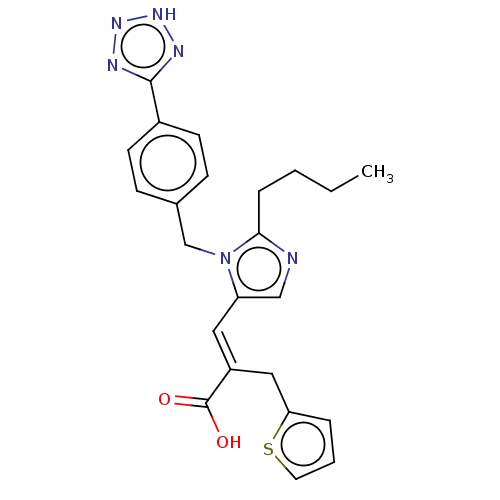
(CHEMBL293091)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)-c1nn[nH]n1 Show InChI InChI=1S/C23H24N6O2S/c1-2-3-6-21-24-14-19(12-18(23(30)31)13-20-5-4-11-32-20)29(21)15-16-7-9-17(10-8-16)22-25-27-28-26-22/h4-5,7-12,14H,2-3,6,13,15H2,1H3,(H,30,31)(H,25,26,27,28)/b18-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230778
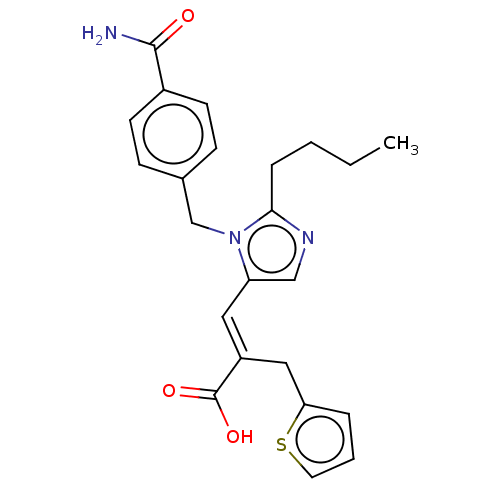
(CHEMBL56211)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)C(N)=O Show InChI InChI=1S/C23H25N3O3S/c1-2-3-6-21-25-14-19(12-18(23(28)29)13-20-5-4-11-30-20)26(21)15-16-7-9-17(10-8-16)22(24)27/h4-5,7-12,14H,2-3,6,13,15H2,1H3,(H2,24,27)(H,28,29)/b18-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data