

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Homo sapiens (Human)) | BDBM10972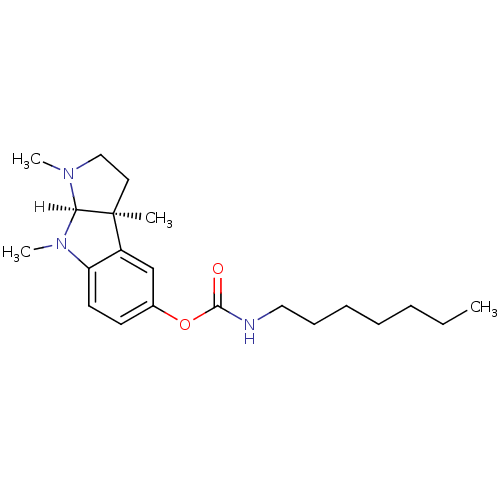 ((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of human serum Butyrylcholinesterase using butyrylthiocholine as substrate; 0.00029-0.001 | Bioorg Med Chem Lett 7: 157-162 (1997) Article DOI: 10.1016/S0960-894X(96)00592-6 BindingDB Entry DOI: 10.7270/Q2CC10P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10972 ((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro in rats for the inhibition of brain (striatal) acetylcholinesterase (AChEI) using acetylthiocholine as substrate; 0.0... | Bioorg Med Chem Lett 7: 157-162 (1997) Article DOI: 10.1016/S0960-894X(96)00592-6 BindingDB Entry DOI: 10.7270/Q2CC10P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10972 ((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase. | Bioorg Med Chem Lett 6: 625-630 (1996) Article DOI: 10.1016/0960-894X(96)00072-8 BindingDB Entry DOI: 10.7270/Q22J6BVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50290392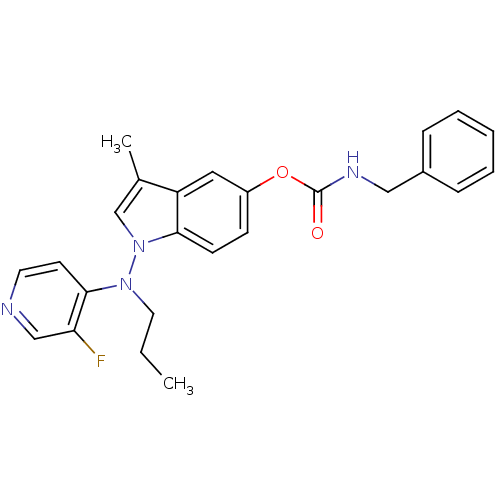 (Benzyl-carbamic acid 1-[(3-fluoro-pyridin-4-yl)-pr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of human serum Butyrylcholinesterase using butyrylthiocholine as substrate; 0.01-0.069 | Bioorg Med Chem Lett 7: 157-162 (1997) Article DOI: 10.1016/S0960-894X(96)00592-6 BindingDB Entry DOI: 10.7270/Q2CC10P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50290391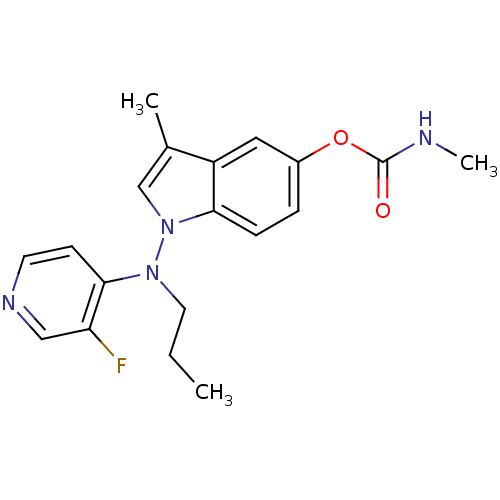 (CHEMBL42531 | Methyl-carbamic acid 1-[(3-fluoro-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of human serum Butyrylcholinesterase using butyrylthiocholine as substrate; 0.023-0.041 | Bioorg Med Chem Lett 7: 157-162 (1997) Article DOI: 10.1016/S0960-894X(96)00592-6 BindingDB Entry DOI: 10.7270/Q2CC10P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50290392 (Benzyl-carbamic acid 1-[(3-fluoro-pyridin-4-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro in rats for the inhibition of brain (striatal) acetylcholinesterase (AChEI) using acetylthiocholine as substrate; 0.0... | Bioorg Med Chem Lett 7: 157-162 (1997) Article DOI: 10.1016/S0960-894X(96)00592-6 BindingDB Entry DOI: 10.7270/Q2CC10P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50290391 (CHEMBL42531 | Methyl-carbamic acid 1-[(3-fluoro-py...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro in rats for the inhibition of brain (striatal) acetylcholinesterase (AChEI) using acetylthiocholine as substrate; 0.0... | Bioorg Med Chem Lett 7: 157-162 (1997) Article DOI: 10.1016/S0960-894X(96)00592-6 BindingDB Entry DOI: 10.7270/Q2CC10P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50231956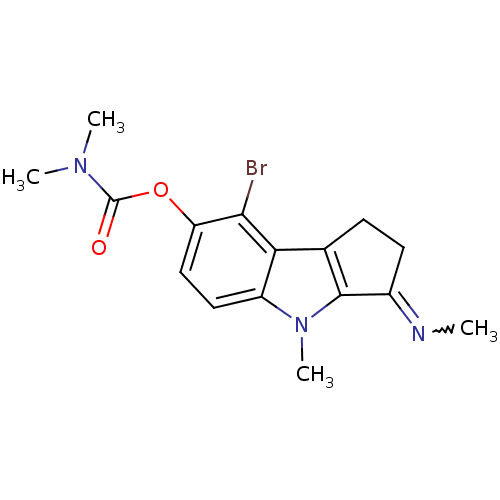 ((Z)-8-bromo-4-methyl-3-(methylimino)-1,2,3,4-tetra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase. | Bioorg Med Chem Lett 6: 625-630 (1996) Article DOI: 10.1016/0960-894X(96)00072-8 BindingDB Entry DOI: 10.7270/Q22J6BVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50342601 (CHEMBL1255901 | Huperzine A) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Acetylcholinesterase inhibitory activity in rat striatal homogenates | J Med Chem 38: 3645-51 (1995) BindingDB Entry DOI: 10.7270/Q2X34Z3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50288976 (CHEMBL155350 | Methyl-carbamic acid 8-chloro-3-[(Z...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of monoamine oxidase-A(MAO-A). | Bioorg Med Chem Lett 6: 625-630 (1996) Article DOI: 10.1016/0960-894X(96)00072-8 BindingDB Entry DOI: 10.7270/Q22J6BVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50290389 (CHEMBL295462 | Methyl-carbamic acid 1-(3-fluoro-py...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro in rats for the inhibition of brain (striatal) acetylcholinesterase (AChEI) using acetylthiocholine as substrate; 0.0... | Bioorg Med Chem Lett 7: 157-162 (1997) Article DOI: 10.1016/S0960-894X(96)00592-6 BindingDB Entry DOI: 10.7270/Q2CC10P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50288977 (CHEMBL160836 | Methyl-carbamic acid 8-bromo-3-[(Z)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase. | Bioorg Med Chem Lett 6: 625-630 (1996) Article DOI: 10.1016/0960-894X(96)00072-8 BindingDB Entry DOI: 10.7270/Q22J6BVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50288981 (CHEMBL160219 | Methyl-carbamic acid 4-methyl-3-[(Z...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase. | Bioorg Med Chem Lett 6: 625-630 (1996) Article DOI: 10.1016/0960-894X(96)00072-8 BindingDB Entry DOI: 10.7270/Q22J6BVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50288976 (CHEMBL155350 | Methyl-carbamic acid 8-chloro-3-[(Z...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase. | Bioorg Med Chem Lett 6: 625-630 (1996) Article DOI: 10.1016/0960-894X(96)00072-8 BindingDB Entry DOI: 10.7270/Q22J6BVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50288966 (CHEMBL156861 | Methyl-carbamic acid 8-bromo-4-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase. | Bioorg Med Chem Lett 6: 625-630 (1996) Article DOI: 10.1016/0960-894X(96)00072-8 BindingDB Entry DOI: 10.7270/Q22J6BVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50288969 (4-(7-Bromo-5-methoxy-benzofuran-2-yl)-piperidine |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of monoamine oxidase-A(MAO-A). | Bioorg Med Chem Lett 6: 625-630 (1996) Article DOI: 10.1016/0960-894X(96)00072-8 BindingDB Entry DOI: 10.7270/Q22J6BVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50290389 (CHEMBL295462 | Methyl-carbamic acid 1-(3-fluoro-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of human serum Butyrylcholinesterase using butyrylthiocholine as substrate; 0.15-0.22 | Bioorg Med Chem Lett 7: 157-162 (1997) Article DOI: 10.1016/S0960-894X(96)00592-6 BindingDB Entry DOI: 10.7270/Q2CC10P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50288977 (CHEMBL160836 | Methyl-carbamic acid 8-bromo-3-[(Z)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of monoamine oxidase-A(MAO-A). | Bioorg Med Chem Lett 6: 625-630 (1996) Article DOI: 10.1016/0960-894X(96)00072-8 BindingDB Entry DOI: 10.7270/Q22J6BVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50288979 (CHEMBL155754 | Methyl-carbamic acid 8-chloro-4-met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase. | Bioorg Med Chem Lett 6: 625-630 (1996) Article DOI: 10.1016/0960-894X(96)00072-8 BindingDB Entry DOI: 10.7270/Q22J6BVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50288975 (CHEMBL445916 | Methyl-carbamic acid 8-bromo-3-[(Z)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase. | Bioorg Med Chem Lett 6: 625-630 (1996) Article DOI: 10.1016/0960-894X(96)00072-8 BindingDB Entry DOI: 10.7270/Q22J6BVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50033519 (5-[2-(4-Hydroxy-phenyl)-ethylamino]-1-(3-methyl-bu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Acetylcholinesterase inhibitory activity in rat striatal homogenates | J Med Chem 38: 3645-51 (1995) BindingDB Entry DOI: 10.7270/Q2X34Z3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50033527 (5-[2-(3,4-Dichloro-phenyl)-ethylamino]-1-(3-methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Acetylcholinesterase inhibitory activity in rat striatal homogenates | J Med Chem 38: 3645-51 (1995) BindingDB Entry DOI: 10.7270/Q2X34Z3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50033521 (5-[2-(3-Chloro-phenyl)-ethylamino]-1-(3-methyl-but...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Acetylcholinesterase inhibitory activity in rat striatal homogenates | J Med Chem 38: 3645-51 (1995) BindingDB Entry DOI: 10.7270/Q2X34Z3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50033491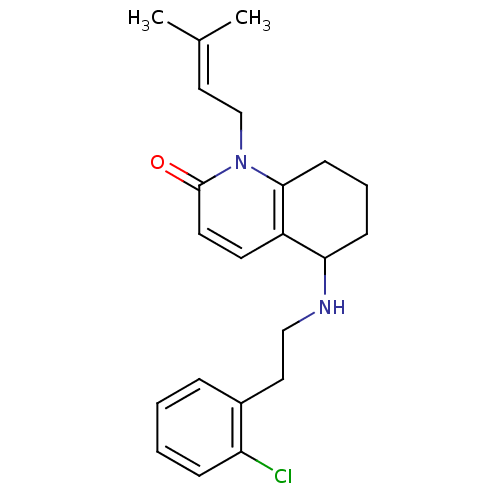 (5-[2-(2-Chloro-phenyl)-ethylamino]-1-(3-methyl-but...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Acetylcholinesterase inhibitory activity in rat striatal homogenates | J Med Chem 38: 3645-51 (1995) BindingDB Entry DOI: 10.7270/Q2X34Z3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50231956 ((Z)-8-bromo-4-methyl-3-(methylimino)-1,2,3,4-tetra...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of monoamine oxidase-A(MAO-A). | Bioorg Med Chem Lett 6: 625-630 (1996) Article DOI: 10.1016/0960-894X(96)00072-8 BindingDB Entry DOI: 10.7270/Q22J6BVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Acetylcholinesterase inhibitory activity in rat striatal homogenates | J Med Chem 38: 3645-51 (1995) BindingDB Entry DOI: 10.7270/Q2X34Z3G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50288966 (CHEMBL156861 | Methyl-carbamic acid 8-bromo-4-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of monoamine oxidase-A(MAO-A). | Bioorg Med Chem Lett 6: 625-630 (1996) Article DOI: 10.1016/0960-894X(96)00072-8 BindingDB Entry DOI: 10.7270/Q22J6BVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50288973 (CHEMBL157182 | Methyl-carbamic acid 6-chloro-4-met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase. | Bioorg Med Chem Lett 6: 625-630 (1996) Article DOI: 10.1016/0960-894X(96)00072-8 BindingDB Entry DOI: 10.7270/Q22J6BVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50288967 (Butyl-carbamic acid 8-bromo-4-methyl-3-[(Z)-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of monoamine oxidase-A(MAO-A). | Bioorg Med Chem Lett 6: 625-630 (1996) Article DOI: 10.1016/0960-894X(96)00072-8 BindingDB Entry DOI: 10.7270/Q22J6BVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50033495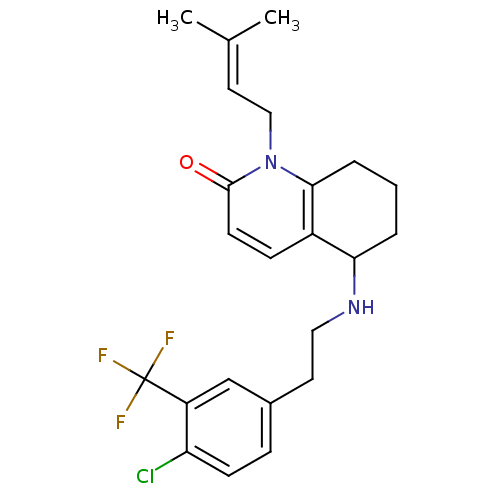 (5-[2-(4-Chloro-3-trifluoromethyl-phenyl)-ethylamin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Acetylcholinesterase inhibitory activity in rat striatal homogenates | J Med Chem 38: 3645-51 (1995) BindingDB Entry DOI: 10.7270/Q2X34Z3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50033496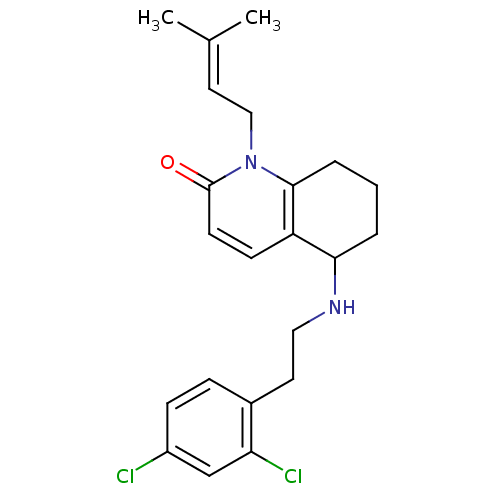 (5-[2-(2,4-Dichloro-phenyl)-ethylamino]-1-(3-methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Acetylcholinesterase inhibitory activity in rat striatal homogenates | J Med Chem 38: 3645-51 (1995) BindingDB Entry DOI: 10.7270/Q2X34Z3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50033512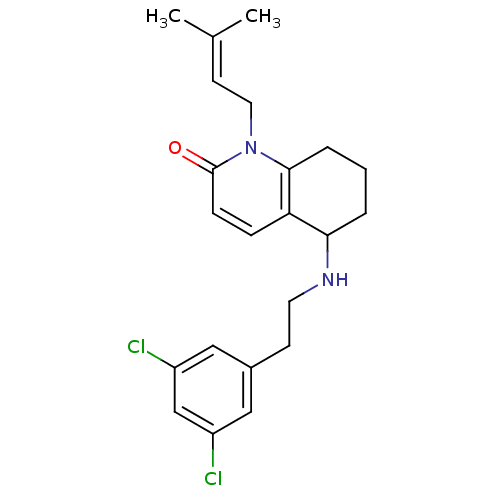 (5-[2-(3,5-Dichloro-phenyl)-ethylamino]-1-(3-methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Acetylcholinesterase inhibitory activity in rat striatal homogenates | J Med Chem 38: 3645-51 (1995) BindingDB Entry DOI: 10.7270/Q2X34Z3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50290390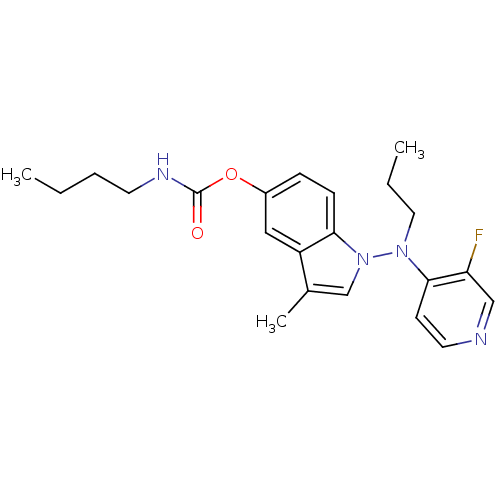 (Butyl-carbamic acid 1-[(3-fluoro-pyridin-4-yl)-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of human serum Butyrylcholinesterase using butyrylthiocholine as substrate; 0.69-1.2 | Bioorg Med Chem Lett 7: 157-162 (1997) Article DOI: 10.1016/S0960-894X(96)00592-6 BindingDB Entry DOI: 10.7270/Q2CC10P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50288970 (CHEMBL346766 | Methyl-[4-methyl-1,4-dihydro-2H-cyc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of monoamine oxidase-A(MAO-A). | Bioorg Med Chem Lett 6: 625-630 (1996) Article DOI: 10.1016/0960-894X(96)00072-8 BindingDB Entry DOI: 10.7270/Q22J6BVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50033510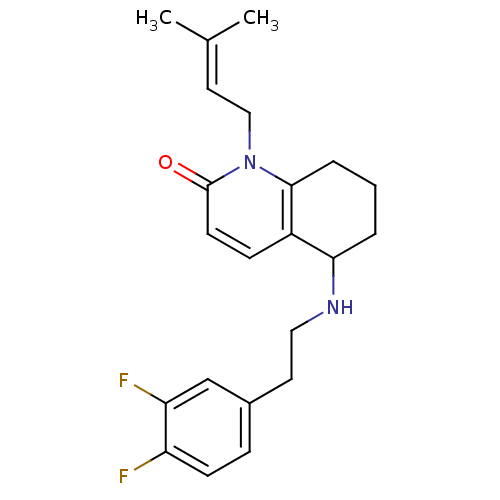 (5-[2-(3,4-Difluoro-phenyl)-ethylamino]-1-(3-methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Acetylcholinesterase inhibitory activity in rat striatal homogenates | J Med Chem 38: 3645-51 (1995) BindingDB Entry DOI: 10.7270/Q2X34Z3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50033492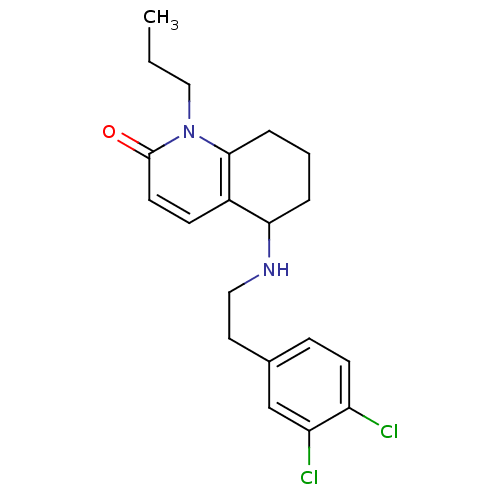 (5-[2-(3,4-Dichloro-phenyl)-ethylamino]-1-propyl-5,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Acetylcholinesterase inhibitory activity in rat striatal homogenates | J Med Chem 38: 3645-51 (1995) BindingDB Entry DOI: 10.7270/Q2X34Z3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50033511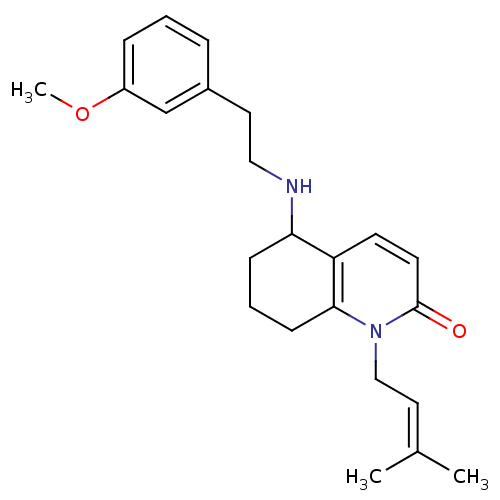 (5-[2-(3-Methoxy-phenyl)-ethylamino]-1-(3-methyl-bu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Acetylcholinesterase inhibitory activity in rat striatal homogenates | J Med Chem 38: 3645-51 (1995) BindingDB Entry DOI: 10.7270/Q2X34Z3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50288979 (CHEMBL155754 | Methyl-carbamic acid 8-chloro-4-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of monoamine oxidase-A(MAO-A). | Bioorg Med Chem Lett 6: 625-630 (1996) Article DOI: 10.1016/0960-894X(96)00072-8 BindingDB Entry DOI: 10.7270/Q22J6BVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50033507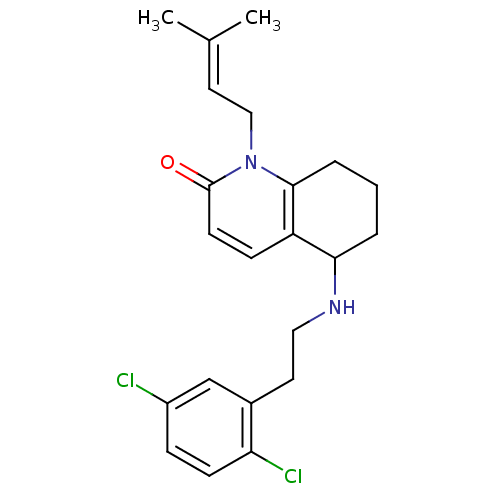 (5-[2-(2,5-Dichloro-phenyl)-ethylamino]-1-(3-methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Acetylcholinesterase inhibitory activity in rat striatal homogenates | J Med Chem 38: 3645-51 (1995) BindingDB Entry DOI: 10.7270/Q2X34Z3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50288971 (CHEMBL156919 | [4-Methyl-1,4-dihydro-2H-cyclopenta...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of monoamine oxidase-A(MAO-A). | Bioorg Med Chem Lett 6: 625-630 (1996) Article DOI: 10.1016/0960-894X(96)00072-8 BindingDB Entry DOI: 10.7270/Q22J6BVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50033523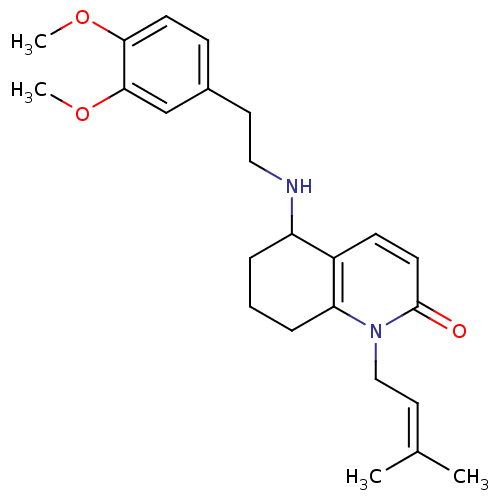 (5-[2-(3,4-Dimethoxy-phenyl)-ethylamino]-1-(3-methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Acetylcholinesterase inhibitory activity in rat striatal homogenates | J Med Chem 38: 3645-51 (1995) BindingDB Entry DOI: 10.7270/Q2X34Z3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50288965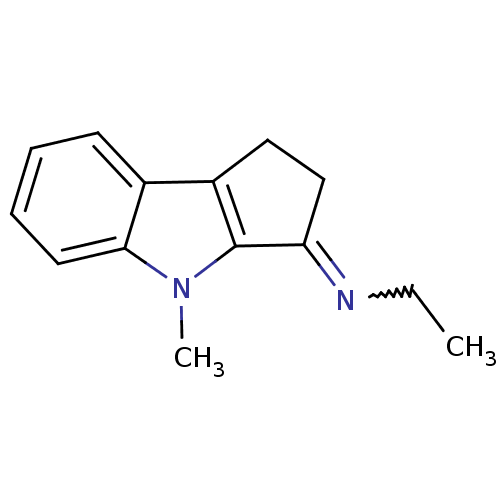 (CHEMBL347197 | Ethyl-[4-methyl-1,4-dihydro-2H-cycl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of monoamine oxidase-A(MAO-A). | Bioorg Med Chem Lett 6: 625-630 (1996) Article DOI: 10.1016/0960-894X(96)00072-8 BindingDB Entry DOI: 10.7270/Q22J6BVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50033494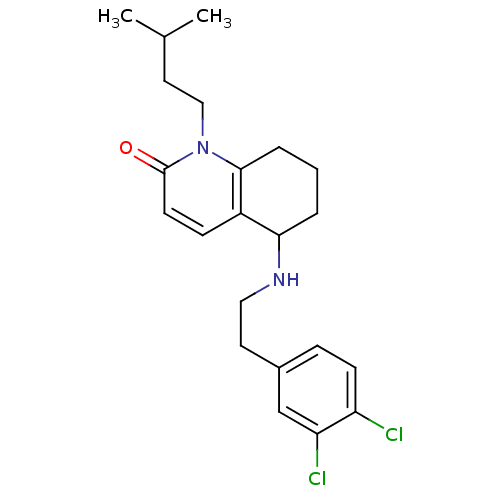 (5-[2-(3,4-Dichloro-phenyl)-ethylamino]-1-(3-methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Acetylcholinesterase inhibitory activity in rat striatal homogenates | J Med Chem 38: 3645-51 (1995) BindingDB Entry DOI: 10.7270/Q2X34Z3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50033527 (5-[2-(3,4-Dichloro-phenyl)-ethylamino]-1-(3-methyl...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc Curated by ChEMBL | Assay Description The compound was tested for Butyrylcholinesterase inhibitory activity in rat striatal homogenates | J Med Chem 38: 3645-51 (1995) BindingDB Entry DOI: 10.7270/Q2X34Z3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50033508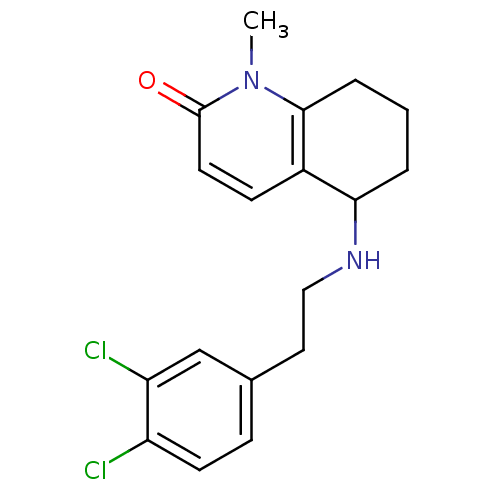 (5-[2-(3,4-Dichloro-phenyl)-ethylamino]-1-methyl-5,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Acetylcholinesterase inhibitory activity in rat striatal homogenates | J Med Chem 38: 3645-51 (1995) BindingDB Entry DOI: 10.7270/Q2X34Z3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50033509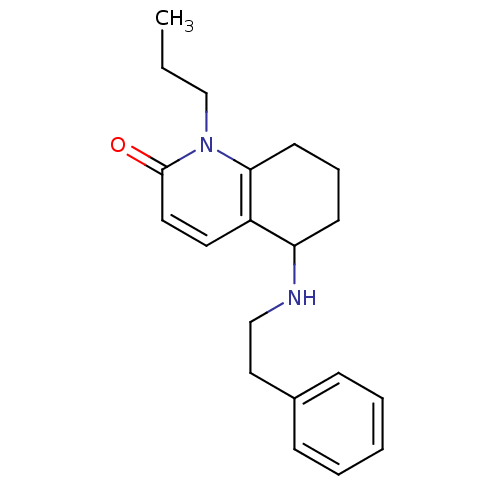 (5-Phenethylamino-1-propyl-5,6,7,8-tetrahydro-1H-qu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Acetylcholinesterase inhibitory activity in rat striatal homogenates | J Med Chem 38: 3645-51 (1995) BindingDB Entry DOI: 10.7270/Q2X34Z3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50033522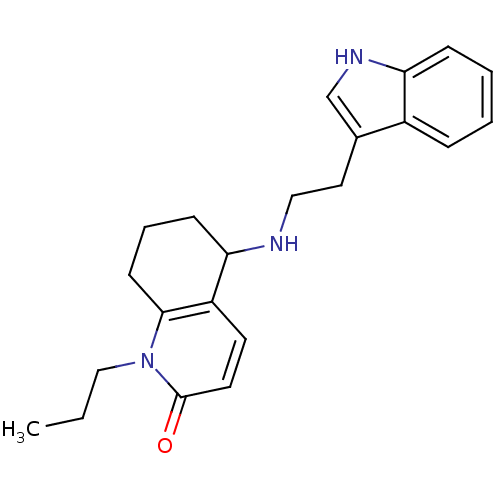 (5-[2-(1H-Indol-3-yl)-ethylamino]-1-propyl-5,6,7,8-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Acetylcholinesterase inhibitory activity in rat striatal homogenates | J Med Chem 38: 3645-51 (1995) BindingDB Entry DOI: 10.7270/Q2X34Z3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50288967 (Butyl-carbamic acid 8-bromo-4-methyl-3-[(Z)-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase. | Bioorg Med Chem Lett 6: 625-630 (1996) Article DOI: 10.1016/0960-894X(96)00072-8 BindingDB Entry DOI: 10.7270/Q22J6BVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50288968 (CHEMBL156931 | Methyl-carbamic acid 4-methyl-3-[(Z...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase. | Bioorg Med Chem Lett 6: 625-630 (1996) Article DOI: 10.1016/0960-894X(96)00072-8 BindingDB Entry DOI: 10.7270/Q22J6BVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50033500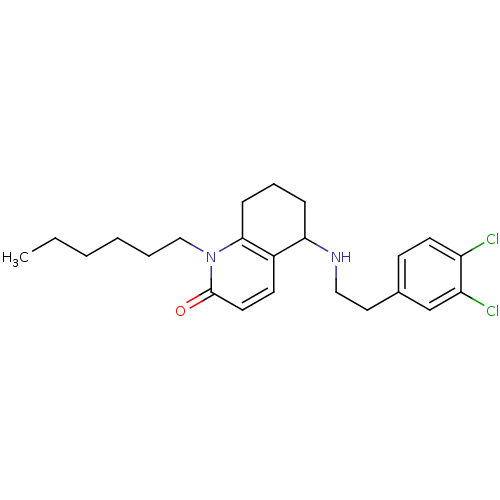 (5-[2-(3,4-Dichloro-phenyl)-ethylamino]-1-hexyl-5,6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Acetylcholinesterase inhibitory activity in rat striatal homogenates | J Med Chem 38: 3645-51 (1995) BindingDB Entry DOI: 10.7270/Q2X34Z3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 115 total ) | Next | Last >> |