

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50352509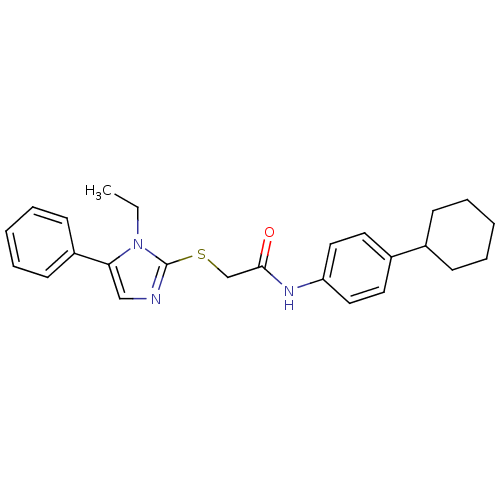 (CHEMBL1824848) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi TryR using trypanothione as substrate preincubated for 10 mins | Bioorg Med Chem Lett 21: 5255-8 (2011) Article DOI: 10.1016/j.bmcl.2011.07.036 BindingDB Entry DOI: 10.7270/Q2ZP46HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50352515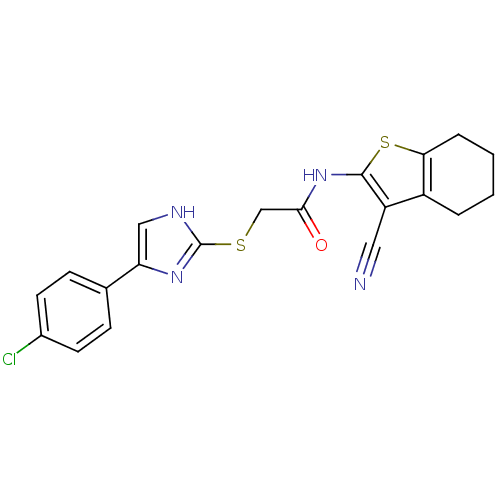 (CHEMBL1824850) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi TryR using trypanothione as substrate preincubated for 10 mins | Bioorg Med Chem Lett 21: 5255-8 (2011) Article DOI: 10.1016/j.bmcl.2011.07.036 BindingDB Entry DOI: 10.7270/Q2ZP46HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM60660 (2-[8-(6-amino-1H-benzimidazol-2-yl)octyl]-3H-benzi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi TryR using trypanothione as substrate preincubated for 10 mins | Bioorg Med Chem Lett 21: 5255-8 (2011) Article DOI: 10.1016/j.bmcl.2011.07.036 BindingDB Entry DOI: 10.7270/Q2ZP46HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50352513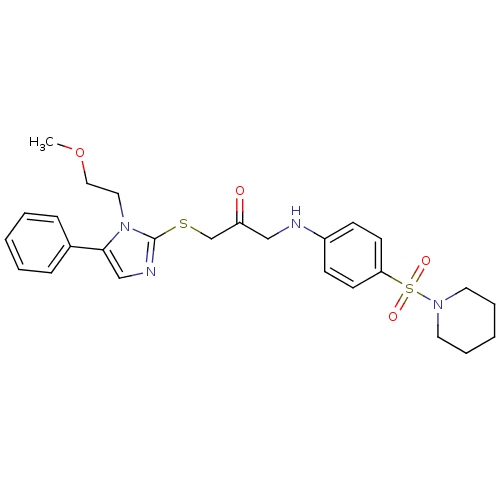 (CHEMBL1824853) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi TryR using trypanothione as substrate preincubated for 10 mins | Bioorg Med Chem Lett 21: 5255-8 (2011) Article DOI: 10.1016/j.bmcl.2011.07.036 BindingDB Entry DOI: 10.7270/Q2ZP46HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50352512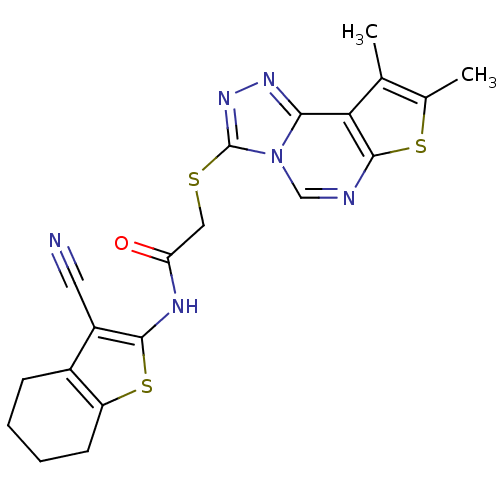 (CHEMBL1824852) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi TryR using trypanothione as substrate preincubated for 10 mins | Bioorg Med Chem Lett 21: 5255-8 (2011) Article DOI: 10.1016/j.bmcl.2011.07.036 BindingDB Entry DOI: 10.7270/Q2ZP46HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50352510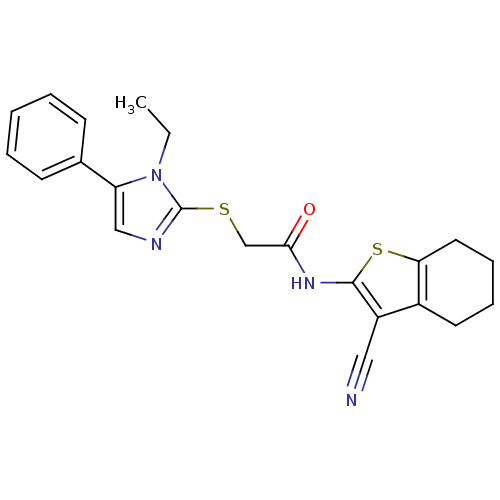 (CHEMBL1824849) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi TryR using trypanothione as substrate preincubated for 10 mins | Bioorg Med Chem Lett 21: 5255-8 (2011) Article DOI: 10.1016/j.bmcl.2011.07.036 BindingDB Entry DOI: 10.7270/Q2ZP46HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50352508 (CHEMBL1824847) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi TryR using trypanothione as substrate preincubated for 10 mins | Bioorg Med Chem Lett 21: 5255-8 (2011) Article DOI: 10.1016/j.bmcl.2011.07.036 BindingDB Entry DOI: 10.7270/Q2ZP46HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50352514 (CHEMBL1824854) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi TryR using trypanothione as substrate preincubated for 10 mins | Bioorg Med Chem Lett 21: 5255-8 (2011) Article DOI: 10.1016/j.bmcl.2011.07.036 BindingDB Entry DOI: 10.7270/Q2ZP46HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50352511 (CHEMBL1824851) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi TryR using trypanothione as substrate preincubated for 10 mins | Bioorg Med Chem Lett 21: 5255-8 (2011) Article DOI: 10.1016/j.bmcl.2011.07.036 BindingDB Entry DOI: 10.7270/Q2ZP46HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Mycobacterium tuberculosis) | BDBM50435857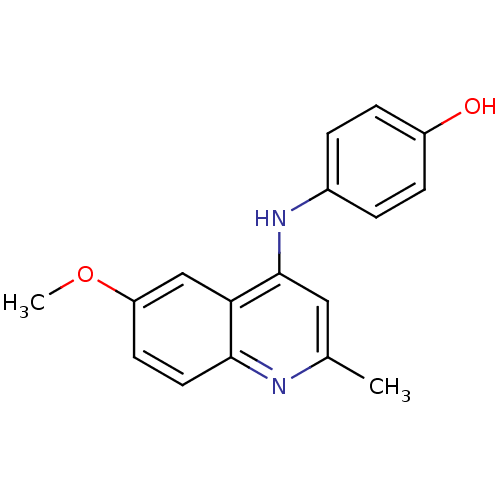 (CHEMBL2393579) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Animal Health Innovation GmbH Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis TrxR after 10 mins by microplate reader analysis | J Med Chem 56: 4849-59 (2013) Article DOI: 10.1021/jm3015734 BindingDB Entry DOI: 10.7270/Q21C1Z88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Mycobacterium tuberculosis) | BDBM50435854 (CHEMBL2393583) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Animal Health Innovation GmbH Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis TrxR after 10 mins by microplate reader analysis | J Med Chem 56: 4849-59 (2013) Article DOI: 10.1021/jm3015734 BindingDB Entry DOI: 10.7270/Q21C1Z88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Mycobacterium tuberculosis) | BDBM50435849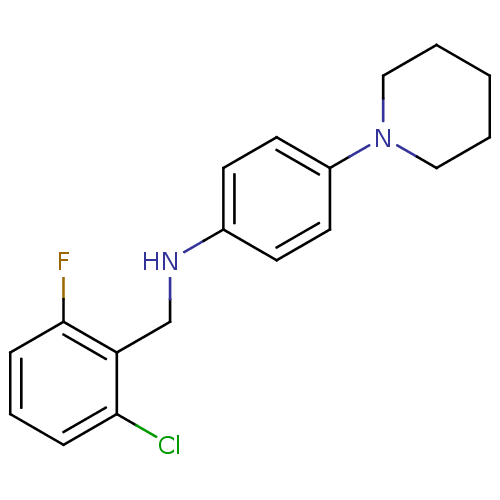 (CHEMBL2391046) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Animal Health Innovation GmbH Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis TrxR after 10 mins by microplate reader analysis | J Med Chem 56: 4849-59 (2013) Article DOI: 10.1021/jm3015734 BindingDB Entry DOI: 10.7270/Q21C1Z88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Mycobacterium tuberculosis) | BDBM50435850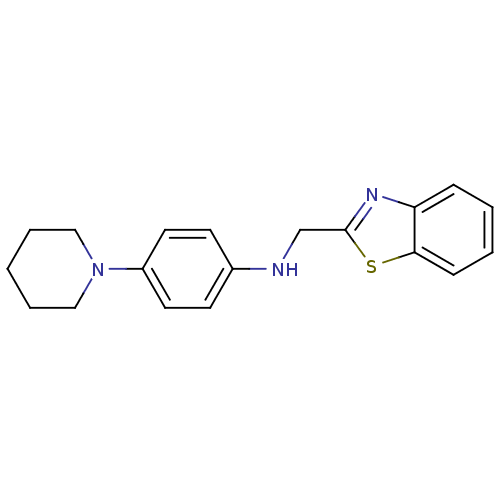 (CHEMBL2391045) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Animal Health Innovation GmbH Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis TrxR after 10 mins by microplate reader analysis | J Med Chem 56: 4849-59 (2013) Article DOI: 10.1021/jm3015734 BindingDB Entry DOI: 10.7270/Q21C1Z88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Mycobacterium tuberculosis) | BDBM50435855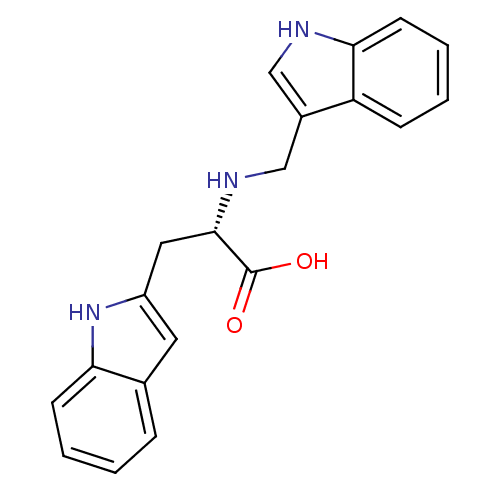 (CHEMBL2393581) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Animal Health Innovation GmbH Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis TrxR after 10 mins by microplate reader analysis | J Med Chem 56: 4849-59 (2013) Article DOI: 10.1021/jm3015734 BindingDB Entry DOI: 10.7270/Q21C1Z88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Mycobacterium tuberculosis) | BDBM50435851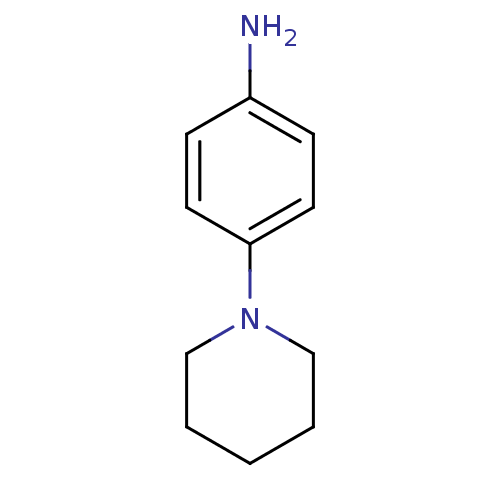 (CHEMBL2391044) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Animal Health Innovation GmbH Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis TrxR after 10 mins by microplate reader analysis | J Med Chem 56: 4849-59 (2013) Article DOI: 10.1021/jm3015734 BindingDB Entry DOI: 10.7270/Q21C1Z88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Mycobacterium tuberculosis) | BDBM50435853 (CHEMBL2393584) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Animal Health Innovation GmbH Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis TrxR after 10 mins by microplate reader analysis | J Med Chem 56: 4849-59 (2013) Article DOI: 10.1021/jm3015734 BindingDB Entry DOI: 10.7270/Q21C1Z88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Mycobacterium tuberculosis) | BDBM50435856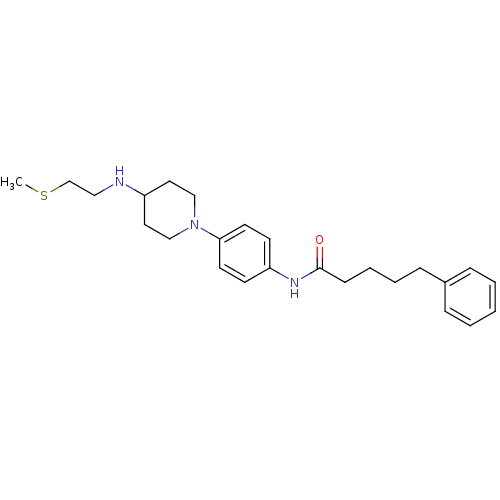 (CHEMBL2393580) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Animal Health Innovation GmbH Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis TrxR after 10 mins by microplate reader analysis | J Med Chem 56: 4849-59 (2013) Article DOI: 10.1021/jm3015734 BindingDB Entry DOI: 10.7270/Q21C1Z88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Mycobacterium tuberculosis) | BDBM50435852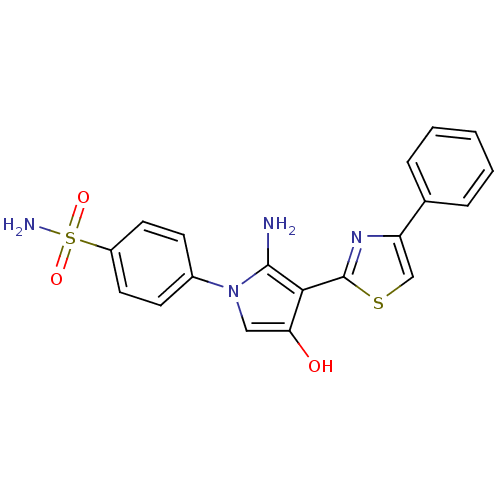 (CHEMBL2393585) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Animal Health Innovation GmbH Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis TrxR after 10 mins by microplate reader analysis | J Med Chem 56: 4849-59 (2013) Article DOI: 10.1021/jm3015734 BindingDB Entry DOI: 10.7270/Q21C1Z88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||