

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50150572 (1-(2-Bromo-phenyl)-3-(2,4-dihydroxy-phenyl)-urea |...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against interleukin-8 receptor of human neutrophils by using [125I]-IL-8 (0.125 nM) as radioligand | Bioorg Med Chem Lett 14: 4307-11 (2004) Article DOI: 10.1016/j.bmcl.2004.05.080 BindingDB Entry DOI: 10.7270/Q23F4P4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50150573 (1-{4-[5-(4-Chloro-phenyl)-isoxazol-3-yl]-phenoxyme...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against interleukin-8 receptor of human neutrophils by using [125I]-IL-8 (0.125 nM) as radioligand | Bioorg Med Chem Lett 14: 4307-11 (2004) Article DOI: 10.1016/j.bmcl.2004.05.080 BindingDB Entry DOI: 10.7270/Q23F4P4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50150561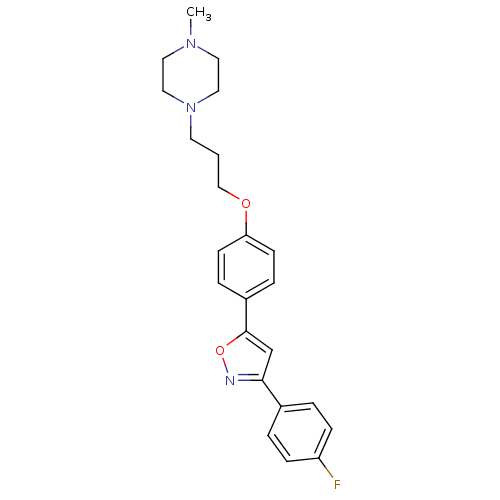 (1-(3-{4-[3-(4-Fluoro-phenyl)-isoxazol-5-yl]-phenox...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against interleukin-8 receptor of human neutrophils by using [125I]-IL-8 (0.125 nM) as radioligand | Bioorg Med Chem Lett 14: 4307-11 (2004) Article DOI: 10.1016/j.bmcl.2004.05.080 BindingDB Entry DOI: 10.7270/Q23F4P4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50150558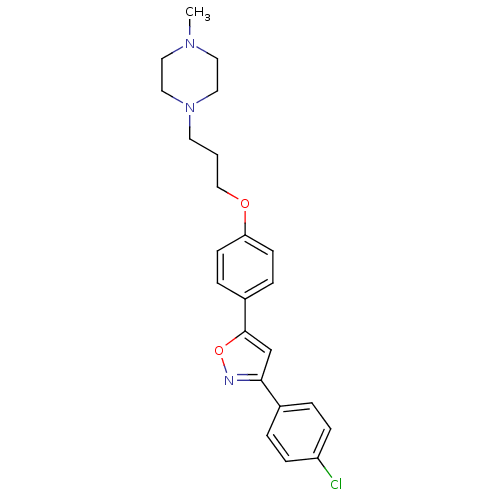 (1-(3-{4-[3-(4-Chloro-phenyl)-isoxazol-5-yl]-phenox...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against interleukin-8 receptor of human neutrophils by using [125I]-IL-8 (0.125 nM) as radioligand | Bioorg Med Chem Lett 14: 4307-11 (2004) Article DOI: 10.1016/j.bmcl.2004.05.080 BindingDB Entry DOI: 10.7270/Q23F4P4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50150555 (1-(3-{4-[3-(4-Chloro-phenyl)-[1,2,4]oxadiazol-5-yl...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against interleukin-8 receptor of human neutrophils by using [125I]-IL-8 (0.125 nM) as radioligand | Bioorg Med Chem Lett 14: 4307-11 (2004) Article DOI: 10.1016/j.bmcl.2004.05.080 BindingDB Entry DOI: 10.7270/Q23F4P4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50150563 (CHEMBL183061 | {4-[5-(4-Chloro-phenyl)-isoxazol-3-...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against interleukin-8 receptor of human neutrophils by using [125I]-IL-8 (0.125 nM) as radioligand | Bioorg Med Chem Lett 14: 4307-11 (2004) Article DOI: 10.1016/j.bmcl.2004.05.080 BindingDB Entry DOI: 10.7270/Q23F4P4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50471725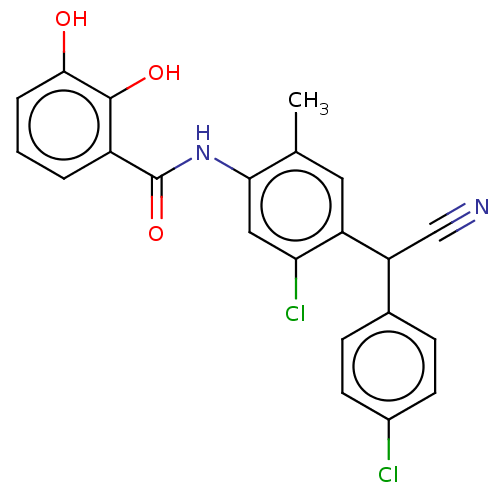 (CHEMBL327734) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of autophosphorylation of the two-component signal transduction system kinase was measured using the KinA/Spo0F regulatory system of Bacil... | J Med Chem 41: 2939-45 (1998) Article DOI: 10.1021/jm9803572 BindingDB Entry DOI: 10.7270/Q2VM4G0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50150553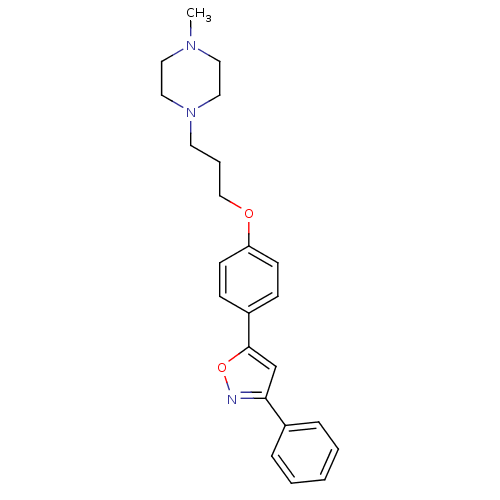 (1-Methyl-4-{3-[4-(3-phenyl-isoxazol-5-yl)-phenoxy]...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against interleukin-8 receptor of human neutrophils by using [125I]-IL-8 (0.125 nM) as radioligand | Bioorg Med Chem Lett 14: 4307-11 (2004) Article DOI: 10.1016/j.bmcl.2004.05.080 BindingDB Entry DOI: 10.7270/Q23F4P4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50150559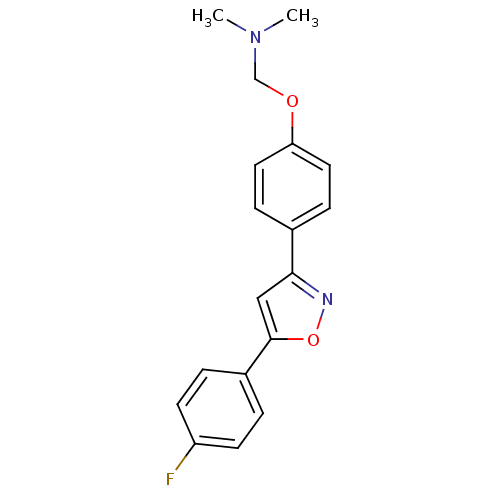 (CHEMBL182361 | {4-[5-(4-Fluoro-phenyl)-isoxazol-3-...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against interleukin-8 receptor of human neutrophils by using [125I]-IL-8 (0.125 nM) as radioligand | Bioorg Med Chem Lett 14: 4307-11 (2004) Article DOI: 10.1016/j.bmcl.2004.05.080 BindingDB Entry DOI: 10.7270/Q23F4P4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50150558 (1-(3-{4-[3-(4-Chloro-phenyl)-isoxazol-5-yl]-phenox...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of interleukin-8 induced elastase release from human neutrophils | Bioorg Med Chem Lett 14: 4307-11 (2004) Article DOI: 10.1016/j.bmcl.2004.05.080 BindingDB Entry DOI: 10.7270/Q23F4P4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50150561 (1-(3-{4-[3-(4-Fluoro-phenyl)-isoxazol-5-yl]-phenox...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of interleukin-8 induced elastase release from human neutrophils | Bioorg Med Chem Lett 14: 4307-11 (2004) Article DOI: 10.1016/j.bmcl.2004.05.080 BindingDB Entry DOI: 10.7270/Q23F4P4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50063753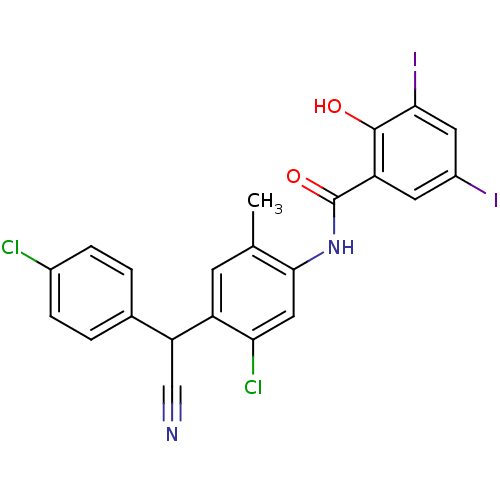 (CHEMBL12131 | Closantel | N-(5-chloro-4-((4-chloro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of autophosphorylation of the two-component signal transduction system kinase was measured using the KinA/Spo0F regulatory system of Bacil... | J Med Chem 41: 2939-45 (1998) Article DOI: 10.1021/jm9803572 BindingDB Entry DOI: 10.7270/Q2VM4G0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50215968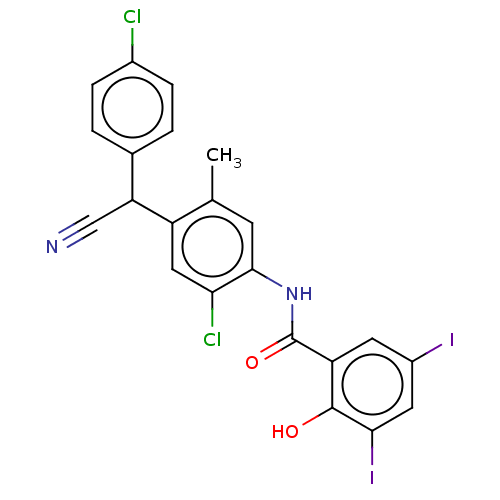 (CHEMBL57324) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of histidine protein kinase (KinA) phosphorylation in the presence of response regulator (Spo0F) | Bioorg Med Chem Lett 8: 1923-8 (1998) BindingDB Entry DOI: 10.7270/Q27S7QZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50150569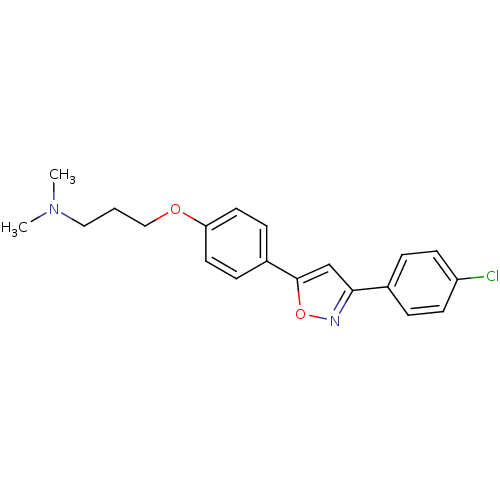 ((3-{4-[3-(4-Chloro-phenyl)-isoxazol-5-yl]-phenoxy}...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against interleukin-8 receptor of human neutrophils by using [125I]-IL-8 (0.125 nM) as radioligand | Bioorg Med Chem Lett 14: 4307-11 (2004) Article DOI: 10.1016/j.bmcl.2004.05.080 BindingDB Entry DOI: 10.7270/Q23F4P4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50150559 (CHEMBL182361 | {4-[5-(4-Fluoro-phenyl)-isoxazol-3-...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of interleukin-8 induced elastase release from human neutrophils | Bioorg Med Chem Lett 14: 4307-11 (2004) Article DOI: 10.1016/j.bmcl.2004.05.080 BindingDB Entry DOI: 10.7270/Q23F4P4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50150574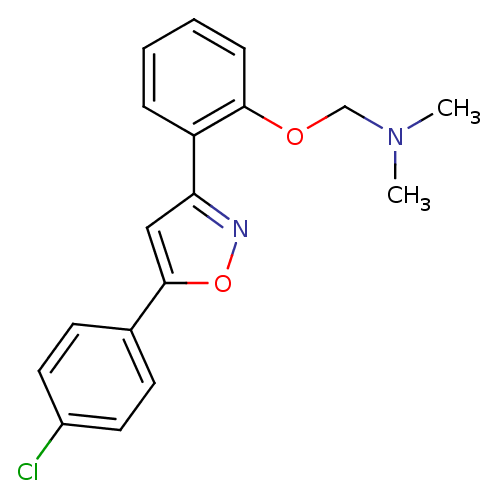 (CHEMBL413959 | {2-[5-(4-Chloro-phenyl)-isoxazol-3-...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against interleukin-8 receptor of human neutrophils by using [125I]-IL-8 (0.125 nM) as radioligand | Bioorg Med Chem Lett 14: 4307-11 (2004) Article DOI: 10.1016/j.bmcl.2004.05.080 BindingDB Entry DOI: 10.7270/Q23F4P4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50150555 (1-(3-{4-[3-(4-Chloro-phenyl)-[1,2,4]oxadiazol-5-yl...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of interleukin-8 induced elastase release from human neutrophils | Bioorg Med Chem Lett 14: 4307-11 (2004) Article DOI: 10.1016/j.bmcl.2004.05.080 BindingDB Entry DOI: 10.7270/Q23F4P4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50471718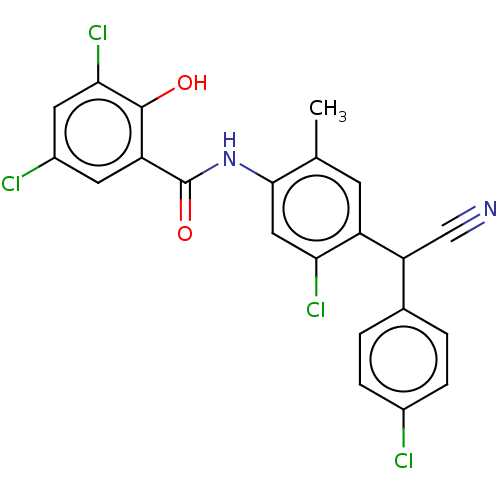 (CHEMBL98026) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of autophosphorylation of the two-component signal transduction system kinase was measured using the KinA/Spo0F regulatory system of Bacil... | J Med Chem 41: 2939-45 (1998) Article DOI: 10.1021/jm9803572 BindingDB Entry DOI: 10.7270/Q2VM4G0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50215967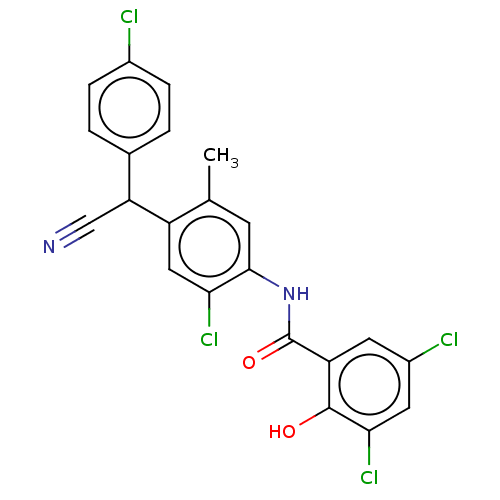 (CHEMBL56579) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of histidine protein kinase (KinA) phosphorylation in the presence of response regulator (Spo0F) | Bioorg Med Chem Lett 8: 1923-8 (1998) BindingDB Entry DOI: 10.7270/Q27S7QZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50045004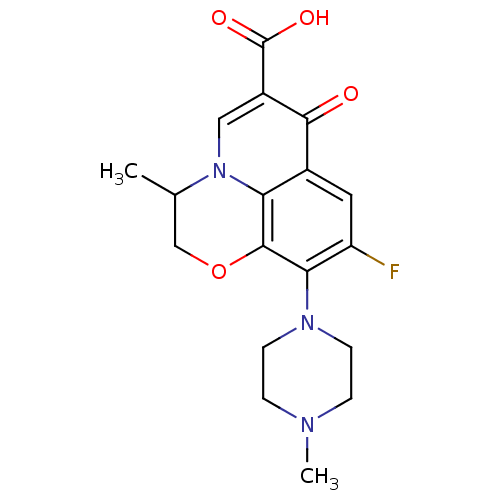 (9-fluoro-3-methyl-10-(4-methylpiperazin-1-yl)-7-ox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 4.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of DNA gyrase supercoiling in Escherichia coli. | Bioorg Med Chem Lett 8: 97-100 (1998) BindingDB Entry DOI: 10.7270/Q25141DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50150567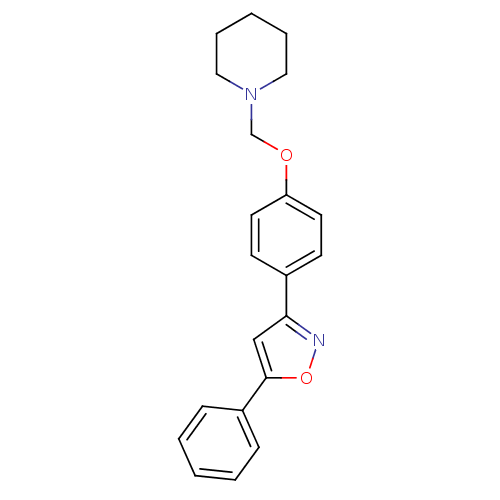 (1-[4-(5-Phenyl-isoxazol-3-yl)-phenoxymethyl]-piper...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against interleukin-8 receptor of human neutrophils by using [125I]-IL-8 (0.125 nM) as radioligand | Bioorg Med Chem Lett 14: 4307-11 (2004) Article DOI: 10.1016/j.bmcl.2004.05.080 BindingDB Entry DOI: 10.7270/Q23F4P4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50150556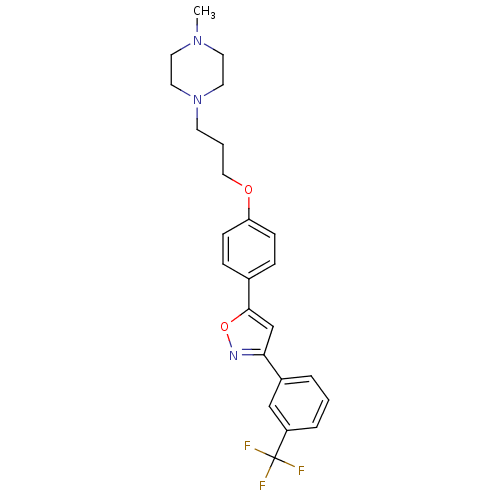 (1-Methyl-4-(3-{4-[3-(3-trifluoromethyl-phenyl)-iso...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against interleukin-8 receptor of human neutrophils by using [125I]-IL-8 (0.125 nM) as radioligand | Bioorg Med Chem Lett 14: 4307-11 (2004) Article DOI: 10.1016/j.bmcl.2004.05.080 BindingDB Entry DOI: 10.7270/Q23F4P4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50218645 (CHEMBL288462) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of KinA/Sp0F system two component (TCS) from Bacillus subtilis. | Bioorg Med Chem Lett 11: 1545-8 (2001) BindingDB Entry DOI: 10.7270/Q2V69MSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50150566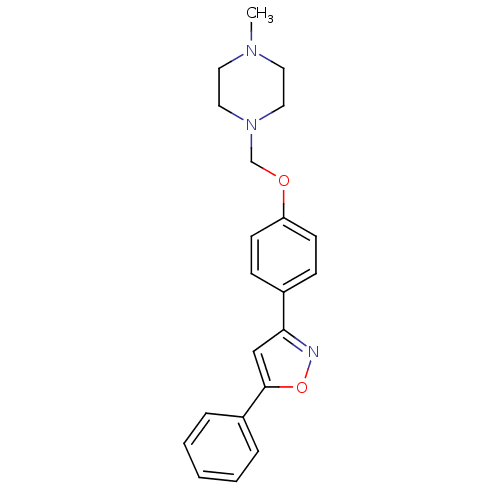 (1-Methyl-4-[4-(5-phenyl-isoxazol-3-yl)-phenoxymeth...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against interleukin-8 receptor of human neutrophils by using [125I]-IL-8 (0.125 nM) as radioligand | Bioorg Med Chem Lett 14: 4307-11 (2004) Article DOI: 10.1016/j.bmcl.2004.05.080 BindingDB Entry DOI: 10.7270/Q23F4P4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50150568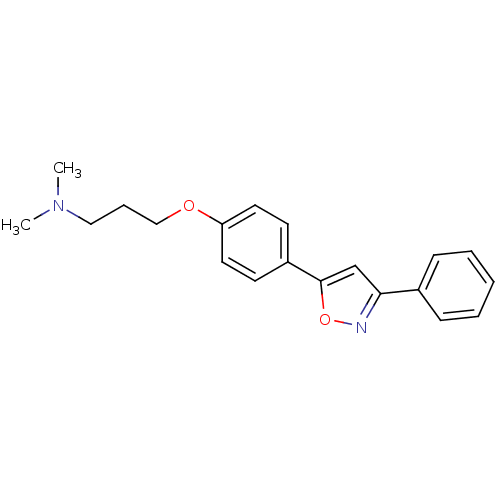 (CHEMBL183819 | Dimethyl-{3-[4-(3-phenyl-isoxazol-5...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against interleukin-8 receptor of human neutrophils by using [125I]-IL-8 (0.125 nM) as radioligand | Bioorg Med Chem Lett 14: 4307-11 (2004) Article DOI: 10.1016/j.bmcl.2004.05.080 BindingDB Entry DOI: 10.7270/Q23F4P4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50150570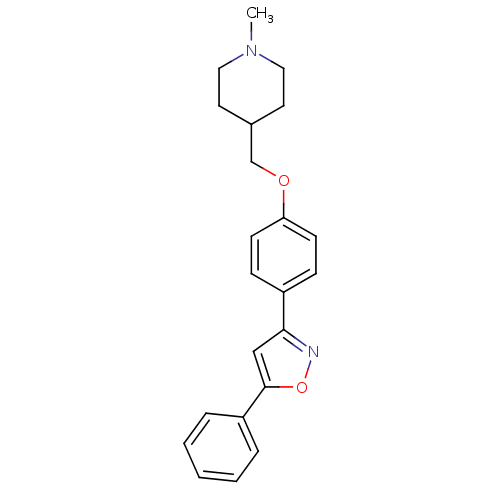 (1-Methyl-4-[4-(5-phenyl-isoxazol-3-yl)-phenoxymeth...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against interleukin-8 receptor of human neutrophils by using [125I]-IL-8 (0.125 nM) as radioligand | Bioorg Med Chem Lett 14: 4307-11 (2004) Article DOI: 10.1016/j.bmcl.2004.05.080 BindingDB Entry DOI: 10.7270/Q23F4P4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50215357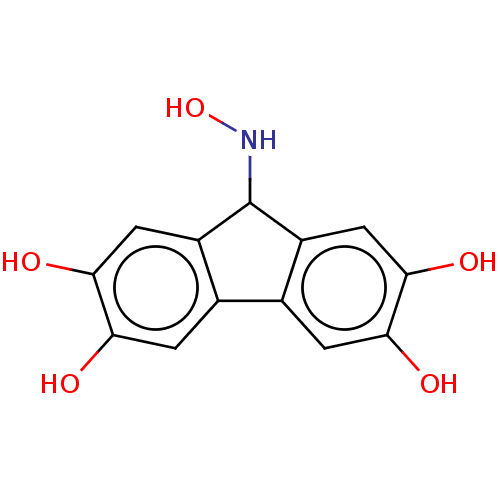 (CHEMBL6353) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of DNA gyrase supercoiling in Escherichia coli. | Bioorg Med Chem Lett 8: 97-100 (1998) BindingDB Entry DOI: 10.7270/Q25141DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50218644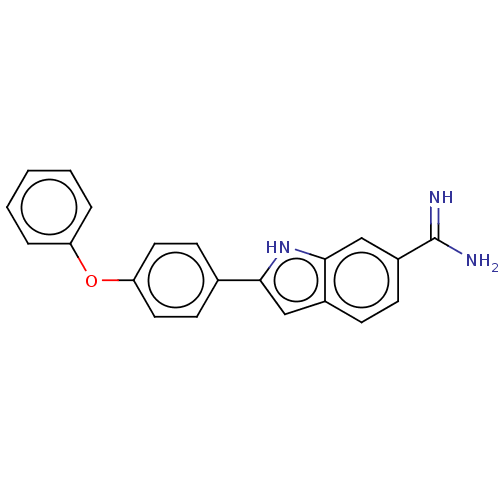 (CHEMBL416972) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of KinA/Sp0F system two component (TCS) from Bacillus subtilis. | Bioorg Med Chem Lett 11: 1545-8 (2001) BindingDB Entry DOI: 10.7270/Q2V69MSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50150560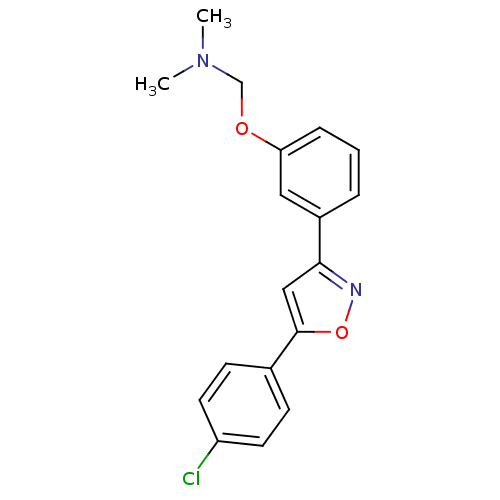 (CHEMBL184583 | {3-[5-(4-Chloro-phenyl)-isoxazol-3-...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against interleukin-8 receptor of human neutrophils by using [125I]-IL-8 (0.125 nM) as radioligand | Bioorg Med Chem Lett 14: 4307-11 (2004) Article DOI: 10.1016/j.bmcl.2004.05.080 BindingDB Entry DOI: 10.7270/Q23F4P4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50150565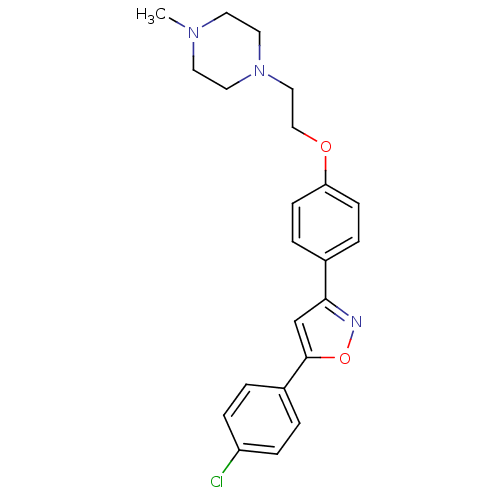 (1-(2-{4-[5-(4-Chloro-phenyl)-isoxazol-3-yl]-phenox...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against interleukin-8 receptor of human neutrophils by using [125I]-IL-8 (0.125 nM) as radioligand | Bioorg Med Chem Lett 14: 4307-11 (2004) Article DOI: 10.1016/j.bmcl.2004.05.080 BindingDB Entry DOI: 10.7270/Q23F4P4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50471730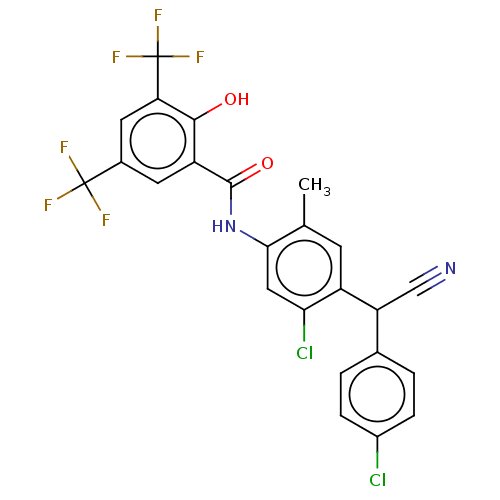 (CHEMBL101786) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of autophosphorylation of the two-component signal transduction system kinase was measured using the KinA/Spo0F regulatory system of Bacil... | J Med Chem 41: 2939-45 (1998) Article DOI: 10.1021/jm9803572 BindingDB Entry DOI: 10.7270/Q2VM4G0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation initiation phosphotransferase F/kinase A (Bacillus subtilis (strain 168)) | BDBM50215963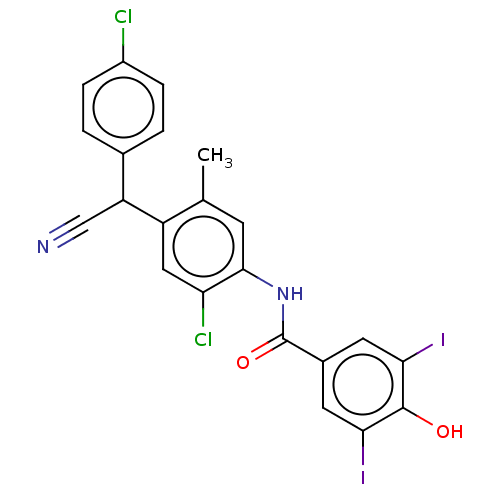 (CHEMBL57687) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of histidine protein kinase (KinA) phosphorylation in the presence of response regulator (Spo0F) | Bioorg Med Chem Lett 8: 1923-8 (1998) BindingDB Entry DOI: 10.7270/Q27S7QZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50471728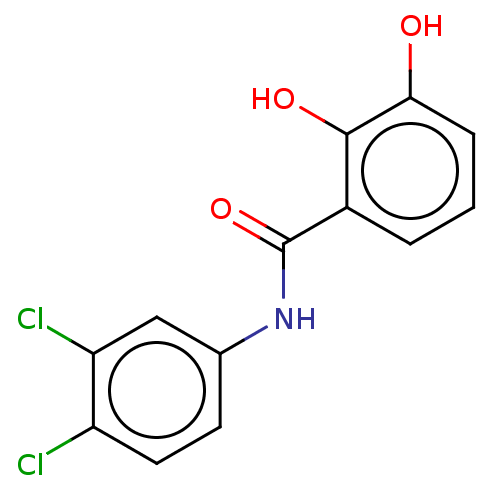 (CHEMBL100719) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of autophosphorylation of the two-component signal transduction system kinase was measured using the KinA/Spo0F regulatory system of Bacil... | J Med Chem 41: 2939-45 (1998) Article DOI: 10.1021/jm9803572 BindingDB Entry DOI: 10.7270/Q2VM4G0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50150571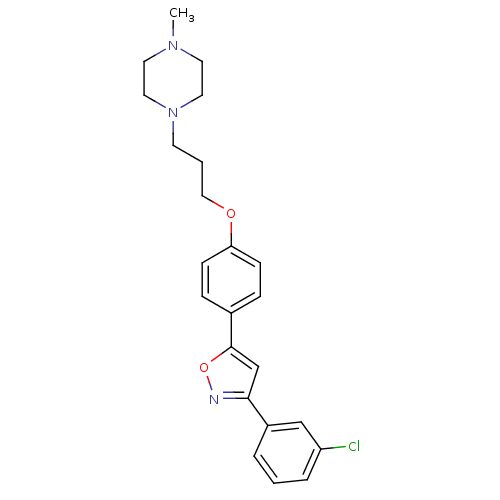 (1-(3-{4-[3-(3-Chloro-phenyl)-isoxazol-5-yl]-phenox...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against interleukin-8 receptor of human neutrophils by using [125I]-IL-8 (0.125 nM) as radioligand | Bioorg Med Chem Lett 14: 4307-11 (2004) Article DOI: 10.1016/j.bmcl.2004.05.080 BindingDB Entry DOI: 10.7270/Q23F4P4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50218640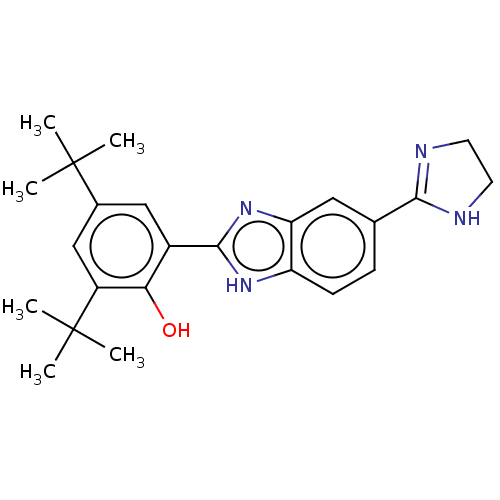 (CHEMBL38683) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of KinA/Sp0F system two component (TCS) from Bacillus subtilis. | Bioorg Med Chem Lett 11: 1545-8 (2001) BindingDB Entry DOI: 10.7270/Q2V69MSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50218648 (CHEMBL290098) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of KinA/Sp0F system two component (TCS) from Bacillus subtilis. | Bioorg Med Chem Lett 11: 1545-8 (2001) BindingDB Entry DOI: 10.7270/Q2V69MSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50150557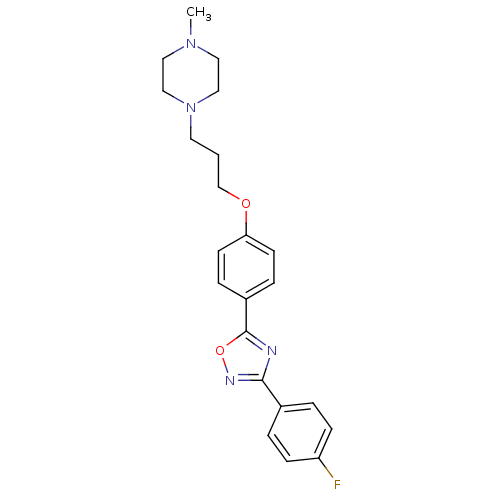 (1-(3-{4-[3-(4-Fluoro-phenyl)-[1,2,4]oxadiazol-5-yl...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against interleukin-8 receptor of human neutrophils by using [125I]-IL-8 (0.125 nM) as radioligand | Bioorg Med Chem Lett 14: 4307-11 (2004) Article DOI: 10.1016/j.bmcl.2004.05.080 BindingDB Entry DOI: 10.7270/Q23F4P4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50150564 (CHEMBL425882 | Dimethyl-[4-(5-phenyl-isoxazol-3-yl...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against interleukin-8 receptor of human neutrophils by using [125I]-IL-8 (0.125 nM) as radioligand | Bioorg Med Chem Lett 14: 4307-11 (2004) Article DOI: 10.1016/j.bmcl.2004.05.080 BindingDB Entry DOI: 10.7270/Q23F4P4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50150575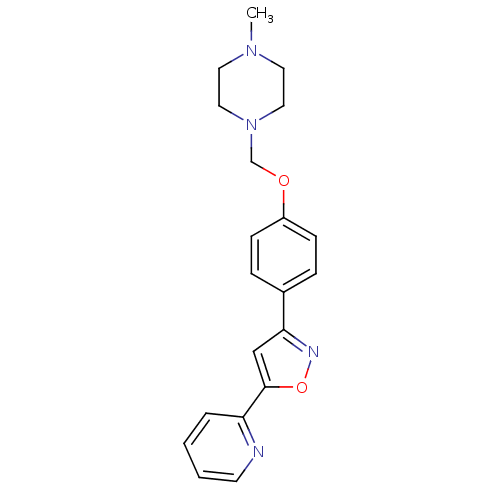 (1-Methyl-4-[4-(5-pyridin-2-yl-isoxazol-3-yl)-pheno...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against interleukin-8 receptor of human neutrophils by using [125I]-IL-8 (0.125 nM) as radioligand | Bioorg Med Chem Lett 14: 4307-11 (2004) Article DOI: 10.1016/j.bmcl.2004.05.080 BindingDB Entry DOI: 10.7270/Q23F4P4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50218643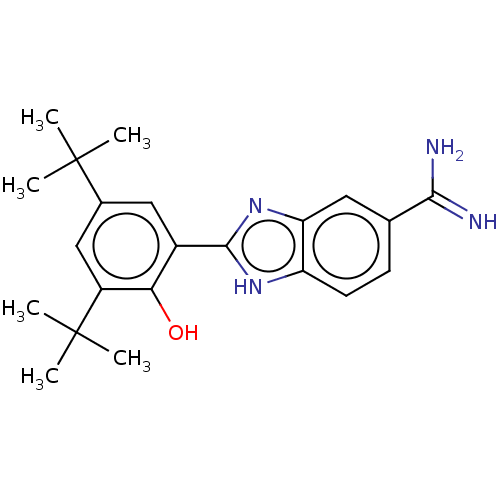 (CHEMBL39634) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of KinA/Sp0F system two component (TCS) from Bacillus subtilis. | Bioorg Med Chem Lett 11: 1545-8 (2001) BindingDB Entry DOI: 10.7270/Q2V69MSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50150562 (CHEMBL184401 | Dimethyl-{4-[5-(4-nitro-phenyl)-iso...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against interleukin-8 receptor of human neutrophils by using [125I]-IL-8 (0.125 nM) as radioligand | Bioorg Med Chem Lett 14: 4307-11 (2004) Article DOI: 10.1016/j.bmcl.2004.05.080 BindingDB Entry DOI: 10.7270/Q23F4P4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50218655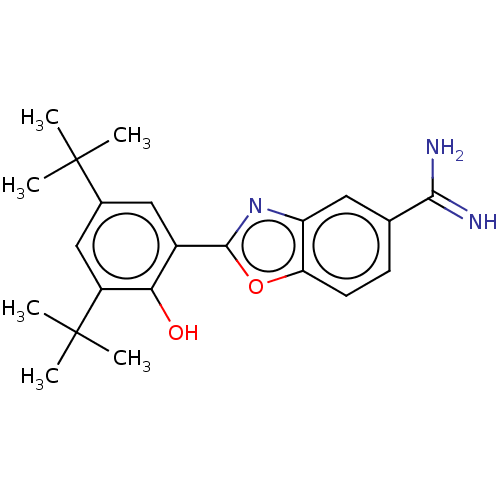 (CHEMBL38914) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of KinA/Sp0F system two component (TCS) from Bacillus subtilis. | Bioorg Med Chem Lett 11: 1545-8 (2001) BindingDB Entry DOI: 10.7270/Q2V69MSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50218647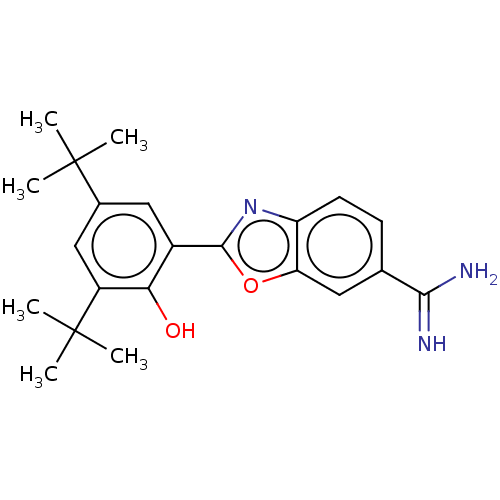 (CHEMBL38867) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of KinA/Sp0F system two component (TCS) from Bacillus subtilis. | Bioorg Med Chem Lett 11: 1545-8 (2001) BindingDB Entry DOI: 10.7270/Q2V69MSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50218659 (CHEMBL43691) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of KinA/Sp0F system two component (TCS) from Bacillus subtilis. | Bioorg Med Chem Lett 11: 1545-8 (2001) BindingDB Entry DOI: 10.7270/Q2V69MSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50471723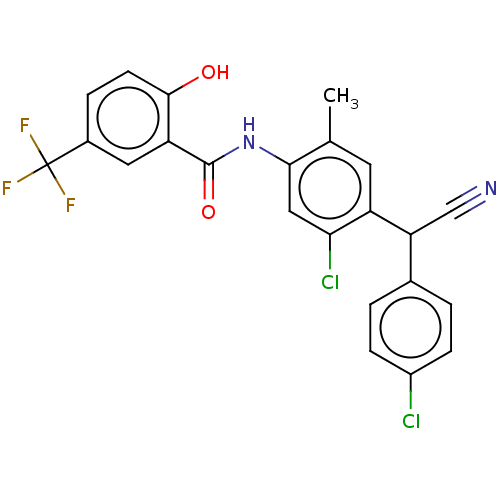 (CHEMBL98273) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of autophosphorylation of the two-component signal transduction system kinase was measured using the KinA/Spo0F regulatory system of Bacil... | J Med Chem 41: 2939-45 (1998) Article DOI: 10.1021/jm9803572 BindingDB Entry DOI: 10.7270/Q2VM4G0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50065970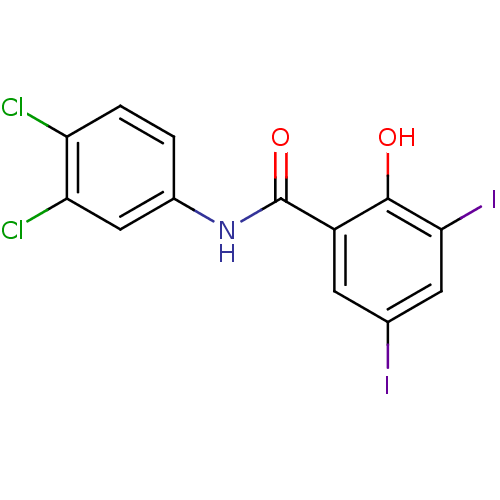 (CHEMBL57656 | N-(3,4-Dichloro-phenyl)-2-hydroxy-3,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of autophosphorylation of the two-component signal transduction system kinase was measured using the KinA/Spo0F regulatory system of Bacil... | J Med Chem 41: 2939-45 (1998) Article DOI: 10.1021/jm9803572 BindingDB Entry DOI: 10.7270/Q2VM4G0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50065970 (CHEMBL57656 | N-(3,4-Dichloro-phenyl)-2-hydroxy-3,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of histidine protein kinase (KinA) phosphorylation in the presence of response regulator (Spo0F) | Bioorg Med Chem Lett 8: 1923-8 (1998) BindingDB Entry DOI: 10.7270/Q27S7QZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50150576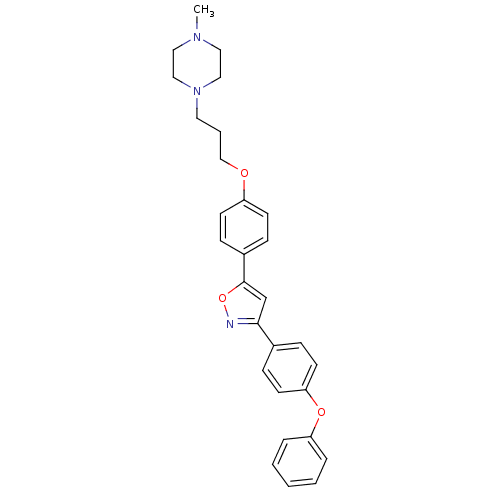 (1-Methyl-4-(3-{4-[3-(4-phenoxy-phenyl)-isoxazol-5-...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against interleukin-8 receptor of human neutrophils by using [125I]-IL-8 (0.125 nM) as radioligand | Bioorg Med Chem Lett 14: 4307-11 (2004) Article DOI: 10.1016/j.bmcl.2004.05.080 BindingDB Entry DOI: 10.7270/Q23F4P4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50150554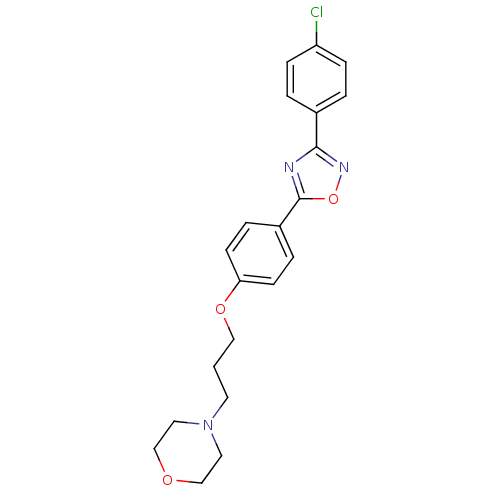 (4-(3-{4-[3-(4-Chloro-phenyl)-[1,2,4]oxadiazol-5-yl...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against interleukin-8 receptor of human neutrophils by using [125I]-IL-8 (0.125 nM) as radioligand | Bioorg Med Chem Lett 14: 4307-11 (2004) Article DOI: 10.1016/j.bmcl.2004.05.080 BindingDB Entry DOI: 10.7270/Q23F4P4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sporulation kinase A (Bacillus subtilis (strain 168)) | BDBM50218653 (CHEMBL39178) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of KinA/Sp0F system two component (TCS) from Bacillus subtilis. | Bioorg Med Chem Lett 11: 1545-8 (2001) BindingDB Entry DOI: 10.7270/Q2V69MSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 106 total ) | Next | Last >> |