 Found 1312 hits with Last Name = 'franco' and Initial = 'l'
Found 1312 hits with Last Name = 'franco' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM18372
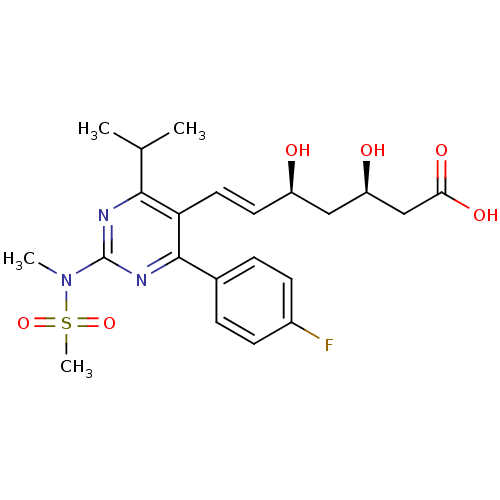
((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-(N-methylmethan...)Show SMILES CC(C)c1nc(nc(-c2ccc(F)cc2)c1\C=C\[C@@H](O)C[C@@H](O)CC(O)=O)N(C)S(C)(=O)=O |r| Show InChI InChI=1S/C22H28FN3O6S/c1-13(2)20-18(10-9-16(27)11-17(28)12-19(29)30)21(14-5-7-15(23)8-6-14)25-22(24-20)26(3)33(4,31)32/h5-10,13,16-17,27-28H,11-12H2,1-4H3,(H,29,30)/b10-9+/t16-,17-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Autónoma de México
Curated by ChEMBL
| Assay Description
Inhibitory constant against HMG-CoA reductase |
Bioorg Med Chem Lett 15: 989-94 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.046
BindingDB Entry DOI: 10.7270/Q2BC3Z1P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50139181

((1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-hydroxy-6-oxotet...)Show SMILES CCC(C)(C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)[C@@H]12 |r,c:14,t:12| Show InChI InChI=1S/C25H38O5/c1-6-25(4,5)24(28)30-21-12-15(2)11-17-8-7-16(3)20(23(17)21)10-9-19-13-18(26)14-22(27)29-19/h7-8,11,15-16,18-21,23,26H,6,9-10,12-14H2,1-5H3/t15-,16-,18+,19+,20-,21-,23-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Autónoma de México
Curated by ChEMBL
| Assay Description
Inhibitory constant against HMG-CoA reductase |
Bioorg Med Chem Lett 15: 989-94 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.046
BindingDB Entry DOI: 10.7270/Q2BC3Z1P |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50160720
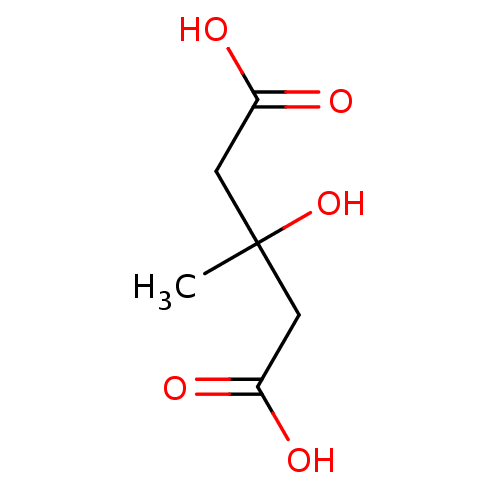
(3-hydroxy-3-methylglutaric acid | 3-hydroxy-3-meth...)Show InChI InChI=1S/C6H10O5/c1-6(11,2-4(7)8)3-5(9)10/h11H,2-3H2,1H3,(H,7,8)(H,9,10) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 23.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Autónoma de México
Curated by ChEMBL
| Assay Description
Inhibitory constant against HMG-CoA reductase |
Bioorg Med Chem Lett 15: 989-94 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.046
BindingDB Entry DOI: 10.7270/Q2BC3Z1P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50148911

((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9| Show InChI InChI=1S/C30H48O3/c1-18-10-15-30(25(32)33)17-16-28(6)20(24(30)19(18)2)8-9-22-27(5)13-12-23(31)26(3,4)21(27)11-14-29(22,28)7/h8,18-19,21-24,31H,9-17H2,1-7H3,(H,32,33)/t18-,19+,21+,22-,23+,24+,27+,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma del Estado de Morelos
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human recombinant PTP-1B expressed in Escherichia coli TB1 using p-nitrophenyl phosphate as substrate |
Eur J Med Chem 46: 2243-51 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.005
BindingDB Entry DOI: 10.7270/Q2FT8N4M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50346601

(NSC-114945 | OLEANOLIC_ACID | Oleanolic acid | Ole...)Show SMILES CC1(C)CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2C1)C(O)=O |r,c:10| Show InChI InChI=1S/C30H48O3/c1-25(2)14-16-30(24(32)33)17-15-28(6)19(20(30)18-25)8-9-22-27(5)12-11-23(31)26(3,4)21(27)10-13-29(22,28)7/h8,20-23,31H,9-18H2,1-7H3,(H,32,33)/t20-,21-,22+,23-,27-,28+,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma del Estado de Morelos
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human recombinant PTP-1B expressed in Escherichia coli TB1 using p-nitrophenyl phosphate as substrate |
Eur J Med Chem 46: 2243-51 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.005
BindingDB Entry DOI: 10.7270/Q2FT8N4M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50385306

(CHEMBL2002784)Show InChI InChI=1S/C12H12N2O3S/c1-3-17-11(16)10(15)14-12-13-8-5-4-7(2)6-9(8)18-12/h4-6H,3H2,1-2H3,(H,13,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma del Estado de Morelos
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of human recombinant GST-tagged PTP1B expressed in Escherichia coli TB1 cells using p-NPP as substrate by double reciprocal plo... |
Eur J Med Chem 53: 346-55 (2012)
Article DOI: 10.1016/j.ejmech.2012.04.025
BindingDB Entry DOI: 10.7270/Q2KP836R |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50241428

(CHEMBL401612 | N-(6-Methyl-1,3-benzothiazol-2-yl)-...)Show SMILES Cc1ccc2nc(NS(=O)(=O)c3ccc(cc3)[N+]([O-])=O)sc2c1 Show InChI InChI=1S/C14H11N3O4S2/c1-9-2-7-12-13(8-9)22-14(15-12)16-23(20,21)11-5-3-10(4-6-11)17(18)19/h2-8H,1H3,(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autónoma del Estado de Morelos
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 by double reciprocal plot analysis |
Bioorg Med Chem 17: 3332-41 (2009)
Article DOI: 10.1016/j.bmc.2009.03.042
BindingDB Entry DOI: 10.7270/Q2JQ10X9 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50391854

(CHEMBL463665)Show SMILES CC1(C)CC[C@@]2(CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)C2=C1)C(O)=O |r,c:32| Show InChI InChI=1S/C30H48O3/c1-25(2)14-16-30(24(32)33)17-15-28(6)19(20(30)18-25)8-9-22-27(5)12-11-23(31)26(3,4)21(27)10-13-29(22,28)7/h18-19,21-23,31H,8-17H2,1-7H3,(H,32,33)/t19-,21+,22-,23+,27+,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma del Estado de Morelos
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of human recombinant PTP-1B expressed in Escherichia coli TB1 using p-nitrophenyl phosphate as substrate |
Eur J Med Chem 46: 2243-51 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.005
BindingDB Entry DOI: 10.7270/Q2FT8N4M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50385307

(CHEMBL2035961)Show InChI InChI=1S/C11H9ClN2O3S/c1-2-17-10(16)9(15)14-11-13-7-4-3-6(12)5-8(7)18-11/h3-5H,2H2,1H3,(H,13,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma del Estado de Morelos
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of human recombinant GST-tagged PTP1B expressed in Escherichia coli TB1 cells using p-NPP as substrate by double reciprocal plo... |
Eur J Med Chem 53: 346-55 (2012)
Article DOI: 10.1016/j.ejmech.2012.04.025
BindingDB Entry DOI: 10.7270/Q2KP836R |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50385308

(CHEMBL2035962)Show InChI InChI=1S/C11H9FN2O3S/c1-2-17-10(16)9(15)14-11-13-7-4-3-6(12)5-8(7)18-11/h3-5H,2H2,1H3,(H,13,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma del Estado de Morelos
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of human recombinant GST-tagged PTP1B expressed in Escherichia coli TB1 cells using p-NPP as substrate by double reciprocal plo... |
Eur J Med Chem 53: 346-55 (2012)
Article DOI: 10.1016/j.ejmech.2012.04.025
BindingDB Entry DOI: 10.7270/Q2KP836R |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM54263

((4aS,6aR,6aR,6bR,8aR,12aR,14aS)-10-keto-2,2,6a,6b,...)Show SMILES CC1(C)CC[C@@]2(CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CCC(=O)C(C)(C)[C@@H]5CC[C@@]34C)C2=C1)C(O)=O |c:32| Show InChI InChI=1S/C30H46O3/c1-25(2)14-16-30(24(32)33)17-15-28(6)19(20(30)18-25)8-9-22-27(5)12-11-23(31)26(3,4)21(27)10-13-29(22,28)7/h18-19,21-22H,8-17H2,1-7H3,(H,32,33)/t19-,21+,22-,27+,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma del Estado de Morelos
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human recombinant PTP-1B expressed in Escherichia coli TB1 using p-nitrophenyl phosphate as substrate |
Eur J Med Chem 46: 2243-51 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.005
BindingDB Entry DOI: 10.7270/Q2FT8N4M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50241429
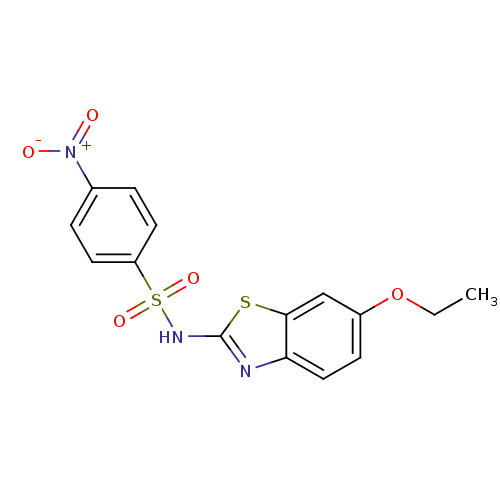
(CHEMBL401525 | N-(6-Ethoxy-1,3-benzothiazol-2-yl)-...)Show SMILES CCOc1ccc2nc(NS(=O)(=O)c3ccc(cc3)[N+]([O-])=O)sc2c1 Show InChI InChI=1S/C15H13N3O5S2/c1-2-23-11-5-8-13-14(9-11)24-15(16-13)17-25(21,22)12-6-3-10(4-7-12)18(19)20/h3-9H,2H2,1H3,(H,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autónoma del Estado de Morelos
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 by double reciprocal plot analysis |
Bioorg Med Chem 17: 3332-41 (2009)
Article DOI: 10.1016/j.bmc.2009.03.042
BindingDB Entry DOI: 10.7270/Q2JQ10X9 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50139181

((1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-hydroxy-6-oxotet...)Show SMILES CCC(C)(C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)[C@@H]12 |r,c:14,t:12| Show InChI InChI=1S/C25H38O5/c1-6-25(4,5)24(28)30-21-12-15(2)11-17-8-7-16(3)20(23(17)21)10-9-19-13-18(26)14-22(27)29-19/h7-8,11,15-16,18-21,23,26H,6,9-10,12-14H2,1-5H3/t15-,16-,18+,19+,20-,21-,23-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Autónoma de México
Curated by ChEMBL
| Assay Description
Inhibitory constant against HMG-CoA reductase with alpha asarone |
Bioorg Med Chem Lett 15: 989-94 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.046
BindingDB Entry DOI: 10.7270/Q2BC3Z1P |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM18372

((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-(N-methylmethan...)Show SMILES CC(C)c1nc(nc(-c2ccc(F)cc2)c1\C=C\[C@@H](O)C[C@@H](O)CC(O)=O)N(C)S(C)(=O)=O |r| Show InChI InChI=1S/C22H28FN3O6S/c1-13(2)20-18(10-9-16(27)11-17(28)12-19(29)30)21(14-5-7-15(23)8-6-14)25-22(24-20)26(3)33(4,31)32/h5-10,13,16-17,27-28H,11-12H2,1-4H3,(H,29,30)/b10-9+/t16-,17-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 7.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Autónoma de México
Curated by ChEMBL
| Assay Description
Inhibitory constant against HMG-CoA reductase with alpha asarone |
Bioorg Med Chem Lett 15: 989-94 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.046
BindingDB Entry DOI: 10.7270/Q2BC3Z1P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50512300

(CHEMBL4575012)Show SMILES CCn1ncc(c1C)-c1nc2c(N[C@H]3CCN(C3)C(=O)C3CCOCC3)ncnc2n1C |r| Show InChI InChI=1S/C22H30N8O2/c1-4-30-14(2)17(11-25-30)20-27-18-19(23-13-24-21(18)28(20)3)26-16-5-8-29(12-16)22(31)15-6-9-32-10-7-15/h11,13,15-16H,4-10,12H2,1-3H3,(H,23,24,26)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged recombinant full length human p110delta/untagged recombinant full length human p85alpha expressed in baculovirus... |
J Med Chem 62: 4370-4382 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01818
BindingDB Entry DOI: 10.7270/Q2V98CDF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50512313
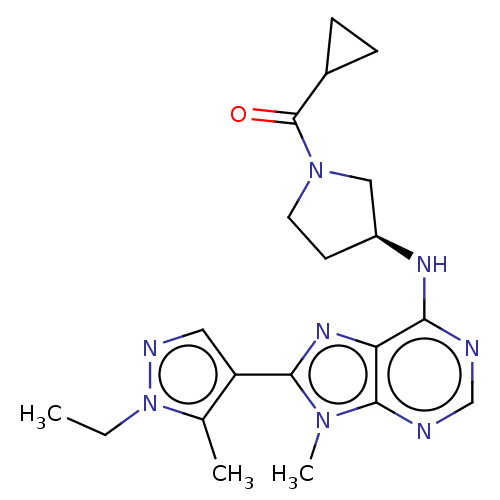
(CHEMBL4435694)Show SMILES CCn1ncc(c1C)-c1nc2c(N[C@H]3CCN(C3)C(=O)C3CC3)ncnc2n1C |r| Show InChI InChI=1S/C20H26N8O/c1-4-28-12(2)15(9-23-28)18-25-16-17(21-11-22-19(16)26(18)3)24-14-7-8-27(10-14)20(29)13-5-6-13/h9,11,13-14H,4-8,10H2,1-3H3,(H,21,22,24)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged recombinant full length human p110delta/untagged recombinant full length human p85alpha expressed in baculovirus... |
J Med Chem 62: 4370-4382 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01818
BindingDB Entry DOI: 10.7270/Q2V98CDF |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50184359

((3S,6S,9R,12S,15S,23S)-15-((S)-2-acetylamino-hexan...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r,wU:21.75,50.52,12.11,4.4,39.41,wD:25.25,61.64,(19.68,-11.7,;18.32,-10.97,;18.27,-9.43,;16.91,-8.71,;16.86,-7.17,;18.17,-6.36,;18.11,-4.82,;19.42,-4,;16.76,-4.09,;15.5,-6.44,;15.45,-4.9,;14.19,-7.26,;12.83,-6.53,;11.53,-7.35,;11.58,-8.88,;12.94,-9.61,;10.27,-9.7,;8.91,-8.97,;7.6,-9.79,;6.25,-9.06,;4.94,-9.87,;3.58,-9.15,;3.53,-7.61,;2.17,-6.88,;.86,-7.7,;2.12,-5.35,;.76,-4.62,;.71,-3.08,;1.93,-2.14,;1.41,-.69,;-.14,-.74,;-1.21,.35,;-2.69,-.02,;-3.1,-1.51,;-2.03,-2.6,;-.56,-2.22,;3.43,-4.53,;4.79,-5.26,;4.84,-6.8,;6.98,-5.22,;8.76,-4.36,;10.12,-5.08,;10.17,-6.62,;8.86,-7.43,;7.5,-6.71,;6.19,-7.52,;8.83,-5.93,;6.09,-4.44,;6.04,-2.9,;4.68,-2.18,;7.35,-2.09,;7.3,-.55,;5.94,.17,;5.89,1.71,;4.53,2.44,;3.23,1.62,;3.28,.07,;4.64,-.64,;8.71,-2.82,;10.02,-2,;9.96,-.46,;11.37,-2.73,;12.68,-1.91,;12.64,-.37,;13.86,.56,;13.35,2.01,;11.81,1.97,;11.37,.5,;11.42,-4.27,;12.78,-4.99,;14.09,-4.18,;2.27,-9.96,;.91,-9.24,;2.32,-11.5,)| Show InChI InChI=1S/C50H69N15O9/c1-3-4-16-36(59-29(2)66)44(69)65-41-25-42(67)55-20-11-10-18-35(43(51)68)60-47(72)39(23-31-26-57-34-17-9-8-15-33(31)34)63-45(70)37(19-12-21-56-50(52)53)61-46(71)38(22-30-13-6-5-7-14-30)62-48(73)40(64-49(41)74)24-32-27-54-28-58-32/h5-9,13-15,17,26-28,35-41,57H,3-4,10-12,16,18-25H2,1-2H3,(H2,51,68)(H,54,58)(H,55,67)(H,59,66)(H,60,72)(H,61,71)(H,62,73)(H,63,70)(H,64,74)(H,65,69)(H4,52,53,56)/t35-,36-,37-,38+,39-,40-,41-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Binding affinity towards human melanocortin receptor (hMC1R) using NDP-MSH as radioligand |
Bioorg Med Chem Lett 13: 1307-11 (2003)
BindingDB Entry DOI: 10.7270/Q21J994C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50512302
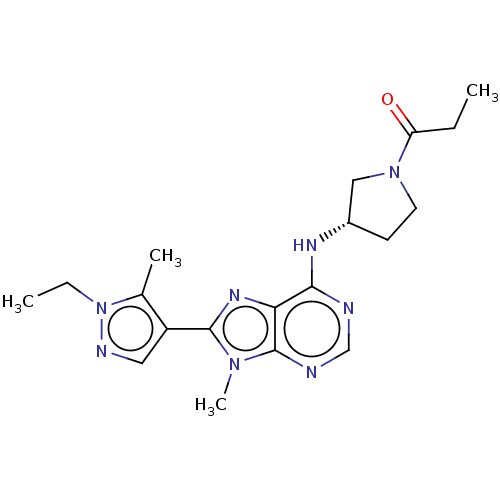
(CHEMBL4441262)Show SMILES CCC(=O)N1CC[C@@H](C1)Nc1ncnc2n(C)c(nc12)-c1cnn(CC)c1C |r| Show InChI InChI=1S/C19H26N8O/c1-5-15(28)26-8-7-13(10-26)23-17-16-19(21-11-20-17)25(4)18(24-16)14-9-22-27(6-2)12(14)3/h9,11,13H,5-8,10H2,1-4H3,(H,20,21,23)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged recombinant full length human p110delta/untagged recombinant full length human p85alpha expressed in baculovirus... |
J Med Chem 62: 4370-4382 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01818
BindingDB Entry DOI: 10.7270/Q2V98CDF |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50212693

(2-(4-(4-(4-(trifluoromethyl)benzyloxy)benzylthio)-...)Show SMILES COc1cc(OCC(O)=O)c(C)cc1SCc1ccc(OCc2ccc(cc2)C(F)(F)F)cc1 Show InChI InChI=1S/C25H23F3O5S/c1-16-11-23(22(31-2)12-21(16)33-14-24(29)30)34-15-18-5-9-20(10-6-18)32-13-17-3-7-19(8-4-17)25(26,27)28/h3-12H,13-15H2,1-2H3,(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta |
Bioorg Med Chem 20: 3523-32 (2012)
Article DOI: 10.1016/j.bmc.2012.04.005
BindingDB Entry DOI: 10.7270/Q2MP54B4 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50212693

(2-(4-(4-(4-(trifluoromethyl)benzyloxy)benzylthio)-...)Show SMILES COc1cc(OCC(O)=O)c(C)cc1SCc1ccc(OCc2ccc(cc2)C(F)(F)F)cc1 Show InChI InChI=1S/C25H23F3O5S/c1-16-11-23(22(31-2)12-21(16)33-14-24(29)30)34-15-18-5-9-20(10-6-18)32-13-17-3-7-19(8-4-17)25(26,27)28/h3-12H,13-15H2,1-2H3,(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta |
Bioorg Med Chem 20: 3523-32 (2012)
Article DOI: 10.1016/j.bmc.2012.04.005
BindingDB Entry DOI: 10.7270/Q2MP54B4 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50184359

((3S,6S,9R,12S,15S,23S)-15-((S)-2-acetylamino-hexan...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r,wU:21.75,50.52,12.11,4.4,39.41,wD:25.25,61.64,(19.68,-11.7,;18.32,-10.97,;18.27,-9.43,;16.91,-8.71,;16.86,-7.17,;18.17,-6.36,;18.11,-4.82,;19.42,-4,;16.76,-4.09,;15.5,-6.44,;15.45,-4.9,;14.19,-7.26,;12.83,-6.53,;11.53,-7.35,;11.58,-8.88,;12.94,-9.61,;10.27,-9.7,;8.91,-8.97,;7.6,-9.79,;6.25,-9.06,;4.94,-9.87,;3.58,-9.15,;3.53,-7.61,;2.17,-6.88,;.86,-7.7,;2.12,-5.35,;.76,-4.62,;.71,-3.08,;1.93,-2.14,;1.41,-.69,;-.14,-.74,;-1.21,.35,;-2.69,-.02,;-3.1,-1.51,;-2.03,-2.6,;-.56,-2.22,;3.43,-4.53,;4.79,-5.26,;4.84,-6.8,;6.98,-5.22,;8.76,-4.36,;10.12,-5.08,;10.17,-6.62,;8.86,-7.43,;7.5,-6.71,;6.19,-7.52,;8.83,-5.93,;6.09,-4.44,;6.04,-2.9,;4.68,-2.18,;7.35,-2.09,;7.3,-.55,;5.94,.17,;5.89,1.71,;4.53,2.44,;3.23,1.62,;3.28,.07,;4.64,-.64,;8.71,-2.82,;10.02,-2,;9.96,-.46,;11.37,-2.73,;12.68,-1.91,;12.64,-.37,;13.86,.56,;13.35,2.01,;11.81,1.97,;11.37,.5,;11.42,-4.27,;12.78,-4.99,;14.09,-4.18,;2.27,-9.96,;.91,-9.24,;2.32,-11.5,)| Show InChI InChI=1S/C50H69N15O9/c1-3-4-16-36(59-29(2)66)44(69)65-41-25-42(67)55-20-11-10-18-35(43(51)68)60-47(72)39(23-31-26-57-34-17-9-8-15-33(31)34)63-45(70)37(19-12-21-56-50(52)53)61-46(71)38(22-30-13-6-5-7-14-30)62-48(73)40(64-49(41)74)24-32-27-54-28-58-32/h5-9,13-15,17,26-28,35-41,57H,3-4,10-12,16,18-25H2,1-2H3,(H2,51,68)(H,54,58)(H,55,67)(H,59,66)(H,60,72)(H,61,71)(H,62,73)(H,63,70)(H,64,74)(H,65,69)(H4,52,53,56)/t35-,36-,37-,38+,39-,40-,41-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Binding affinity towards human melanocortin receptor (hMC4R) using NDP-MSH as radioligand |
Bioorg Med Chem Lett 13: 1307-11 (2003)
BindingDB Entry DOI: 10.7270/Q21J994C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50512303

(CHEMBL4582480)Show SMILES CC[C@H](Nc1ncnc2n(C)c(nc12)-c1cnn(CC)c1C)C(=O)N1C[C@H](C)O[C@H](C)C1 |r| Show InChI InChI=1S/C22H32N8O2/c1-7-17(22(31)29-10-13(3)32-14(4)11-29)26-19-18-21(24-12-23-19)28(6)20(27-18)16-9-25-30(8-2)15(16)5/h9,12-14,17H,7-8,10-11H2,1-6H3,(H,23,24,26)/t13-,14+,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged recombinant full length human p110delta/untagged recombinant full length human p85alpha expressed in baculovirus... |
J Med Chem 62: 4370-4382 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01818
BindingDB Entry DOI: 10.7270/Q2V98CDF |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50227738

(2-(4-(4-fluoro-3-((2-fluoro-4-(trifluoromethyl)ben...)Show SMILES Cc1cc(Oc2ccc(F)c(CNC(=O)c3ccc(cc3F)C(F)(F)F)c2)ccc1OC(C)(C)C(O)=O Show InChI InChI=1S/C26H22F5NO5/c1-14-10-17(6-9-22(14)37-25(2,3)24(34)35)36-18-5-8-20(27)15(11-18)13-32-23(33)19-7-4-16(12-21(19)28)26(29,30)31/h4-12H,13H2,1-3H3,(H,32,33)(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta |
Bioorg Med Chem 20: 3523-32 (2012)
Article DOI: 10.1016/j.bmc.2012.04.005
BindingDB Entry DOI: 10.7270/Q2MP54B4 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50227736

(2-(4-(5-((2-fluoro-4-(trifluoromethyl)benzamido)me...)Show SMILES Cc1ccc(CNC(=O)c2ccc(cc2F)C(F)(F)F)cc1Oc1ccc(OC(C)(C)C(O)=O)c(C)c1 Show InChI InChI=1S/C27H25F4NO5/c1-15-5-6-17(14-32-24(33)20-9-7-18(13-21(20)28)27(29,30)31)12-23(15)36-19-8-10-22(16(2)11-19)37-26(3,4)25(34)35/h5-13H,14H2,1-4H3,(H,32,33)(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta |
Bioorg Med Chem 20: 3523-32 (2012)
Article DOI: 10.1016/j.bmc.2012.04.005
BindingDB Entry DOI: 10.7270/Q2MP54B4 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50227745

(2-(4-(3-((2-bromo-4-(trifluoromethyl)benzamido)met...)Show SMILES Cc1cc(Oc2cccc(CNC(=O)c3ccc(cc3Br)C(F)(F)F)c2)ccc1OC(C)(C)C(O)=O Show InChI InChI=1S/C26H23BrF3NO5/c1-15-11-19(8-10-22(15)36-25(2,3)24(33)34)35-18-6-4-5-16(12-18)14-31-23(32)20-9-7-17(13-21(20)27)26(28,29)30/h4-13H,14H2,1-3H3,(H,31,32)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta |
Bioorg Med Chem 20: 3523-32 (2012)
Article DOI: 10.1016/j.bmc.2012.04.005
BindingDB Entry DOI: 10.7270/Q2MP54B4 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50227743
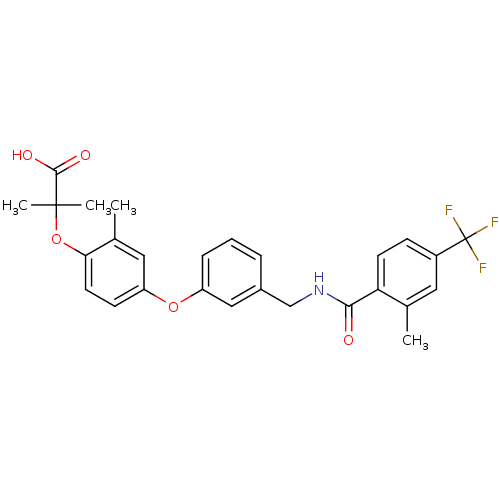
(2-methyl-2-(2-methyl-4-(3-((2-methyl-4-(trifluorom...)Show SMILES Cc1cc(Oc2cccc(CNC(=O)c3ccc(cc3C)C(F)(F)F)c2)ccc1OC(C)(C)C(O)=O Show InChI InChI=1S/C27H26F3NO5/c1-16-12-19(27(28,29)30)8-10-22(16)24(32)31-15-18-6-5-7-20(14-18)35-21-9-11-23(17(2)13-21)36-26(3,4)25(33)34/h5-14H,15H2,1-4H3,(H,31,32)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta |
Bioorg Med Chem 20: 3523-32 (2012)
Article DOI: 10.1016/j.bmc.2012.04.005
BindingDB Entry DOI: 10.7270/Q2MP54B4 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50227738

(2-(4-(4-fluoro-3-((2-fluoro-4-(trifluoromethyl)ben...)Show SMILES Cc1cc(Oc2ccc(F)c(CNC(=O)c3ccc(cc3F)C(F)(F)F)c2)ccc1OC(C)(C)C(O)=O Show InChI InChI=1S/C26H22F5NO5/c1-14-10-17(6-9-22(14)37-25(2,3)24(34)35)36-18-5-8-20(27)15(11-18)13-32-23(33)19-7-4-16(12-21(19)28)26(29,30)31/h4-12H,13H2,1-3H3,(H,32,33)(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta |
Bioorg Med Chem 20: 3523-32 (2012)
Article DOI: 10.1016/j.bmc.2012.04.005
BindingDB Entry DOI: 10.7270/Q2MP54B4 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50199781
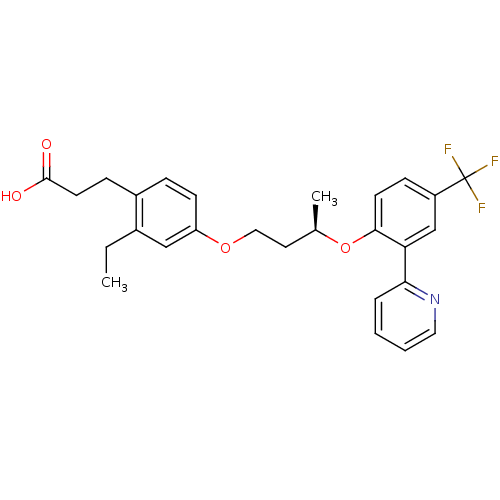
(3-(2-ethyl-4-((R)-3-(2-(pyridin-2-yl)-4-(trifluoro...)Show SMILES CCc1cc(OCC[C@@H](C)Oc2ccc(cc2-c2ccccn2)C(F)(F)F)ccc1CCC(O)=O Show InChI InChI=1S/C27H28F3NO4/c1-3-19-16-22(10-7-20(19)8-12-26(32)33)34-15-13-18(2)35-25-11-9-21(27(28,29)30)17-23(25)24-6-4-5-14-31-24/h4-7,9-11,14,16-18H,3,8,12-13,15H2,1-2H3,(H,32,33)/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta |
Bioorg Med Chem 20: 3523-32 (2012)
Article DOI: 10.1016/j.bmc.2012.04.005
BindingDB Entry DOI: 10.7270/Q2MP54B4 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50227765

(3-(4-(3-((5-chloro-3-methyl-1H-indole-2-carboxamid...)Show SMILES Cc1c([nH]c2ccc(Cl)cc12)C(=O)NCc1cccc(Oc2ccc(CCC(O)=O)c(C)c2)c1 Show InChI InChI=1S/C27H25ClN2O4/c1-16-12-22(9-6-19(16)7-11-25(31)32)34-21-5-3-4-18(13-21)15-29-27(33)26-17(2)23-14-20(28)8-10-24(23)30-26/h3-6,8-10,12-14,30H,7,11,15H2,1-2H3,(H,29,33)(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta |
Bioorg Med Chem 20: 3523-32 (2012)
Article DOI: 10.1016/j.bmc.2012.04.005
BindingDB Entry DOI: 10.7270/Q2MP54B4 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50199778

(3-(4-((R)-3-(4-ethyl-2-(thiazol-4-yl)phenoxy)butox...)Show SMILES CCc1ccc(O[C@H](C)CCOc2ccc(CCC(O)=O)c(C)c2)c(c1)-c1cscn1 Show InChI InChI=1S/C25H29NO4S/c1-4-19-5-9-24(22(14-19)23-15-31-16-26-23)30-18(3)11-12-29-21-8-6-20(17(2)13-21)7-10-25(27)28/h5-6,8-9,13-16,18H,4,7,10-12H2,1-3H3,(H,27,28)/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta |
Bioorg Med Chem 20: 3523-32 (2012)
Article DOI: 10.1016/j.bmc.2012.04.005
BindingDB Entry DOI: 10.7270/Q2MP54B4 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50199779
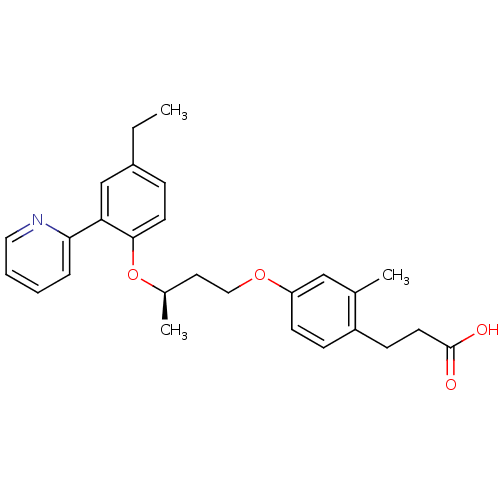
(3-(4-((R)-3-(4-ethyl-2-(pyridin-2-yl)phenoxy)butox...)Show SMILES CCc1ccc(O[C@H](C)CCOc2ccc(CCC(O)=O)c(C)c2)c(c1)-c1ccccn1 Show InChI InChI=1S/C27H31NO4/c1-4-21-8-12-26(24(18-21)25-7-5-6-15-28-25)32-20(3)14-16-31-23-11-9-22(19(2)17-23)10-13-27(29)30/h5-9,11-12,15,17-18,20H,4,10,13-14,16H2,1-3H3,(H,29,30)/t20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta |
Bioorg Med Chem 20: 3523-32 (2012)
Article DOI: 10.1016/j.bmc.2012.04.005
BindingDB Entry DOI: 10.7270/Q2MP54B4 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50199776
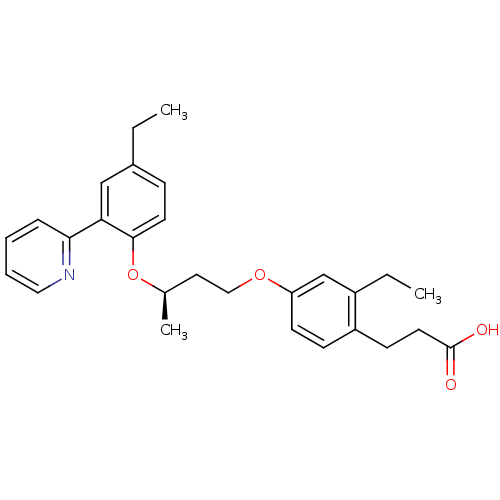
(3-(2-ethyl-4-((R)-3-(4-ethyl-2-(pyridin-2-yl)pheno...)Show SMILES CCc1ccc(O[C@H](C)CCOc2ccc(CCC(O)=O)c(CC)c2)c(c1)-c1ccccn1 Show InChI InChI=1S/C28H33NO4/c1-4-21-9-13-27(25(18-21)26-8-6-7-16-29-26)33-20(3)15-17-32-24-12-10-23(11-14-28(30)31)22(5-2)19-24/h6-10,12-13,16,18-20H,4-5,11,14-15,17H2,1-3H3,(H,30,31)/t20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta |
Bioorg Med Chem 20: 3523-32 (2012)
Article DOI: 10.1016/j.bmc.2012.04.005
BindingDB Entry DOI: 10.7270/Q2MP54B4 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50227759

(3-(4-(3-((6-chloro-1H-indole-2-carboxamido)methyl)...)Show SMILES Cc1cc(Oc2cccc(CNC(=O)c3cc4ccc(Cl)cc4[nH]3)c2)ccc1CCC(O)=O Show InChI InChI=1S/C26H23ClN2O4/c1-16-11-22(9-6-18(16)7-10-25(30)31)33-21-4-2-3-17(12-21)15-28-26(32)24-13-19-5-8-20(27)14-23(19)29-24/h2-6,8-9,11-14,29H,7,10,15H2,1H3,(H,28,32)(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta |
Bioorg Med Chem 20: 3523-32 (2012)
Article DOI: 10.1016/j.bmc.2012.04.005
BindingDB Entry DOI: 10.7270/Q2MP54B4 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50227752
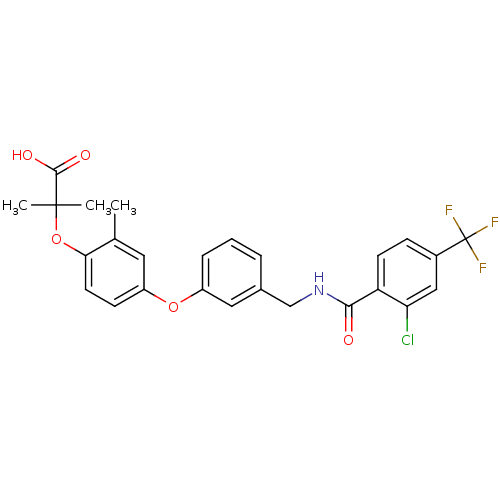
(2-(4-(3-((2-chloro-4-(trifluoromethyl)benzamido)me...)Show SMILES Cc1cc(Oc2cccc(CNC(=O)c3ccc(cc3Cl)C(F)(F)F)c2)ccc1OC(C)(C)C(O)=O Show InChI InChI=1S/C26H23ClF3NO5/c1-15-11-19(8-10-22(15)36-25(2,3)24(33)34)35-18-6-4-5-16(12-18)14-31-23(32)20-9-7-17(13-21(20)27)26(28,29)30/h4-13H,14H2,1-3H3,(H,31,32)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta |
Bioorg Med Chem 20: 3523-32 (2012)
Article DOI: 10.1016/j.bmc.2012.04.005
BindingDB Entry DOI: 10.7270/Q2MP54B4 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50227761

(2-(4-(3-fluoro-5-((2-fluoro-4-(trifluoromethyl)ben...)Show SMILES Cc1cc(Oc2cc(F)cc(CNC(=O)c3ccc(cc3F)C(F)(F)F)c2)ccc1OC(C)(C)C(O)=O Show InChI InChI=1S/C26H22F5NO5/c1-14-8-18(5-7-22(14)37-25(2,3)24(34)35)36-19-10-15(9-17(27)12-19)13-32-23(33)20-6-4-16(11-21(20)28)26(29,30)31/h4-12H,13H2,1-3H3,(H,32,33)(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta |
Bioorg Med Chem 20: 3523-32 (2012)
Article DOI: 10.1016/j.bmc.2012.04.005
BindingDB Entry DOI: 10.7270/Q2MP54B4 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50227736

(2-(4-(5-((2-fluoro-4-(trifluoromethyl)benzamido)me...)Show SMILES Cc1ccc(CNC(=O)c2ccc(cc2F)C(F)(F)F)cc1Oc1ccc(OC(C)(C)C(O)=O)c(C)c1 Show InChI InChI=1S/C27H25F4NO5/c1-15-5-6-17(14-32-24(33)20-9-7-18(13-21(20)28)27(29,30)31)12-23(15)36-19-8-10-22(16(2)11-19)37-26(3,4)25(34)35/h5-13H,14H2,1-4H3,(H,32,33)(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta |
Bioorg Med Chem 20: 3523-32 (2012)
Article DOI: 10.1016/j.bmc.2012.04.005
BindingDB Entry DOI: 10.7270/Q2MP54B4 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50227746
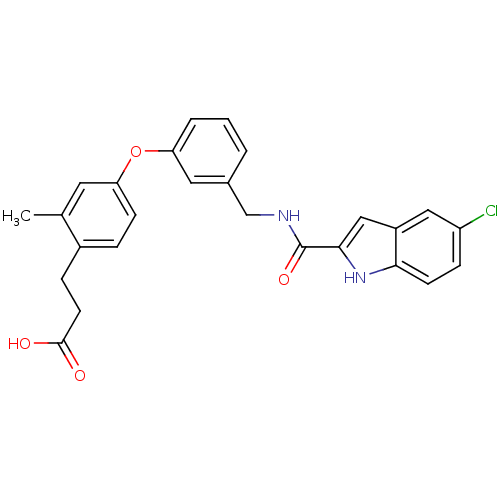
(3-(4-(3-((5-chloro-1H-indole-2-carboxamido)methyl)...)Show SMILES Cc1cc(Oc2cccc(CNC(=O)c3cc4cc(Cl)ccc4[nH]3)c2)ccc1CCC(O)=O Show InChI InChI=1S/C26H23ClN2O4/c1-16-11-22(8-5-18(16)6-10-25(30)31)33-21-4-2-3-17(12-21)15-28-26(32)24-14-19-13-20(27)7-9-23(19)29-24/h2-5,7-9,11-14,29H,6,10,15H2,1H3,(H,28,32)(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta |
Bioorg Med Chem 20: 3523-32 (2012)
Article DOI: 10.1016/j.bmc.2012.04.005
BindingDB Entry DOI: 10.7270/Q2MP54B4 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50213330
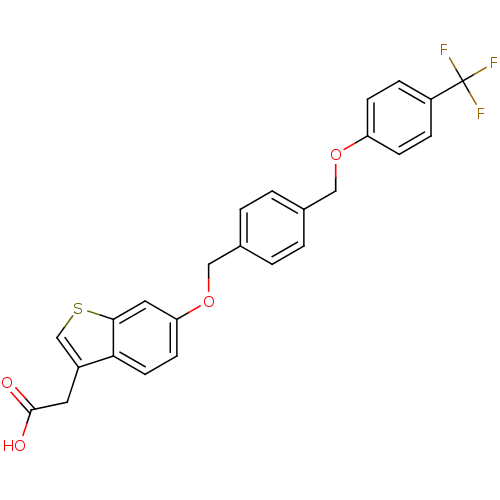
(2-(6-(4-((4-(trifluoromethyl)phenoxy)methyl)benzyl...)Show SMILES OC(=O)Cc1csc2cc(OCc3ccc(COc4ccc(cc4)C(F)(F)F)cc3)ccc12 Show InChI InChI=1S/C25H19F3O4S/c26-25(27,28)19-5-7-20(8-6-19)31-13-16-1-3-17(4-2-16)14-32-21-9-10-22-18(11-24(29)30)15-33-23(22)12-21/h1-10,12,15H,11,13-14H2,(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta |
Bioorg Med Chem 20: 3523-32 (2012)
Article DOI: 10.1016/j.bmc.2012.04.005
BindingDB Entry DOI: 10.7270/Q2MP54B4 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50512301
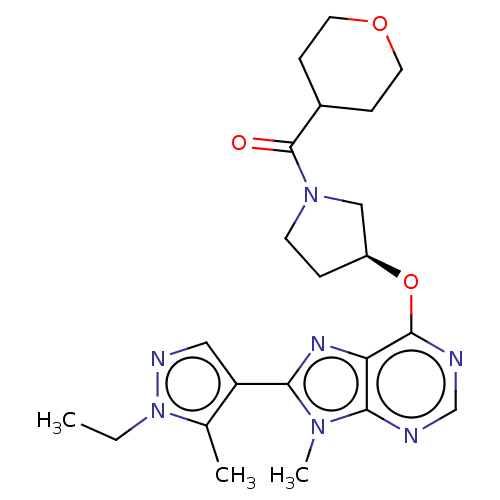
(CHEMBL4570812)Show SMILES CCn1ncc(c1C)-c1nc2c(O[C@H]3CCN(C3)C(=O)C3CCOCC3)ncnc2n1C |r| Show InChI InChI=1S/C22H29N7O3/c1-4-29-14(2)17(11-25-29)19-26-18-20(27(19)3)23-13-24-21(18)32-16-5-8-28(12-16)22(30)15-6-9-31-10-7-15/h11,13,15-16H,4-10,12H2,1-3H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged recombinant full length human p110delta/untagged recombinant full length human p85alpha expressed in baculovirus... |
J Med Chem 62: 4370-4382 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01818
BindingDB Entry DOI: 10.7270/Q2V98CDF |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50194630

((R)-3-{4-[3-(4-chloro-2-phenoxy-phenoxy)-butoxy]-2...)Show SMILES CCc1cc(OCC[C@@H](C)Oc2ccc(Cl)cc2Oc2ccccc2)ccc1CCC(O)=O Show InChI InChI=1S/C27H29ClO5/c1-3-20-17-24(12-9-21(20)10-14-27(29)30)31-16-15-19(2)32-25-13-11-22(28)18-26(25)33-23-7-5-4-6-8-23/h4-9,11-13,17-19H,3,10,14-16H2,1-2H3,(H,29,30)/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta |
Bioorg Med Chem 20: 3523-32 (2012)
Article DOI: 10.1016/j.bmc.2012.04.005
BindingDB Entry DOI: 10.7270/Q2MP54B4 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50194637
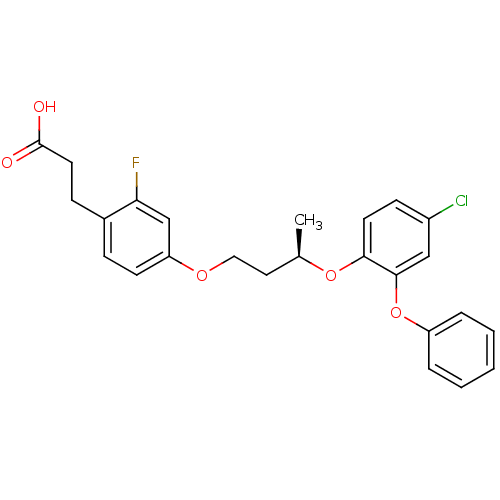
((R)-3-{4-[3-(4-chloro-2-phenoxy-phenoxy)-butoxy]-2...)Show SMILES C[C@H](CCOc1ccc(CCC(O)=O)c(F)c1)Oc1ccc(Cl)cc1Oc1ccccc1 Show InChI InChI=1S/C25H24ClFO5/c1-17(13-14-30-21-10-7-18(22(27)16-21)8-12-25(28)29)31-23-11-9-19(26)15-24(23)32-20-5-3-2-4-6-20/h2-7,9-11,15-17H,8,12-14H2,1H3,(H,28,29)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta |
Bioorg Med Chem 20: 3523-32 (2012)
Article DOI: 10.1016/j.bmc.2012.04.005
BindingDB Entry DOI: 10.7270/Q2MP54B4 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50126016
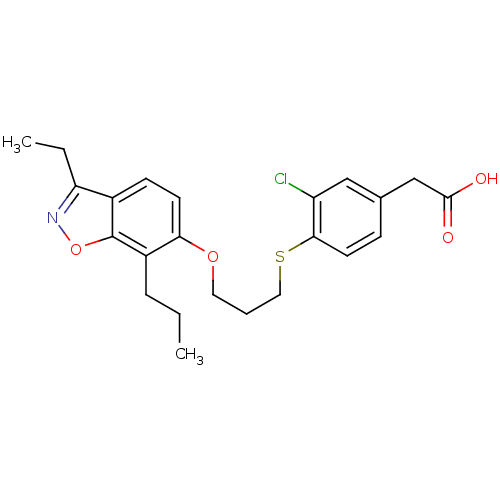
(2-(3-chloro-4-(3-(3-ethyl-7-propylbenzo[d]isoxazol...)Show SMILES CCCc1c(OCCCSc2ccc(CC(O)=O)cc2Cl)ccc2c(CC)noc12 Show InChI InChI=1S/C23H26ClNO4S/c1-3-6-17-20(9-8-16-19(4-2)25-29-23(16)17)28-11-5-12-30-21-10-7-15(13-18(21)24)14-22(26)27/h7-10,13H,3-6,11-12,14H2,1-2H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta |
Bioorg Med Chem 20: 3523-32 (2012)
Article DOI: 10.1016/j.bmc.2012.04.005
BindingDB Entry DOI: 10.7270/Q2MP54B4 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50194635
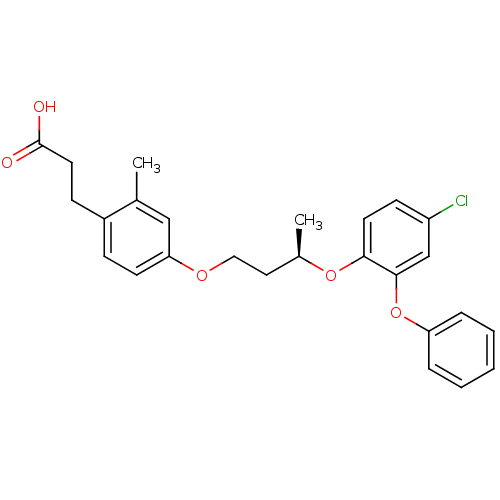
((R)-3-{4-[3-(4-chloro-2-phenoxy-phenoxy)-butoxy]-2...)Show SMILES C[C@H](CCOc1ccc(CCC(O)=O)c(C)c1)Oc1ccc(Cl)cc1Oc1ccccc1 Show InChI InChI=1S/C26H27ClO5/c1-18-16-23(11-8-20(18)9-13-26(28)29)30-15-14-19(2)31-24-12-10-21(27)17-25(24)32-22-6-4-3-5-7-22/h3-8,10-12,16-17,19H,9,13-15H2,1-2H3,(H,28,29)/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta |
Bioorg Med Chem 20: 3523-32 (2012)
Article DOI: 10.1016/j.bmc.2012.04.005
BindingDB Entry DOI: 10.7270/Q2MP54B4 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM28691
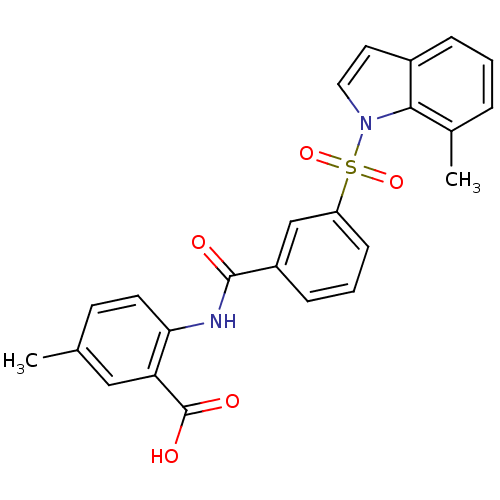
(5-methyl-2-({3-[(7-methyl-1H-indole-1-)sulfonyl]be...)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)S(=O)(=O)n2ccc3cccc(C)c23)c(c1)C(O)=O Show InChI InChI=1S/C24H20N2O5S/c1-15-9-10-21(20(13-15)24(28)29)25-23(27)18-7-4-8-19(14-18)32(30,31)26-12-11-17-6-3-5-16(2)22(17)26/h3-14H,1-2H3,(H,25,27)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta |
Bioorg Med Chem 20: 3523-32 (2012)
Article DOI: 10.1016/j.bmc.2012.04.005
BindingDB Entry DOI: 10.7270/Q2MP54B4 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM28674
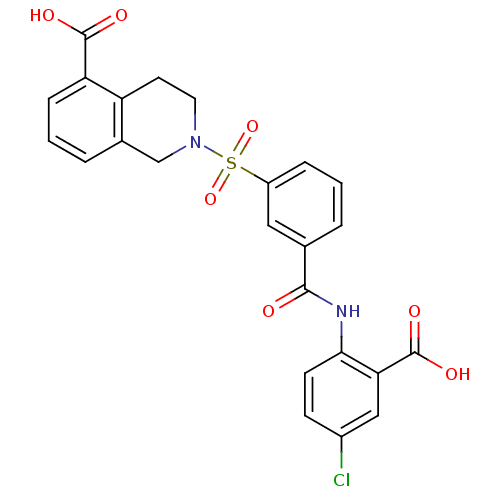
(2-({3-[(2-carboxy-4-chlorophenyl)carbamoyl]benzene...)Show SMILES OC(=O)c1cc(Cl)ccc1NC(=O)c1cccc(c1)S(=O)(=O)N1CCc2c(C1)cccc2C(O)=O Show InChI InChI=1S/C24H19ClN2O7S/c25-16-7-8-21(20(12-16)24(31)32)26-22(28)14-3-1-5-17(11-14)35(33,34)27-10-9-18-15(13-27)4-2-6-19(18)23(29)30/h1-8,11-12H,9-10,13H2,(H,26,28)(H,29,30)(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta |
Bioorg Med Chem 20: 3523-32 (2012)
Article DOI: 10.1016/j.bmc.2012.04.005
BindingDB Entry DOI: 10.7270/Q2MP54B4 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50126018
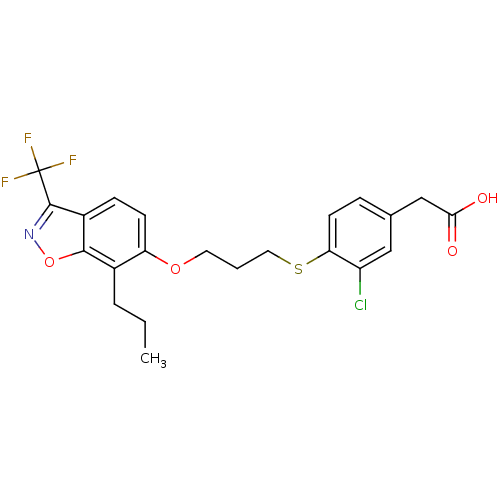
(2-(3-chloro-4-(3-(7-propyl-3-(trifluoromethyl)benz...)Show SMILES CCCc1c(OCCCSc2ccc(CC(O)=O)cc2Cl)ccc2c(noc12)C(F)(F)F Show InChI InChI=1S/C22H21ClF3NO4S/c1-2-4-14-17(7-6-15-20(14)31-27-21(15)22(24,25)26)30-9-3-10-32-18-8-5-13(11-16(18)23)12-19(28)29/h5-8,11H,2-4,9-10,12H2,1H3,(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta |
Bioorg Med Chem 20: 3523-32 (2012)
Article DOI: 10.1016/j.bmc.2012.04.005
BindingDB Entry DOI: 10.7270/Q2MP54B4 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50126018

(2-(3-chloro-4-(3-(7-propyl-3-(trifluoromethyl)benz...)Show SMILES CCCc1c(OCCCSc2ccc(CC(O)=O)cc2Cl)ccc2c(noc12)C(F)(F)F Show InChI InChI=1S/C22H21ClF3NO4S/c1-2-4-14-17(7-6-15-20(14)31-27-21(15)22(24,25)26)30-9-3-10-32-18-8-5-13(11-16(18)23)12-19(28)29/h5-8,11H,2-4,9-10,12H2,1H3,(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta |
Bioorg Med Chem 20: 3523-32 (2012)
Article DOI: 10.1016/j.bmc.2012.04.005
BindingDB Entry DOI: 10.7270/Q2MP54B4 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50227757

(2-(4-(3-((2-fluoro-4-(trifluoromethyl)benzamido)me...)Show SMILES Cc1cc(Oc2cccc(CNC(=O)c3ccc(cc3F)C(F)(F)F)c2)ccc1OC(C)(C)C(O)=O Show InChI InChI=1S/C26H23F4NO5/c1-15-11-19(8-10-22(15)36-25(2,3)24(33)34)35-18-6-4-5-16(12-18)14-31-23(32)20-9-7-17(13-21(20)27)26(28,29)30/h4-13H,14H2,1-3H3,(H,31,32)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta |
Bioorg Med Chem 20: 3523-32 (2012)
Article DOI: 10.1016/j.bmc.2012.04.005
BindingDB Entry DOI: 10.7270/Q2MP54B4 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50227766
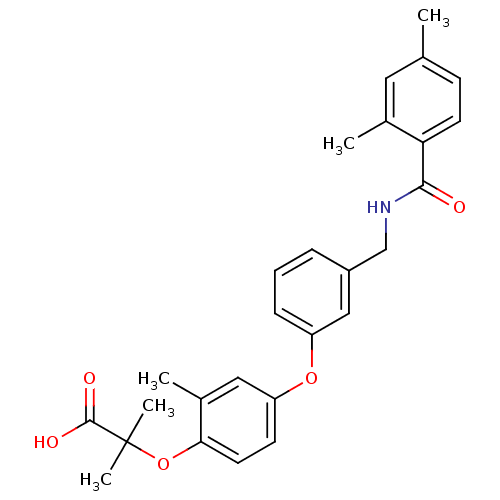
(2-(4-(3-((2,4-dimethylbenzamido)methyl)phenoxy)-2-...)Show SMILES Cc1ccc(C(=O)NCc2cccc(Oc3ccc(OC(C)(C)C(O)=O)c(C)c3)c2)c(C)c1 Show InChI InChI=1S/C27H29NO5/c1-17-9-11-23(18(2)13-17)25(29)28-16-20-7-6-8-21(15-20)32-22-10-12-24(19(3)14-22)33-27(4,5)26(30)31/h6-15H,16H2,1-5H3,(H,28,29)(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta |
Bioorg Med Chem 20: 3523-32 (2012)
Article DOI: 10.1016/j.bmc.2012.04.005
BindingDB Entry DOI: 10.7270/Q2MP54B4 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50227766

(2-(4-(3-((2,4-dimethylbenzamido)methyl)phenoxy)-2-...)Show SMILES Cc1ccc(C(=O)NCc2cccc(Oc3ccc(OC(C)(C)C(O)=O)c(C)c3)c2)c(C)c1 Show InChI InChI=1S/C27H29NO5/c1-17-9-11-23(18(2)13-17)25(29)28-16-20-7-6-8-21(15-20)32-22-10-12-24(19(3)14-22)33-27(4,5)26(30)31/h6-15H,16H2,1-5H3,(H,28,29)(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Aut£noma de M£xico
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta |
Bioorg Med Chem 20: 3523-32 (2012)
Article DOI: 10.1016/j.bmc.2012.04.005
BindingDB Entry DOI: 10.7270/Q2MP54B4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data