

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50254293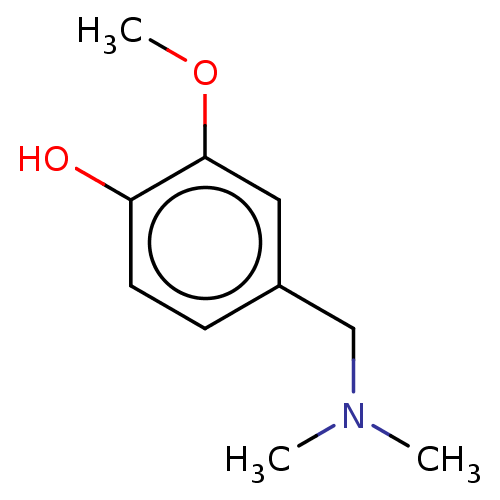 (CHEMBL4070283) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MMDB PC cid PC sid UniChem Similars | Article PubMed | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry and Biochemistry, The Ohio State University, Marion Campus, 1465 Mt. Vernon Avenue, Marion, Ohio 43302, United States. Curated by ChEMBL | Assay Description Mixed type inhibition of Electric eel AChE assessed as inhibition constant using acetylthiocholine as substrate after 7 min by Ellman's method | ACS Med Chem Lett 8: 622-627 (2017) Article DOI: 10.1021/acsmedchemlett.7b00037 BindingDB Entry DOI: 10.7270/Q20V8G76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50254289 (CHEMBL4091215) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 9.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry and Biochemistry, The Ohio State University, Marion Campus, 1465 Mt. Vernon Avenue, Marion, Ohio 43302, United States. Curated by ChEMBL | Assay Description Mixed type inhibition of Electric eel AChE assessed as inhibition constant using acetylthiocholine as substrate after 7 min by Ellman's method | ACS Med Chem Lett 8: 622-627 (2017) Article DOI: 10.1021/acsmedchemlett.7b00037 BindingDB Entry DOI: 10.7270/Q20V8G76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50254288 (CHEMBL4074057) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry and Biochemistry, The Ohio State University, Marion Campus, 1465 Mt. Vernon Avenue, Marion, Ohio 43302, United States. Curated by ChEMBL | Assay Description Mixed type inhibition of Electric eel AChE assessed as inhibition constant using acetylthiocholine as substrate after 7 min by Ellman's method | ACS Med Chem Lett 8: 622-627 (2017) Article DOI: 10.1021/acsmedchemlett.7b00037 BindingDB Entry DOI: 10.7270/Q20V8G76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50254287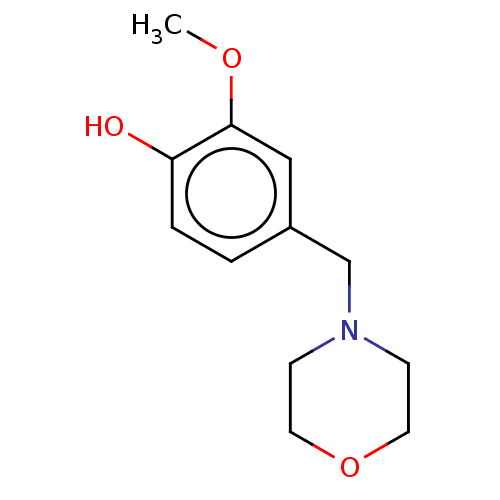 (CHEMBL4094588) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 1.57E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry and Biochemistry, The Ohio State University, Marion Campus, 1465 Mt. Vernon Avenue, Marion, Ohio 43302, United States. Curated by ChEMBL | Assay Description Mixed type inhibition of Electric eel AChE assessed as inhibition constant using acetylthiocholine as substrate after 7 min by Ellman's method | ACS Med Chem Lett 8: 622-627 (2017) Article DOI: 10.1021/acsmedchemlett.7b00037 BindingDB Entry DOI: 10.7270/Q20V8G76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50254288 (CHEMBL4074057) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.27E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry and Biochemistry, The Ohio State University, Marion Campus, 1465 Mt. Vernon Avenue, Marion, Ohio 43302, United States. Curated by ChEMBL | Assay Description Inhibition of Electric eel AChE using acetylthiocholine as substrate after 7 min by Ellman's method | ACS Med Chem Lett 8: 622-627 (2017) Article DOI: 10.1021/acsmedchemlett.7b00037 BindingDB Entry DOI: 10.7270/Q20V8G76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50254289 (CHEMBL4091215) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4.03E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry and Biochemistry, The Ohio State University, Marion Campus, 1465 Mt. Vernon Avenue, Marion, Ohio 43302, United States. Curated by ChEMBL | Assay Description Inhibition of Electric eel AChE using acetylthiocholine as substrate after 7 min by Ellman's method | ACS Med Chem Lett 8: 622-627 (2017) Article DOI: 10.1021/acsmedchemlett.7b00037 BindingDB Entry DOI: 10.7270/Q20V8G76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50254293 (CHEMBL4070283) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.19E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry and Biochemistry, The Ohio State University, Marion Campus, 1465 Mt. Vernon Avenue, Marion, Ohio 43302, United States. Curated by ChEMBL | Assay Description Inhibition of Electric eel AChE using acetylthiocholine as substrate after 7 min by Ellman's method | ACS Med Chem Lett 8: 622-627 (2017) Article DOI: 10.1021/acsmedchemlett.7b00037 BindingDB Entry DOI: 10.7270/Q20V8G76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50254287 (CHEMBL4094588) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.83E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry and Biochemistry, The Ohio State University, Marion Campus, 1465 Mt. Vernon Avenue, Marion, Ohio 43302, United States. Curated by ChEMBL | Assay Description Inhibition of Electric eel AChE using acetylthiocholine as substrate after 7 min by Ellman's method | ACS Med Chem Lett 8: 622-627 (2017) Article DOI: 10.1021/acsmedchemlett.7b00037 BindingDB Entry DOI: 10.7270/Q20V8G76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50465496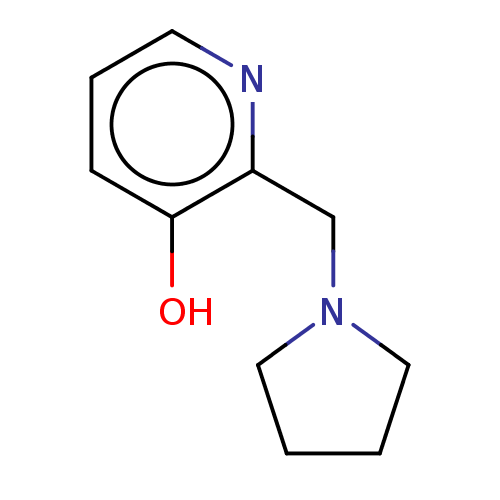 (CHEMBL4287206) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.02E+6 | n/a | n/a | n/a | n/a |
The Ohio State University-Marion Curated by ChEMBL | Assay Description Resurrection of isopropyl phosphate-aged electric eel AChE assessed as enzyme reactivation using acetylthiocholine as substrate at pH 9 after 1 day b... | J Med Chem 61: 7034-7042 (2018) Article DOI: 10.1021/acs.jmedchem.7b01620 BindingDB Entry DOI: 10.7270/Q23B62TV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50465496 (CHEMBL4287206) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.21E+6 | n/a | n/a | n/a | n/a |
The Ohio State University-Marion Curated by ChEMBL | Assay Description Resurrection of methylphosphonate-aged electric eel AChE assessed as enzyme reactivation using acetylthiocholine as substrate at pH 9 after 1 day by ... | J Med Chem 61: 7034-7042 (2018) Article DOI: 10.1021/acs.jmedchem.7b01620 BindingDB Entry DOI: 10.7270/Q23B62TV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||