 Found 93 hits with Last Name = 'freedman' and Initial = 'jm'
Found 93 hits with Last Name = 'freedman' and Initial = 'jm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM20461
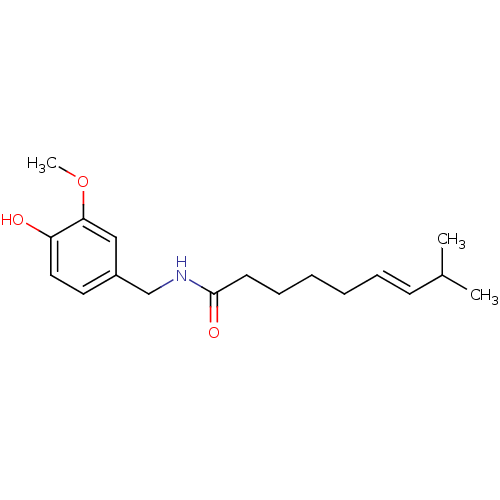
((6E)-N-[(4-hydroxy-3-methoxyphenyl)methyl]-8-methy...)Show InChI InChI=1S/C18H27NO3/c1-14(2)8-6-4-5-7-9-18(21)19-13-15-10-11-16(20)17(12-15)22-3/h6,8,10-12,14,20H,4-5,7,9,13H2,1-3H3,(H,19,21)/b8-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant TRPV1 |
Bioorg Med Chem Lett 20: 7137-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.023
BindingDB Entry DOI: 10.7270/Q29W0FQ5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50052442
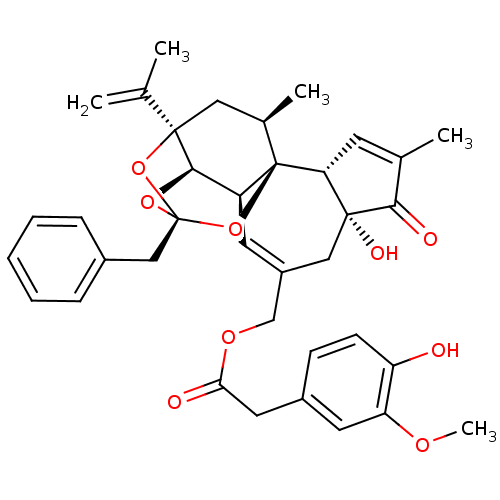
((4-Hydroxy-3-methoxy-phenyl)-acetic acid (2R,3S,3a...)Show SMILES COc1cc(CC(=O)OCC2=C[C@H]3[C@H]4O[C@]5(Cc6ccccc6)O[C@]4(C[C@@H](C)[C@]3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)ccc1O |r,t:10,35,THB:23:15:26.25.24:12| Show InChI InChI=1S/C37H40O9/c1-21(2)35-17-23(4)37-27(33(35)44-36(45-35,46-37)19-24-9-7-6-8-10-24)14-26(18-34(41)30(37)13-22(3)32(34)40)20-43-31(39)16-25-11-12-28(38)29(15-25)42-5/h6-15,23,27,30,33,38,41H,1,16-20H2,2-5H3/t23-,27+,30-,33-,34-,35-,36-,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant TRPV1 |
Bioorg Med Chem Lett 20: 7137-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.023
BindingDB Entry DOI: 10.7270/Q29W0FQ5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50196190
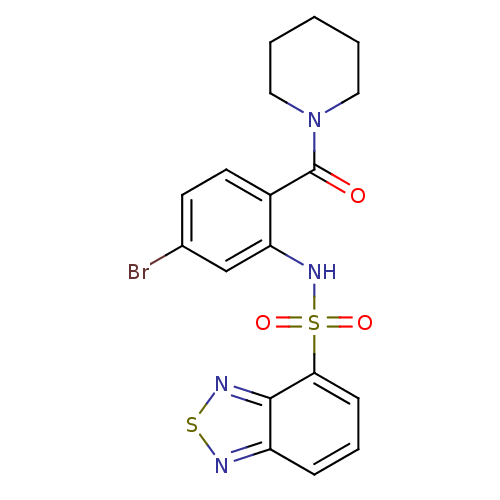
(1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...)Show SMILES Brc1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2cccc3nsnc23)c1 Show InChI InChI=1S/C18H17BrN4O3S2/c19-12-7-8-13(18(24)23-9-2-1-3-10-23)15(11-12)22-28(25,26)16-6-4-5-14-17(16)21-27-20-14/h4-8,11,22H,1-3,9-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK2R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50196191
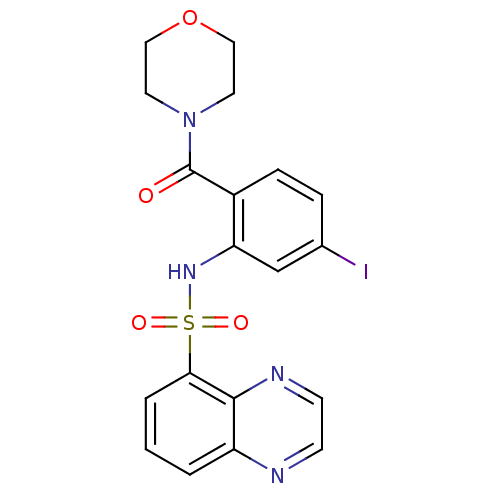
(4-[4-iodo-2-[(5-quinoxalinylsulfonyl)amino]benzoyl...)Show SMILES Ic1ccc(C(=O)N2CCOCC2)c(NS(=O)(=O)c2cccc3nccnc23)c1 Show InChI InChI=1S/C19H17IN4O4S/c20-13-4-5-14(19(25)24-8-10-28-11-9-24)16(12-13)23-29(26,27)17-3-1-2-15-18(17)22-7-6-21-15/h1-7,12,23H,8-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK2R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50196157
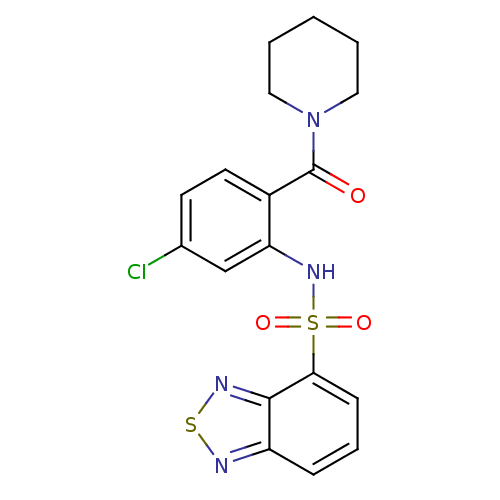
(1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...)Show SMILES Clc1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2cccc3nsnc23)c1 Show InChI InChI=1S/C18H17ClN4O3S2/c19-12-7-8-13(18(24)23-9-2-1-3-10-23)15(11-12)22-28(25,26)16-6-4-5-14-17(16)21-27-20-14/h4-8,11,22H,1-3,9-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK2R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50478086
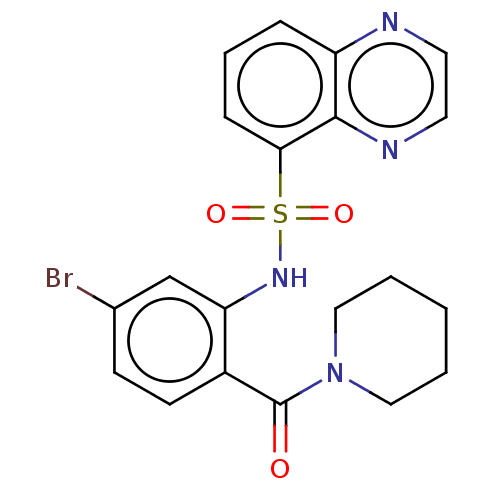
(CHEMBL261682)Show SMILES Brc1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2cccc3nccnc23)c1 Show InChI InChI=1S/C20H19BrN4O3S/c21-14-7-8-15(20(26)25-11-2-1-3-12-25)17(13-14)24-29(27,28)18-6-4-5-16-19(18)23-10-9-22-16/h4-10,13,24H,1-3,11-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK2R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50478090
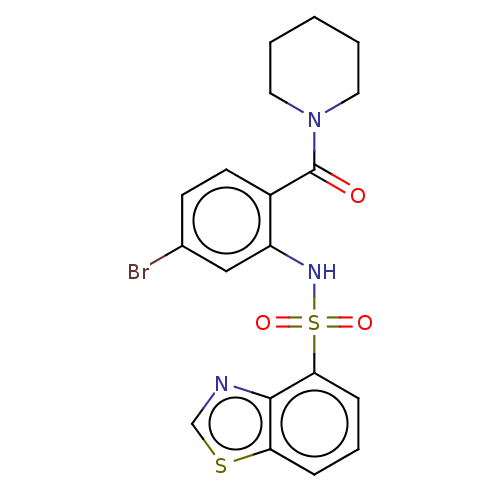
(CHEMBL259762)Show SMILES Brc1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2cccc3scnc23)c1 Show InChI InChI=1S/C19H18BrN3O3S2/c20-13-7-8-14(19(24)23-9-2-1-3-10-23)15(11-13)22-28(25,26)17-6-4-5-16-18(17)21-12-27-16/h4-8,11-12,22H,1-3,9-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK2R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50478094
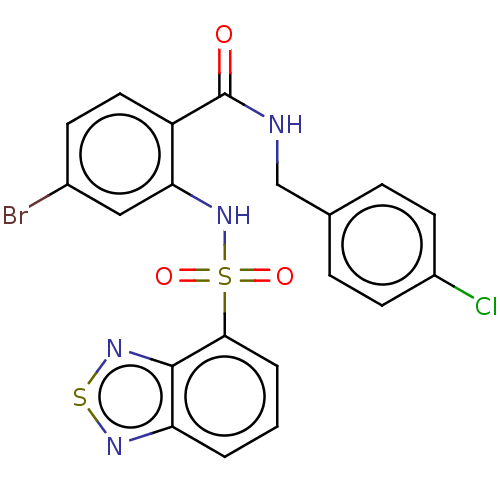
(CHEMBL408452)Show SMILES Clc1ccc(CNC(=O)c2ccc(Br)cc2NS(=O)(=O)c2cccc3nsnc23)cc1 Show InChI InChI=1S/C20H14BrClN4O3S2/c21-13-6-9-15(20(27)23-11-12-4-7-14(22)8-5-12)17(10-13)26-31(28,29)18-3-1-2-16-19(18)25-30-24-16/h1-10,26H,11H2,(H,23,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK2R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50478089
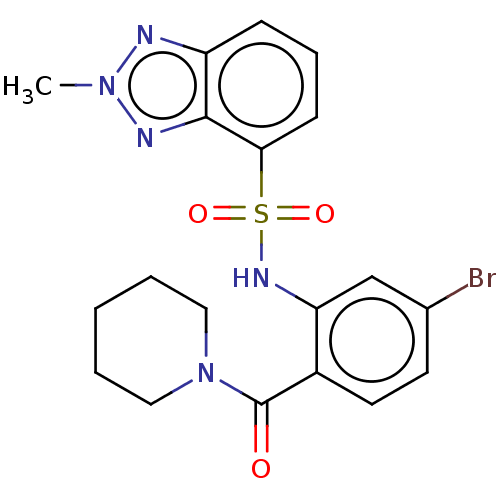
(CHEMBL261636)Show SMILES Cn1nc2cccc(c2n1)S(=O)(=O)Nc1cc(Br)ccc1C(=O)N1CCCCC1 Show InChI InChI=1S/C19H20BrN5O3S/c1-24-21-15-6-5-7-17(18(15)22-24)29(27,28)23-16-12-13(20)8-9-14(16)19(26)25-10-3-2-4-11-25/h5-9,12,23H,2-4,10-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK2R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50478092
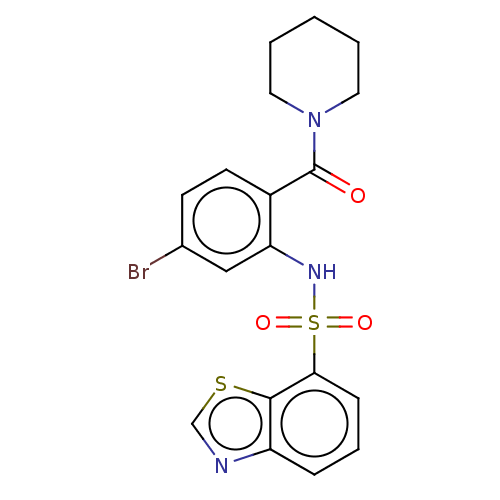
(CHEMBL410708)Show SMILES Brc1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2cccc3ncsc23)c1 Show InChI InChI=1S/C19H18BrN3O3S2/c20-13-7-8-14(19(24)23-9-2-1-3-10-23)16(11-13)22-28(25,26)17-6-4-5-15-18(17)27-12-21-15/h4-8,11-12,22H,1-3,9-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK2R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50196192
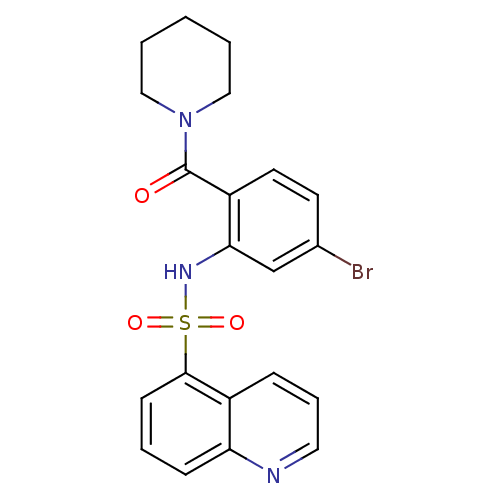
(1-[4-bromo-2-[(quinolin-5-ylsulfonyl)amino]-benzoy...)Show SMILES Brc1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2cccc3ncccc23)c1 Show InChI InChI=1S/C21H20BrN3O3S/c22-15-9-10-17(21(26)25-12-2-1-3-13-25)19(14-15)24-29(27,28)20-8-4-7-18-16(20)6-5-11-23-18/h4-11,14,24H,1-3,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK2R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50478085
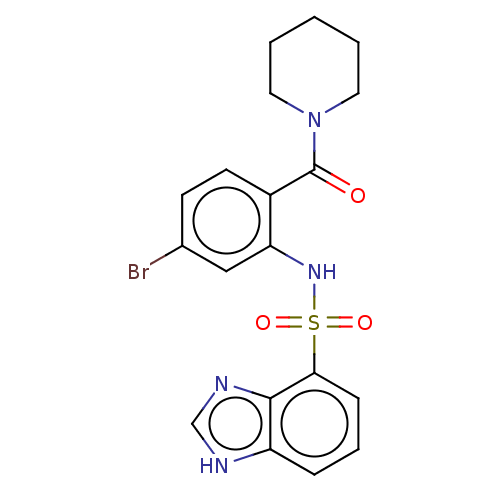
(CHEMBL259224)Show SMILES Brc1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2cccc3[nH]cnc23)c1 Show InChI InChI=1S/C19H19BrN4O3S/c20-13-7-8-14(19(25)24-9-2-1-3-10-24)16(11-13)23-28(26,27)17-6-4-5-15-18(17)22-12-21-15/h4-8,11-12,23H,1-3,9-10H2,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK2R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50196171
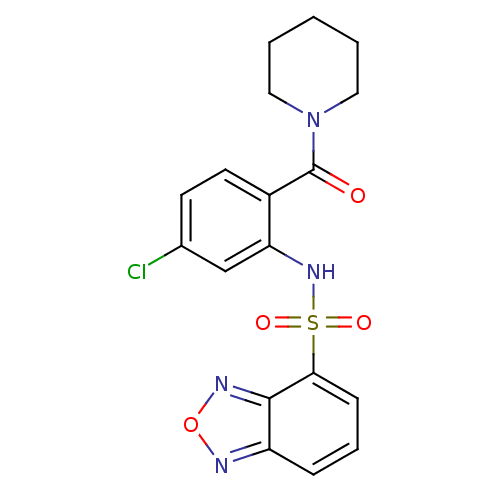
(1-[2-[(2,1,3-benzooxadiazol-4-ylsulfonyl)amino]-4-...)Show SMILES Clc1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2cccc3nonc23)c1 Show InChI InChI=1S/C18H17ClN4O4S/c19-12-7-8-13(18(24)23-9-2-1-3-10-23)15(11-12)22-28(25,26)16-6-4-5-14-17(16)21-27-20-14/h4-8,11,22H,1-3,9-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK2R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50478091
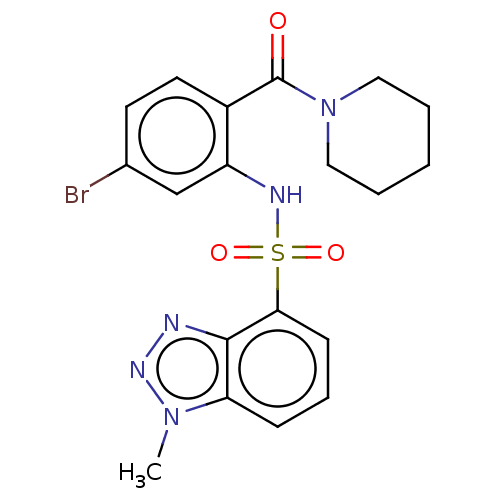
(CHEMBL259751)Show SMILES Cn1nnc2c(cccc12)S(=O)(=O)Nc1cc(Br)ccc1C(=O)N1CCCCC1 Show InChI InChI=1S/C19H20BrN5O3S/c1-24-16-6-5-7-17(18(16)21-23-24)29(27,28)22-15-12-13(20)8-9-14(15)19(26)25-10-3-2-4-11-25/h5-9,12,22H,2-4,10-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK2R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50478087
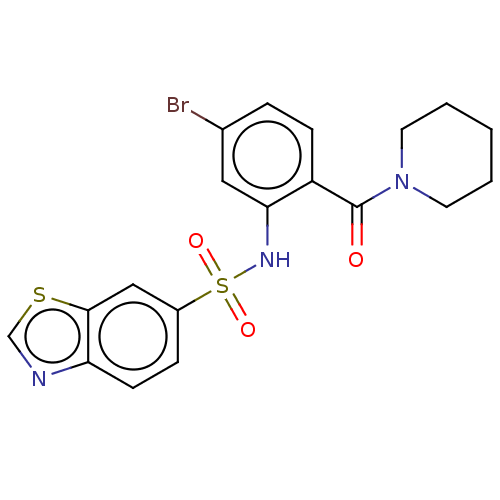
(CHEMBL409479)Show SMILES Brc1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2ccc3ncsc3c2)c1 Show InChI InChI=1S/C19H18BrN3O3S2/c20-13-4-6-15(19(24)23-8-2-1-3-9-23)17(10-13)22-28(25,26)14-5-7-16-18(11-14)27-12-21-16/h4-7,10-12,22H,1-3,8-9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK2R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50478093
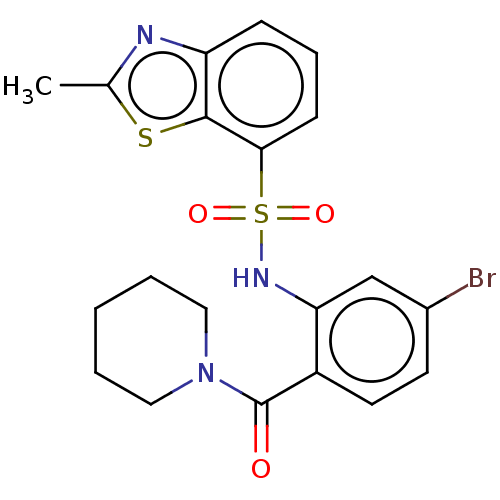
(CHEMBL410905)Show SMILES Cc1nc2cccc(c2s1)S(=O)(=O)Nc1cc(Br)ccc1C(=O)N1CCCCC1 Show InChI InChI=1S/C20H20BrN3O3S2/c1-13-22-16-6-5-7-18(19(16)28-13)29(26,27)23-17-12-14(21)8-9-15(17)20(25)24-10-3-2-4-11-24/h5-9,12,23H,2-4,10-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK2R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50478088
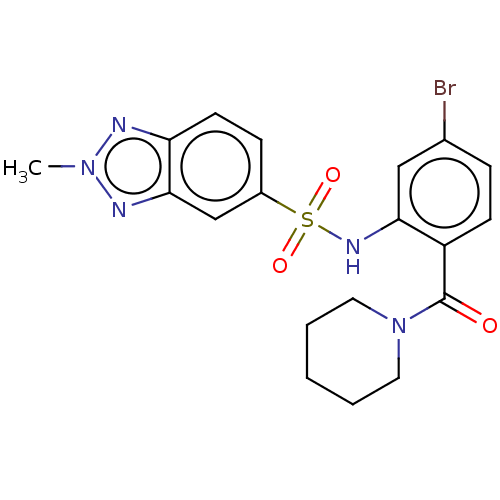
(CHEMBL412282)Show SMILES Cn1nc2ccc(cc2n1)S(=O)(=O)Nc1cc(Br)ccc1C(=O)N1CCCCC1 Show InChI InChI=1S/C19H20BrN5O3S/c1-24-21-16-8-6-14(12-18(16)22-24)29(27,28)23-17-11-13(20)5-7-15(17)19(26)25-9-3-2-4-10-25/h5-8,11-12,23H,2-4,9-10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK1R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50478090

(CHEMBL259762)Show SMILES Brc1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2cccc3scnc23)c1 Show InChI InChI=1S/C19H18BrN3O3S2/c20-13-7-8-14(19(24)23-9-2-1-3-10-23)15(11-13)22-28(25,26)17-6-4-5-16-18(17)21-12-27-16/h4-8,11-12,22H,1-3,9-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK1R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50478093

(CHEMBL410905)Show SMILES Cc1nc2cccc(c2s1)S(=O)(=O)Nc1cc(Br)ccc1C(=O)N1CCCCC1 Show InChI InChI=1S/C20H20BrN3O3S2/c1-13-22-16-6-5-7-18(19(16)28-13)29(26,27)23-17-12-14(21)8-9-15(17)20(25)24-10-3-2-4-11-24/h5-9,12,23H,2-4,10-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK1R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50478094

(CHEMBL408452)Show SMILES Clc1ccc(CNC(=O)c2ccc(Br)cc2NS(=O)(=O)c2cccc3nsnc23)cc1 Show InChI InChI=1S/C20H14BrClN4O3S2/c21-13-6-9-15(20(27)23-11-12-4-7-14(22)8-5-12)17(10-13)26-31(28,29)18-3-1-2-16-19(18)25-30-24-16/h1-10,26H,11H2,(H,23,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK1R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50196152
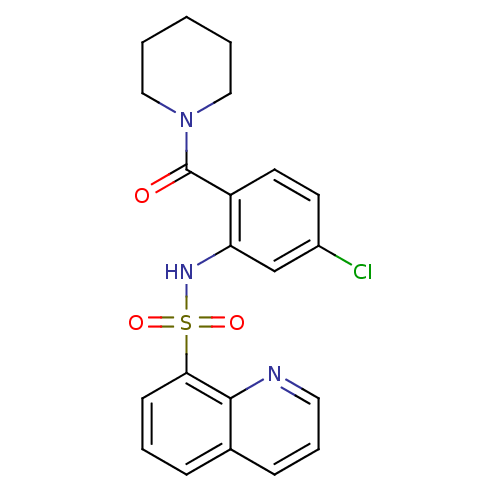
(1-[4-chloro-2-[(quinolin-8-ylsulfonyl)amino]-benzo...)Show SMILES Clc1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2cccc3cccnc23)c1 Show InChI InChI=1S/C21H20ClN3O3S/c22-16-9-10-17(21(26)25-12-2-1-3-13-25)18(14-16)24-29(27,28)19-8-4-6-15-7-5-11-23-20(15)19/h4-11,14,24H,1-3,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK1R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50196191

(4-[4-iodo-2-[(5-quinoxalinylsulfonyl)amino]benzoyl...)Show SMILES Ic1ccc(C(=O)N2CCOCC2)c(NS(=O)(=O)c2cccc3nccnc23)c1 Show InChI InChI=1S/C19H17IN4O4S/c20-13-4-5-14(19(25)24-8-10-28-11-9-24)16(12-13)23-29(26,27)17-3-1-2-15-18(17)22-7-6-21-15/h1-7,12,23H,8-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK1R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50478091

(CHEMBL259751)Show SMILES Cn1nnc2c(cccc12)S(=O)(=O)Nc1cc(Br)ccc1C(=O)N1CCCCC1 Show InChI InChI=1S/C19H20BrN5O3S/c1-24-16-6-5-7-17(18(16)21-23-24)29(27,28)22-15-12-13(20)8-9-14(15)19(26)25-10-3-2-4-11-25/h5-9,12,22H,2-4,10-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK1R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50196190

(1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...)Show SMILES Brc1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2cccc3nsnc23)c1 Show InChI InChI=1S/C18H17BrN4O3S2/c19-12-7-8-13(18(24)23-9-2-1-3-10-23)15(11-12)22-28(25,26)16-6-4-5-14-17(16)21-27-20-14/h4-8,11,22H,1-3,9-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK1R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50196157

(1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...)Show SMILES Clc1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2cccc3nsnc23)c1 Show InChI InChI=1S/C18H17ClN4O3S2/c19-12-7-8-13(18(24)23-9-2-1-3-10-23)15(11-12)22-28(25,26)16-6-4-5-14-17(16)21-27-20-14/h4-8,11,22H,1-3,9-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK1R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50196160
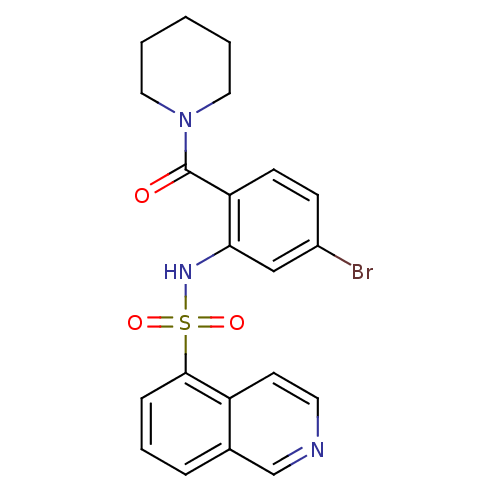
(1-[4-bromo-2-[(isoquinolin-5-ylsulfonyl)amino]-ben...)Show SMILES Brc1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2cccc3cnccc23)c1 Show InChI InChI=1S/C21H20BrN3O3S/c22-16-7-8-18(21(26)25-11-2-1-3-12-25)19(13-16)24-29(27,28)20-6-4-5-15-14-23-10-9-17(15)20/h4-10,13-14,24H,1-3,11-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK1R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50478092

(CHEMBL410708)Show SMILES Brc1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2cccc3ncsc23)c1 Show InChI InChI=1S/C19H18BrN3O3S2/c20-13-7-8-14(19(24)23-9-2-1-3-10-23)16(11-13)22-28(25,26)17-6-4-5-15-18(17)27-12-21-15/h4-8,11-12,22H,1-3,9-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK1R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50478088

(CHEMBL412282)Show SMILES Cn1nc2ccc(cc2n1)S(=O)(=O)Nc1cc(Br)ccc1C(=O)N1CCCCC1 Show InChI InChI=1S/C19H20BrN5O3S/c1-24-21-16-8-6-14(12-18(16)22-24)29(27,28)23-17-11-13(20)5-7-15(17)19(26)25-9-3-2-4-10-25/h5-8,11-12,23H,2-4,9-10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK2R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50196172
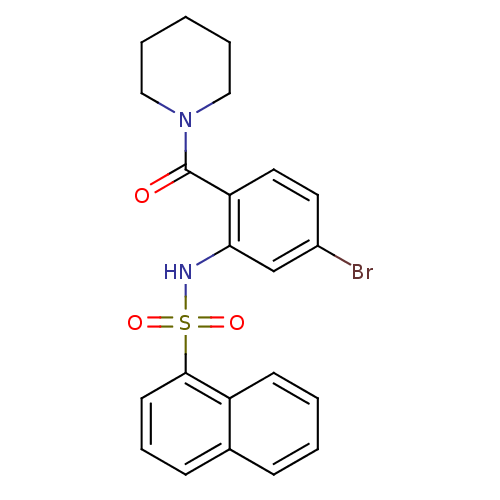
(1-[4-bromo-2-[[naphthalen-1-ylsulfonyl]amino]benzo...)Show SMILES Brc1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2cccc3ccccc23)c1 Show InChI InChI=1S/C22H21BrN2O3S/c23-17-11-12-19(22(26)25-13-4-1-5-14-25)20(15-17)24-29(27,28)21-10-6-8-16-7-2-3-9-18(16)21/h2-3,6-12,15,24H,1,4-5,13-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK2R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50196152

(1-[4-chloro-2-[(quinolin-8-ylsulfonyl)amino]-benzo...)Show SMILES Clc1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2cccc3cccnc23)c1 Show InChI InChI=1S/C21H20ClN3O3S/c22-16-9-10-17(21(26)25-12-2-1-3-13-25)18(14-16)24-29(27,28)19-8-4-6-15-7-5-11-23-20(15)19/h4-11,14,24H,1-3,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK2R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50196160

(1-[4-bromo-2-[(isoquinolin-5-ylsulfonyl)amino]-ben...)Show SMILES Brc1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2cccc3cnccc23)c1 Show InChI InChI=1S/C21H20BrN3O3S/c22-16-7-8-18(21(26)25-11-2-1-3-12-25)19(13-16)24-29(27,28)20-6-4-5-15-14-23-10-9-17(15)20/h4-10,13-14,24H,1-3,11-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK2R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50478089

(CHEMBL261636)Show SMILES Cn1nc2cccc(c2n1)S(=O)(=O)Nc1cc(Br)ccc1C(=O)N1CCCCC1 Show InChI InChI=1S/C19H20BrN5O3S/c1-24-21-15-6-5-7-17(18(15)22-24)29(27,28)23-16-12-13(20)8-9-14(16)19(26)25-10-3-2-4-11-25/h5-9,12,23H,2-4,10-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK1R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50196171

(1-[2-[(2,1,3-benzooxadiazol-4-ylsulfonyl)amino]-4-...)Show SMILES Clc1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2cccc3nonc23)c1 Show InChI InChI=1S/C18H17ClN4O4S/c19-12-7-8-13(18(24)23-9-2-1-3-10-23)15(11-12)22-28(25,26)16-6-4-5-14-17(16)21-27-20-14/h4-8,11,22H,1-3,9-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK1R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50478087

(CHEMBL409479)Show SMILES Brc1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2ccc3ncsc3c2)c1 Show InChI InChI=1S/C19H18BrN3O3S2/c20-13-4-6-15(19(24)23-8-2-1-3-9-23)17(10-13)22-28(25,26)14-5-7-16-18(11-14)27-12-21-16/h4-7,10-12,22H,1-3,8-9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK1R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50478086

(CHEMBL261682)Show SMILES Brc1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2cccc3nccnc23)c1 Show InChI InChI=1S/C20H19BrN4O3S/c21-14-7-8-15(20(26)25-11-2-1-3-12-25)17(13-14)24-29(27,28)18-6-4-5-16-19(18)23-10-9-22-16/h4-10,13,24H,1-3,11-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK1R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50196192

(1-[4-bromo-2-[(quinolin-5-ylsulfonyl)amino]-benzoy...)Show SMILES Brc1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2cccc3ncccc23)c1 Show InChI InChI=1S/C21H20BrN3O3S/c22-15-9-10-17(21(26)25-12-2-1-3-13-25)19(14-15)24-29(27,28)20-8-4-7-18-16(20)6-5-11-23-18/h4-11,14,24H,1-3,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK1R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50478085

(CHEMBL259224)Show SMILES Brc1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2cccc3[nH]cnc23)c1 Show InChI InChI=1S/C19H19BrN4O3S/c20-13-7-8-14(19(25)24-9-2-1-3-10-24)16(11-13)23-28(26,27)17-6-4-5-15-18(17)22-12-21-15/h4-8,11-12,23H,1-3,9-10H2,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK1R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50196172

(1-[4-bromo-2-[[naphthalen-1-ylsulfonyl]amino]benzo...)Show SMILES Brc1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2cccc3ccccc23)c1 Show InChI InChI=1S/C22H21BrN2O3S/c23-17-11-12-19(22(26)25-13-4-1-5-14-25)20(15-17)24-29(27,28)21-10-6-8-16-7-2-3-9-18(16)21/h2-3,6-12,15,24H,1,4-5,13-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8S from human CCK1R |
Bioorg Med Chem 16: 3917-25 (2008)
Article DOI: 10.1016/j.bmc.2008.01.059
BindingDB Entry DOI: 10.7270/Q2D79F5S |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50331116

(2-morpholino-N-(4-(trifluoromethyl)phenyl)-7-(3-(t...)Show SMILES FC(F)(F)c1ccc(Nc2nc(nc3CCN(CCc23)c2ncccc2C(F)(F)F)N2CCOCC2)cc1 Show InChI InChI=1S/C25H24F6N6O/c26-24(27,28)16-3-5-17(6-4-16)33-21-18-7-10-36(22-19(25(29,30)31)2-1-9-32-22)11-8-20(18)34-23(35-21)37-12-14-38-15-13-37/h1-6,9H,7-8,10-15H2,(H,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 assessed as inhibition of capsaicin-induced in intracellular calcium levels by cell-based FLIPR assay |
Bioorg Med Chem Lett 20: 7137-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.023
BindingDB Entry DOI: 10.7270/Q29W0FQ5 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50331150

(CHEMBL1290369 | N-(4-tert-butylphenyl)-7-(3-(trifl...)Show SMILES CC(C)(C)c1ccc(Nc2ncnc3CN(CCc23)c2ncccc2C(F)(F)F)cc1 Show InChI InChI=1S/C23H24F3N5/c1-22(2,3)15-6-8-16(9-7-15)30-20-17-10-12-31(13-19(17)28-14-29-20)21-18(23(24,25)26)5-4-11-27-21/h4-9,11,14H,10,12-13H2,1-3H3,(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 assessed as inhibition of capsaicin-induced in intracellular calcium levels by cell-based FLIPR assay |
Bioorg Med Chem Lett 20: 7137-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.023
BindingDB Entry DOI: 10.7270/Q29W0FQ5 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50254114
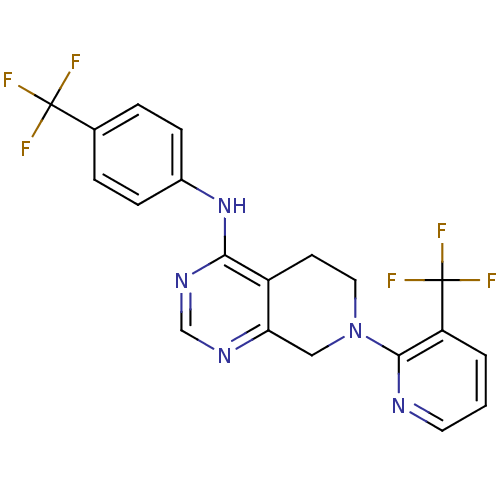
(CHEMBL461658 | N-(4-(trifluoromethyl)phenyl)-7-(3-...)Show SMILES FC(F)(F)c1ccc(Nc2ncnc3CN(CCc23)c2ncccc2C(F)(F)F)cc1 Show InChI InChI=1S/C20H15F6N5/c21-19(22,23)12-3-5-13(6-4-12)30-17-14-7-9-31(10-16(14)28-11-29-17)18-15(20(24,25)26)2-1-8-27-18/h1-6,8,11H,7,9-10H2,(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 assessed as inhibition of capsaicin-induced in intracellular calcium levels by cell-based FLIPR assay |
Bioorg Med Chem Lett 20: 7137-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.023
BindingDB Entry DOI: 10.7270/Q29W0FQ5 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50331131
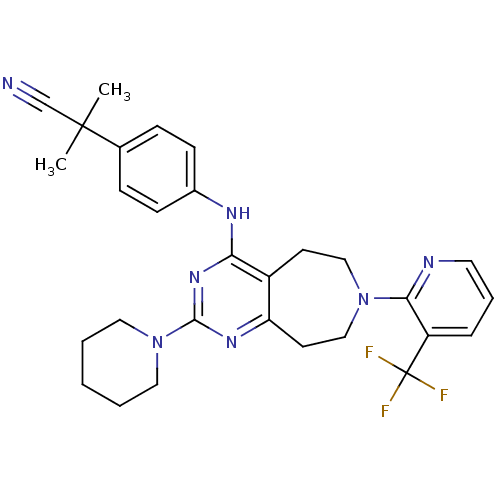
(2-methyl-2-(4-(2-(piperidin-1-yl)-7-(3-(trifluorom...)Show SMILES CC(C)(C#N)c1ccc(Nc2nc(nc3CCN(CCc23)c2ncccc2C(F)(F)F)N2CCCCC2)cc1 Show InChI InChI=1S/C29H32F3N7/c1-28(2,19-33)20-8-10-21(11-9-20)35-25-22-12-17-38(26-23(29(30,31)32)7-6-14-34-26)18-13-24(22)36-27(37-25)39-15-4-3-5-16-39/h6-11,14H,3-5,12-13,15-18H2,1-2H3,(H,35,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 assessed as inhibition of capsaicin-induced in intracellular calcium levels by cell-based FLIPR assay |
Bioorg Med Chem Lett 20: 7137-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.023
BindingDB Entry DOI: 10.7270/Q29W0FQ5 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50331154
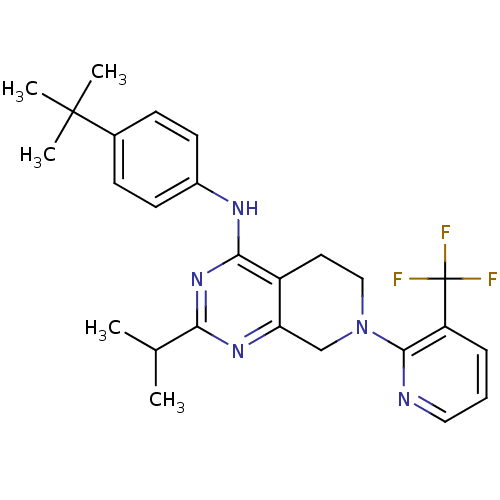
(CHEMBL1290584 | N-(4-tert-butylphenyl)-2-isopropyl...)Show SMILES CC(C)c1nc2CN(CCc2c(Nc2ccc(cc2)C(C)(C)C)n1)c1ncccc1C(F)(F)F Show InChI InChI=1S/C26H30F3N5/c1-16(2)22-32-21-15-34(24-20(26(27,28)29)7-6-13-30-24)14-12-19(21)23(33-22)31-18-10-8-17(9-11-18)25(3,4)5/h6-11,13,16H,12,14-15H2,1-5H3,(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 assessed as inhibition of capsaicin-induced in intracellular calcium levels by cell-based FLIPR assay |
Bioorg Med Chem Lett 20: 7137-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.023
BindingDB Entry DOI: 10.7270/Q29W0FQ5 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20461

((6E)-N-[(4-hydroxy-3-methoxyphenyl)methyl]-8-methy...)Show InChI InChI=1S/C18H27NO3/c1-14(2)8-6-4-5-7-9-18(21)19-13-15-10-11-16(20)17(12-15)22-3/h6,8,10-12,14,20H,4-5,7,9,13H2,1-3H3,(H,19,21)/b8-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat TRPV1 |
Bioorg Med Chem Lett 20: 7137-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.023
BindingDB Entry DOI: 10.7270/Q29W0FQ5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50331117
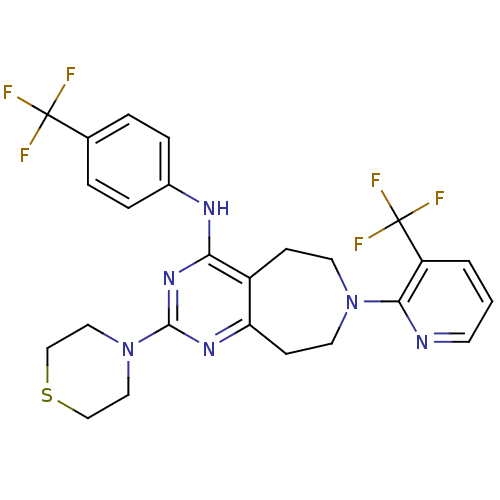
(2-thiomorpholino-N-(4-(trifluoromethyl)phenyl)-7-(...)Show SMILES FC(F)(F)c1ccc(Nc2nc(nc3CCN(CCc23)c2ncccc2C(F)(F)F)N2CCSCC2)cc1 Show InChI InChI=1S/C25H24F6N6S/c26-24(27,28)16-3-5-17(6-4-16)33-21-18-7-10-36(22-19(25(29,30)31)2-1-9-32-22)11-8-20(18)34-23(35-21)37-12-14-38-15-13-37/h1-6,9H,7-8,10-15H2,(H,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 assessed as inhibition of capsaicin-induced in intracellular calcium levels by cell-based FLIPR assay |
Bioorg Med Chem Lett 20: 7137-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.023
BindingDB Entry DOI: 10.7270/Q29W0FQ5 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50331124
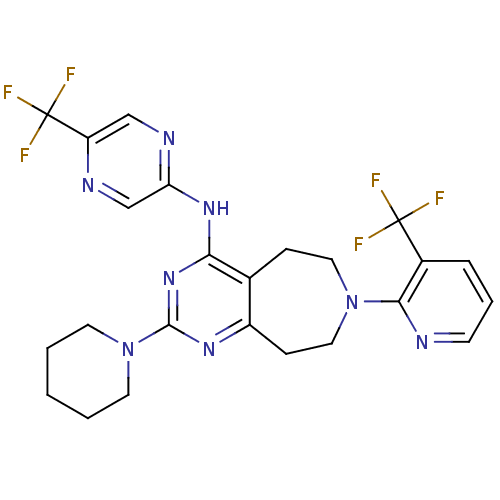
(2-(piperidin-1-yl)-N-(5-(trifluoromethyl)pyrazin-2...)Show SMILES FC(F)(F)c1cnc(Nc2nc(nc3CCN(CCc23)c2ncccc2C(F)(F)F)N2CCCCC2)cn1 Show InChI InChI=1S/C24H24F6N8/c25-23(26,27)16-5-4-8-31-21(16)37-11-6-15-17(7-12-37)34-22(38-9-2-1-3-10-38)36-20(15)35-19-14-32-18(13-33-19)24(28,29)30/h4-5,8,13-14H,1-3,6-7,9-12H2,(H,33,34,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 assessed as inhibition of capsaicin-induced in intracellular calcium levels by cell-based FLIPR assay |
Bioorg Med Chem Lett 20: 7137-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.023
BindingDB Entry DOI: 10.7270/Q29W0FQ5 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50331157

(CHEMBL1290704 | N-(4-tert-butylphenyl)-7-(3-(trifl...)Show SMILES CC(C)(C)c1ccc(Nc2ncnc3CCN(CCc23)c2ncccc2C(F)(F)F)cc1 Show InChI InChI=1S/C24H26F3N5/c1-23(2,3)16-6-8-17(9-7-16)31-21-18-10-13-32(14-11-20(18)29-15-30-21)22-19(24(25,26)27)5-4-12-28-22/h4-9,12,15H,10-11,13-14H2,1-3H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 assessed as inhibition of capsaicin-induced in intracellular calcium levels by cell-based FLIPR assay |
Bioorg Med Chem Lett 20: 7137-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.023
BindingDB Entry DOI: 10.7270/Q29W0FQ5 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50331137
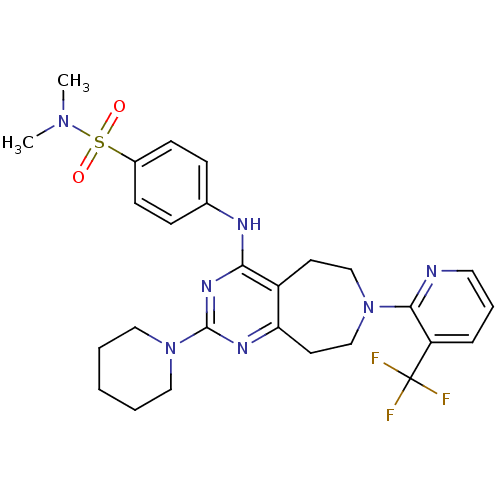
(CHEMBL1289818 | N,N-dimethyl-4-(2-(piperidin-1-yl)...)Show SMILES CN(C)S(=O)(=O)c1ccc(Nc2nc(nc3CCN(CCc23)c2ncccc2C(F)(F)F)N2CCCCC2)cc1 Show InChI InChI=1S/C27H32F3N7O2S/c1-35(2)40(38,39)20-10-8-19(9-11-20)32-24-21-12-17-36(25-22(27(28,29)30)7-6-14-31-25)18-13-23(21)33-26(34-24)37-15-4-3-5-16-37/h6-11,14H,3-5,12-13,15-18H2,1-2H3,(H,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 assessed as inhibition of capsaicin-induced in intracellular calcium levels by cell-based FLIPR assay |
Bioorg Med Chem Lett 20: 7137-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.023
BindingDB Entry DOI: 10.7270/Q29W0FQ5 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50331160
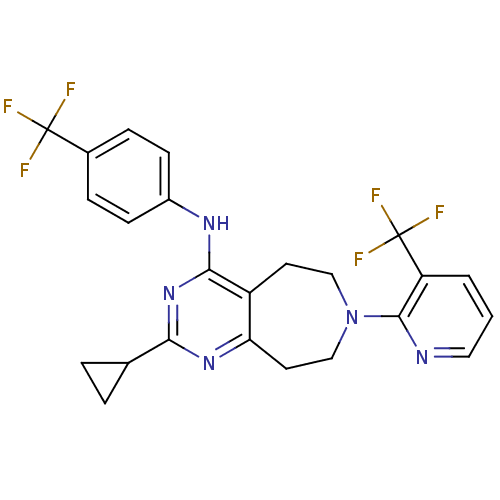
(2-cyclopropyl-N-(4-(trifluoromethyl)phenyl)-7-(3-(...)Show SMILES FC(F)(F)c1ccc(Nc2nc(nc3CCN(CCc23)c2ncccc2C(F)(F)F)C2CC2)cc1 Show InChI InChI=1S/C24H21F6N5/c25-23(26,27)15-5-7-16(8-6-15)32-21-17-9-12-35(22-18(24(28,29)30)2-1-11-31-22)13-10-19(17)33-20(34-21)14-3-4-14/h1-2,5-8,11,14H,3-4,9-10,12-13H2,(H,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 assessed as inhibition of capsaicin-induced in intracellular calcium levels by cell-based FLIPR assay |
Bioorg Med Chem Lett 20: 7137-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.023
BindingDB Entry DOI: 10.7270/Q29W0FQ5 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50331111
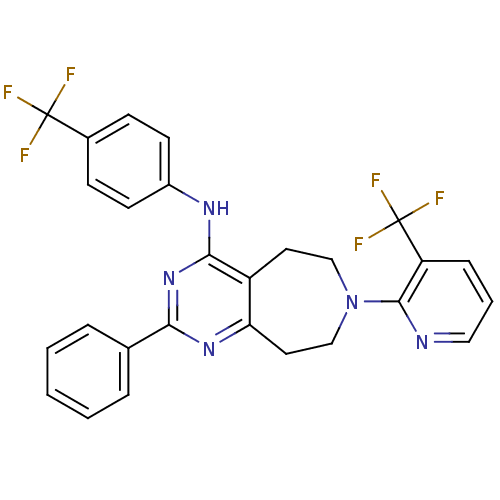
(2-phenyl-N-(4-(trifluoromethyl)phenyl)-7-(3-(trifl...)Show SMILES FC(F)(F)c1ccc(Nc2nc(nc3CCN(CCc23)c2ncccc2C(F)(F)F)-c2ccccc2)cc1 Show InChI InChI=1S/C27H21F6N5/c28-26(29,30)18-8-10-19(11-9-18)35-24-20-12-15-38(25-21(27(31,32)33)7-4-14-34-25)16-13-22(20)36-23(37-24)17-5-2-1-3-6-17/h1-11,14H,12-13,15-16H2,(H,35,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 assessed as inhibition of capsaicin-induced in intracellular calcium levels by cell-based FLIPR assay |
Bioorg Med Chem Lett 20: 7137-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.023
BindingDB Entry DOI: 10.7270/Q29W0FQ5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data