 Found 42 hits with Last Name = 'frimpong' and Initial = 'k'
Found 42 hits with Last Name = 'frimpong' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243396
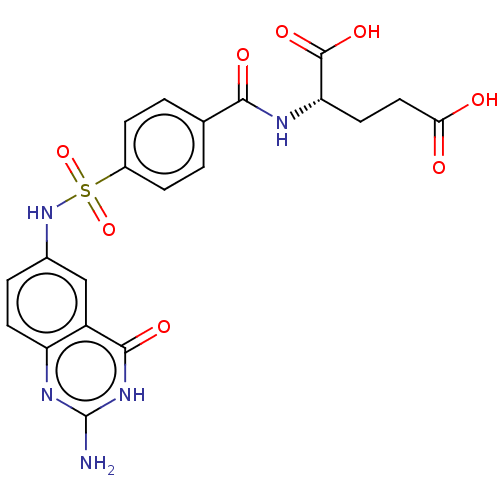
(CHEMBL1231520)Show SMILES Nc1nc2ccc(NS(=O)(=O)c3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C20H19N5O8S/c21-20-23-14-6-3-11(9-13(14)18(29)24-20)25-34(32,33)12-4-1-10(2-5-12)17(28)22-15(19(30)31)7-8-16(26)27/h1-6,9,15,25H,7-8H2,(H,22,28)(H,26,27)(H,30,31)(H3,21,23,24,29)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human AICARFT |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243463
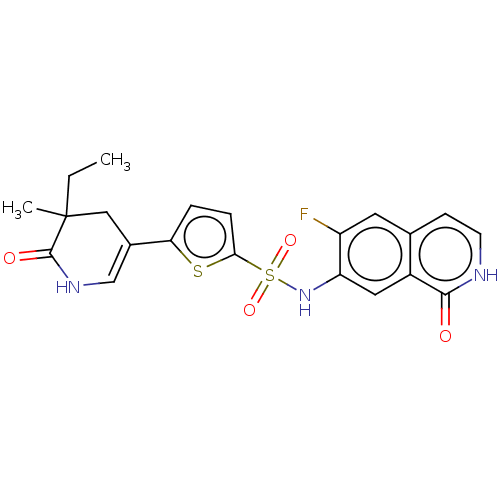
(CHEMBL4100363)Show SMILES CCC1(C)CC(=CNC1=O)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |c:5| Show InChI InChI=1S/C21H20FN3O4S2/c1-3-21(2)10-13(11-24-20(21)27)17-4-5-18(30-17)31(28,29)25-16-9-14-12(8-15(16)22)6-7-23-19(14)26/h4-9,11,25H,3,10H2,1-2H3,(H,23,26)(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243462
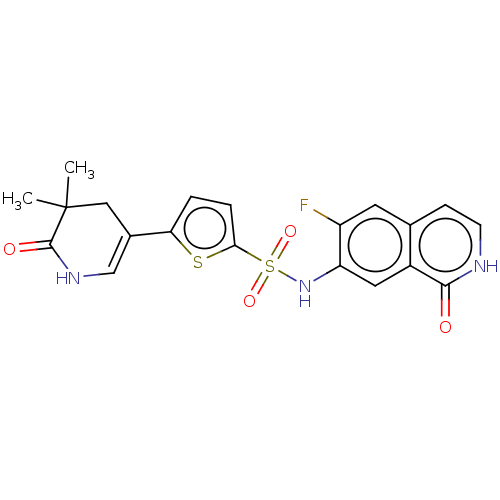
(CHEMBL4083899)Show SMILES CC1(C)CC(=CNC1=O)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |c:4| Show InChI InChI=1S/C20H18FN3O4S2/c1-20(2)9-12(10-23-19(20)26)16-3-4-17(29-16)30(27,28)24-15-8-13-11(7-14(15)21)5-6-22-18(13)25/h3-8,10,24H,9H2,1-2H3,(H,22,25)(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243461
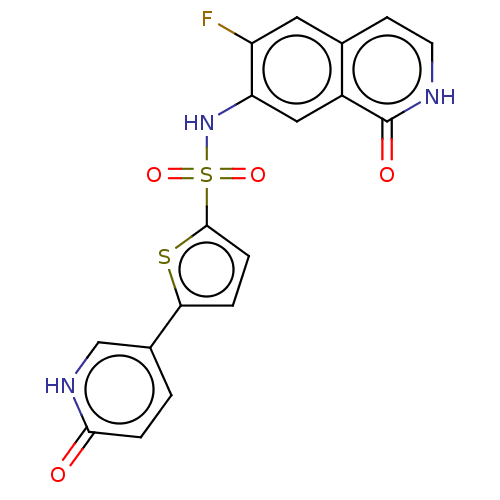
(CHEMBL4075503)Show SMILES Fc1cc2cc[nH]c(=O)c2cc1NS(=O)(=O)c1ccc(s1)-c1ccc(=O)[nH]c1 Show InChI InChI=1S/C18H12FN3O4S2/c19-13-7-10-5-6-20-18(24)12(10)8-14(13)22-28(25,26)17-4-2-15(27-17)11-1-3-16(23)21-9-11/h1-9,22H,(H,20,24)(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243486
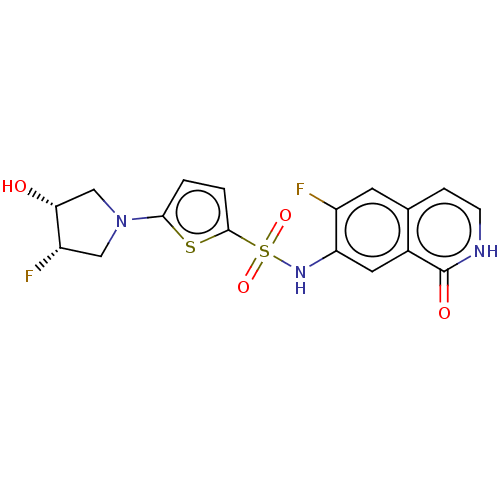
(CHEMBL4081385)Show SMILES O[C@@H]1CN(C[C@@H]1F)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C17H15F2N3O4S2/c18-11-5-9-3-4-20-17(24)10(9)6-13(11)21-28(25,26)16-2-1-15(27-16)22-7-12(19)14(23)8-22/h1-6,12,14,21,23H,7-8H2,(H,20,24)/t12-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243487

(CHEMBL4091668)Show SMILES O[C@@H]1CN(CC1(F)F)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C17H14F3N3O4S2/c18-11-5-9-3-4-21-16(25)10(9)6-12(11)22-29(26,27)15-2-1-14(28-15)23-7-13(24)17(19,20)8-23/h1-6,13,22,24H,7-8H2,(H,21,25)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243441
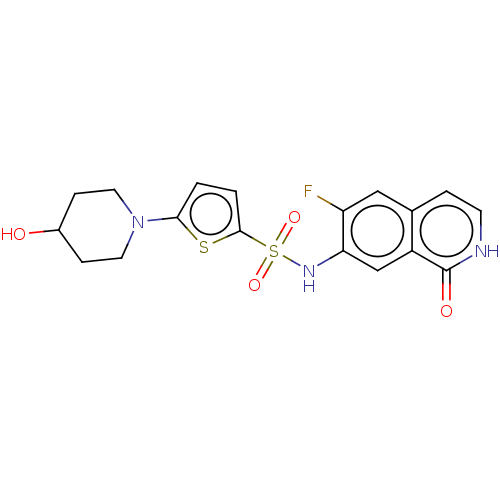
(CHEMBL4076500)Show SMILES OC1CCN(CC1)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F Show InChI InChI=1S/C18H18FN3O4S2/c19-14-9-11-3-6-20-18(24)13(11)10-15(14)21-28(25,26)17-2-1-16(27-17)22-7-4-12(23)5-8-22/h1-3,6,9-10,12,21,23H,4-5,7-8H2,(H,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243443
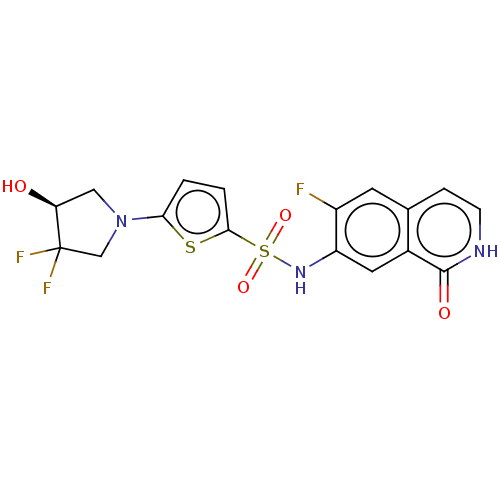
(CHEMBL4070790)Show SMILES O[C@H]1CN(CC1(F)F)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C17H14F3N3O4S2/c18-11-5-9-3-4-21-16(25)10(9)6-12(11)22-29(26,27)15-2-1-14(28-15)23-7-13(24)17(19,20)8-23/h1-6,13,22,24H,7-8H2,(H,21,25)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243485
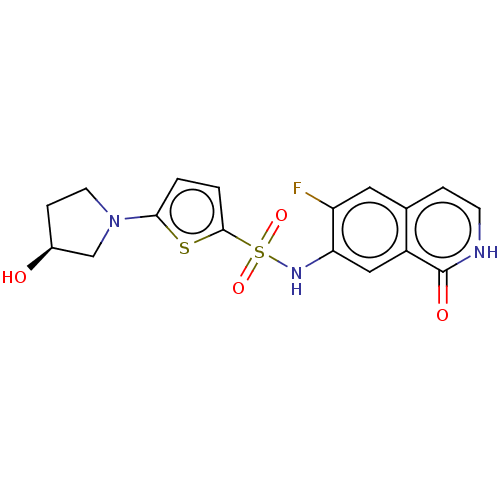
(CHEMBL4074469)Show SMILES O[C@H]1CCN(C1)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C17H16FN3O4S2/c18-13-7-10-3-5-19-17(23)12(10)8-14(13)20-27(24,25)16-2-1-15(26-16)21-6-4-11(22)9-21/h1-3,5,7-8,11,20,22H,4,6,9H2,(H,19,23)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243415
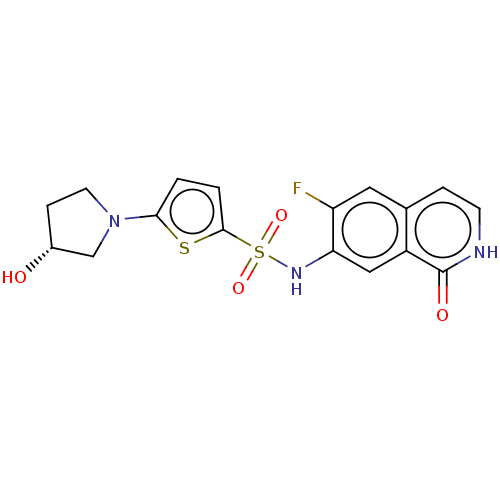
(CHEMBL4063104)Show SMILES O[C@@H]1CCN(C1)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C17H16FN3O4S2/c18-13-7-10-3-5-19-17(23)12(10)8-14(13)20-27(24,25)16-2-1-15(26-16)21-6-4-11(22)9-21/h1-3,5,7-8,11,20,22H,4,6,9H2,(H,19,23)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243434
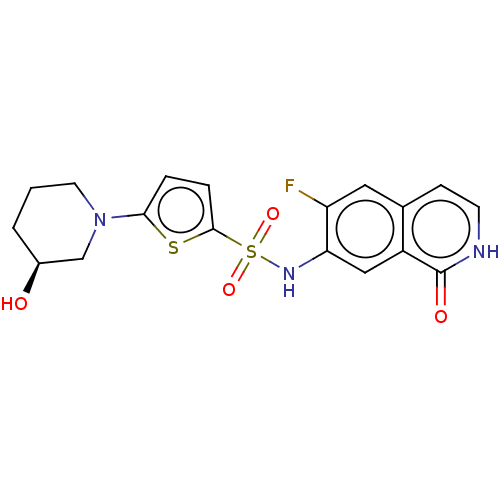
(CHEMBL4079085)Show SMILES O[C@H]1CCCN(C1)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C18H18FN3O4S2/c19-14-8-11-5-6-20-18(24)13(11)9-15(14)21-28(25,26)17-4-3-16(27-17)22-7-1-2-12(23)10-22/h3-6,8-9,12,21,23H,1-2,7,10H2,(H,20,24)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243442
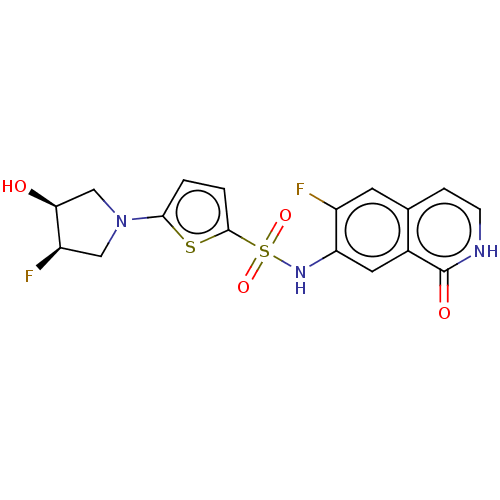
(CHEMBL4099409)Show SMILES O[C@H]1CN(C[C@H]1F)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C17H15F2N3O4S2/c18-11-5-9-3-4-20-17(24)10(9)6-13(11)21-28(25,26)16-2-1-15(27-16)22-7-12(19)14(23)8-22/h1-6,12,14,21,23H,7-8H2,(H,20,24)/t12-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243433
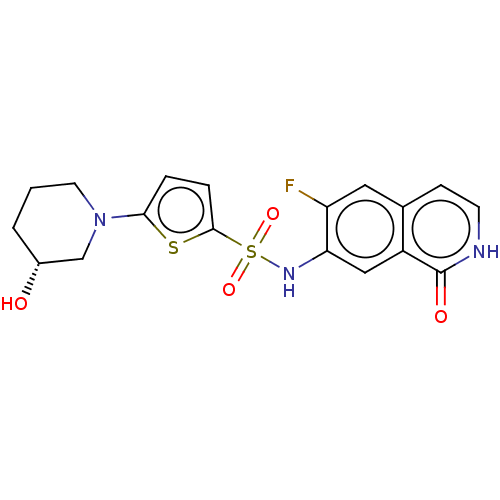
(CHEMBL4101204)Show SMILES O[C@@H]1CCCN(C1)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C18H18FN3O4S2/c19-14-8-11-5-6-20-18(24)13(11)9-15(14)21-28(25,26)17-4-3-16(27-17)22-7-1-2-12(23)10-22/h3-6,8-9,12,21,23H,1-2,7,10H2,(H,20,24)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243477
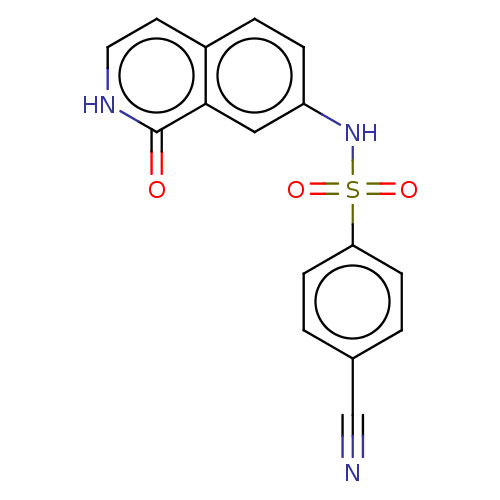
(CHEMBL4092503)Show SMILES O=c1[nH]ccc2ccc(NS(=O)(=O)c3ccc(cc3)C#N)cc12 Show InChI InChI=1S/C16H11N3O3S/c17-10-11-1-5-14(6-2-11)23(21,22)19-13-4-3-12-7-8-18-16(20)15(12)9-13/h1-9,19H,(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 608 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243414
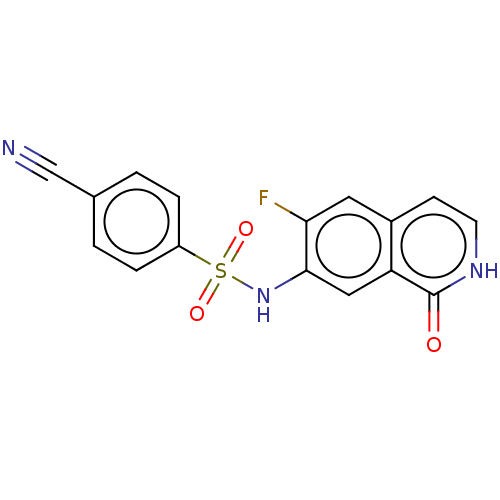
(CHEMBL4084757)Show SMILES Fc1cc2cc[nH]c(=O)c2cc1NS(=O)(=O)c1ccc(cc1)C#N Show InChI InChI=1S/C16H10FN3O3S/c17-14-7-11-5-6-19-16(21)13(11)8-15(14)20-24(22,23)12-3-1-10(9-18)2-4-12/h1-8,20H,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 723 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243396

(CHEMBL1231520)Show SMILES Nc1nc2ccc(NS(=O)(=O)c3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C20H19N5O8S/c21-20-23-14-6-3-11(9-13(14)18(29)24-20)25-34(32,33)12-4-1-10(2-5-12)17(28)22-15(19(30)31)7-8-16(26)27/h1-6,9,15,25H,7-8H2,(H,22,28)(H,26,27)(H,30,31)(H3,21,23,24,29)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243413
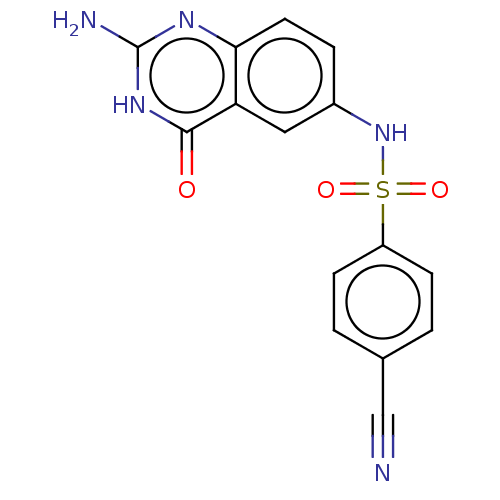
(CHEMBL4077027)Show SMILES Nc1nc2ccc(NS(=O)(=O)c3ccc(cc3)C#N)cc2c(=O)[nH]1 Show InChI InChI=1S/C15H11N5O3S/c16-8-9-1-4-11(5-2-9)24(22,23)20-10-3-6-13-12(7-10)14(21)19-15(17)18-13/h1-7,20H,(H3,17,18,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243508
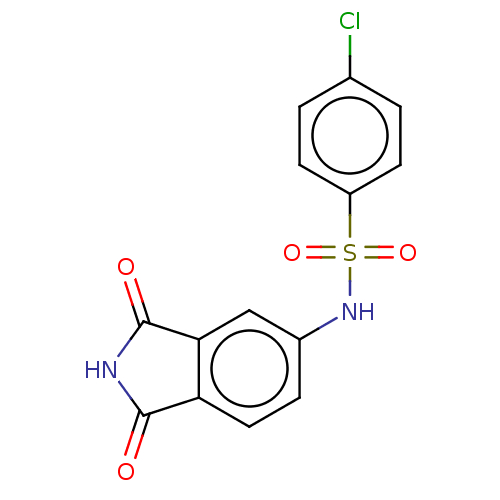
(CHEMBL4101760)Show SMILES Clc1ccc(cc1)S(=O)(=O)Nc1ccc2C(=O)NC(=O)c2c1 Show InChI InChI=1S/C14H9ClN2O4S/c15-8-1-4-10(5-2-8)22(20,21)17-9-3-6-11-12(7-9)14(19)16-13(11)18/h1-7,17H,(H,16,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243397
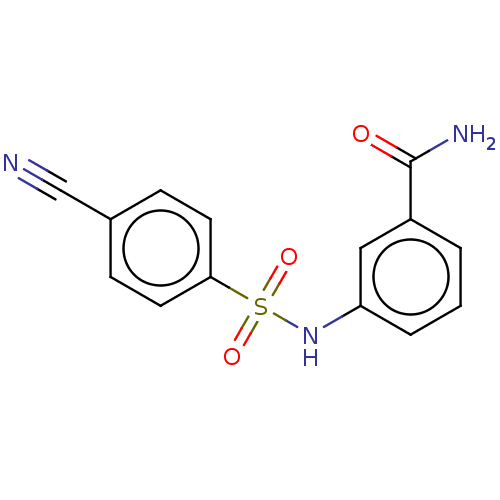
(CHEMBL4100255)Show InChI InChI=1S/C14H11N3O3S/c15-9-10-4-6-13(7-5-10)21(19,20)17-12-3-1-2-11(8-12)14(16)18/h1-8,17H,(H2,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Serine hydroxymethyltransferase, cytosolic
(Homo sapiens (Human)) | BDBM50243415

(CHEMBL4063104)Show SMILES O[C@@H]1CCN(C1)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C17H16FN3O4S2/c18-13-7-10-3-5-19-17(23)12(10)8-14(13)20-27(24,25)16-2-1-15(26-16)21-6-4-11(22)9-21/h1-3,5,7-8,11,20,22H,4,6,9H2,(H,19,23)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of SHMT1 (unknown origin) using serine/THF as substrate after 50 mins by HPLC method |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243398
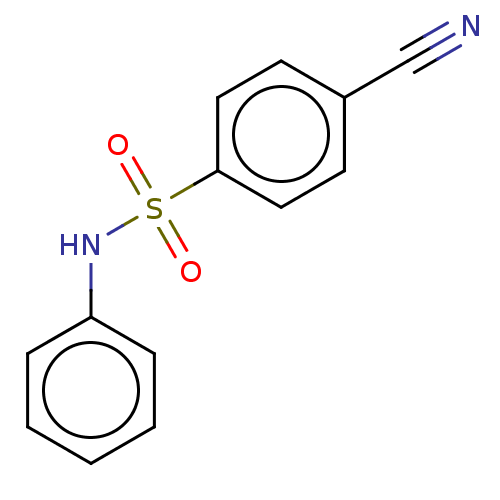
(CHEMBL4082237)Show InChI InChI=1S/C13H10N2O2S/c14-10-11-6-8-13(9-7-11)18(16,17)15-12-4-2-1-3-5-12/h1-9,15H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM50243415

(CHEMBL4063104)Show SMILES O[C@@H]1CCN(C1)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C17H16FN3O4S2/c18-13-7-10-3-5-19-17(23)12(10)8-14(13)20-27(24,25)16-2-1-15(26-16)21-6-4-11(22)9-21/h1-3,5,7-8,11,20,22H,4,6,9H2,(H,19,23)/t11-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MTHFD2 (unknown origin) using NAD/5,10-methylene THF as substrate after 30 mins by HPLC method |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase 2, mitochondrial
(Homo sapiens) | BDBM50243415

(CHEMBL4063104)Show SMILES O[C@@H]1CCN(C1)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C17H16FN3O4S2/c18-13-7-10-3-5-19-17(23)12(10)8-14(13)20-27(24,25)16-2-1-15(26-16)21-6-4-11(22)9-21/h1-3,5,7-8,11,20,22H,4,6,9H2,(H,19,23)/t11-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MTHFD2L (unknown origin) using NAD/5,10-methylene THF as substrate after 2 hrs by HPLC method |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
C-1-tetrahydrofolate synthase, cytoplasmic
(Homo sapiens (Human)) | BDBM50243415

(CHEMBL4063104)Show SMILES O[C@@H]1CCN(C1)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C17H16FN3O4S2/c18-13-7-10-3-5-19-17(23)12(10)8-14(13)20-27(24,25)16-2-1-15(26-16)21-6-4-11(22)9-21/h1-3,5,7-8,11,20,22H,4,6,9H2,(H,19,23)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MTHFD1 (unknown origin) using NADP/5,10-methylene THF as substrate after 30 mins by HPLC method |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15B
(Rattus norvegicus) | BDBM50243415

(CHEMBL4063104)Show SMILES O[C@@H]1CCN(C1)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C17H16FN3O4S2/c18-13-7-10-3-5-19-17(23)12(10)8-14(13)20-27(24,25)16-2-1-15(26-16)21-6-4-11(22)9-21/h1-3,5,7-8,11,20,22H,4,6,9H2,(H,19,23)/t11-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of thymidylate synthase (unknown origin) using dUMP/5,10-methylene THF as substrate after 1 hr by mass spectrometric method |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243415

(CHEMBL4063104)Show SMILES O[C@@H]1CCN(C1)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C17H16FN3O4S2/c18-13-7-10-3-5-19-17(23)12(10)8-14(13)20-27(24,25)16-2-1-15(26-16)21-6-4-11(22)9-21/h1-3,5,7-8,11,20,22H,4,6,9H2,(H,19,23)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 356 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AICARFT in human NCI-H460 cells assessed as increase in ZMP levels using regular folate media after 16 hrs by LC-MS method |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243434

(CHEMBL4079085)Show SMILES O[C@H]1CCCN(C1)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C18H18FN3O4S2/c19-14-8-11-5-6-20-18(24)13(11)9-15(14)21-28(25,26)17-4-3-16(27-17)22-7-1-2-12(23)10-22/h3-6,8-9,12,21,23H,1-2,7,10H2,(H,20,24)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AICARFT in human NCI-H460 cells assessed as increase in ZMP levels using low folate media after 16 hrs by LC-MS method |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243463

(CHEMBL4100363)Show SMILES CCC1(C)CC(=CNC1=O)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |c:5| Show InChI InChI=1S/C21H20FN3O4S2/c1-3-21(2)10-13(11-24-20(21)27)17-4-5-18(30-17)31(28,29)25-16-9-14-12(8-15(16)22)6-7-23-19(14)26/h4-9,11,25H,3,10H2,1-2H3,(H,23,26)(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AICARFT in human NCI-H460 cells assessed as increase in ZMP levels using low folate media after 16 hrs by LC-MS method |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243477

(CHEMBL4092503)Show SMILES O=c1[nH]ccc2ccc(NS(=O)(=O)c3ccc(cc3)C#N)cc12 Show InChI InChI=1S/C16H11N3O3S/c17-10-11-1-5-14(6-2-11)23(21,22)19-13-4-3-12-7-8-18-16(20)15(12)9-13/h1-9,19H,(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 567 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AICARFT in human NCI-H460 cells assessed as increase in ZMP levels using low folate media after 16 hrs by LC-MS method |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243433

(CHEMBL4101204)Show SMILES O[C@@H]1CCCN(C1)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C18H18FN3O4S2/c19-14-8-11-5-6-20-18(24)13(11)9-15(14)21-28(25,26)17-4-3-16(27-17)22-7-1-2-12(23)10-22/h3-6,8-9,12,21,23H,1-2,7,10H2,(H,20,24)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 68 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AICARFT in human NCI-H460 cells assessed as increase in ZMP levels using low folate media after 16 hrs by LC-MS method |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243485

(CHEMBL4074469)Show SMILES O[C@H]1CCN(C1)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C17H16FN3O4S2/c18-13-7-10-3-5-19-17(23)12(10)8-14(13)20-27(24,25)16-2-1-15(26-16)21-6-4-11(22)9-21/h1-3,5,7-8,11,20,22H,4,6,9H2,(H,19,23)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AICARFT in human NCI-H460 cells assessed as increase in ZMP levels using low folate media after 16 hrs by LC-MS method |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243414

(CHEMBL4084757)Show SMILES Fc1cc2cc[nH]c(=O)c2cc1NS(=O)(=O)c1ccc(cc1)C#N Show InChI InChI=1S/C16H10FN3O3S/c17-14-7-11-5-6-19-16(21)13(11)8-15(14)20-24(22,23)12-3-1-10(9-18)2-4-12/h1-8,20H,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.81E+3 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AICARFT in human NCI-H460 cells assessed as increase in ZMP levels using low folate media after 16 hrs by LC-MS method |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243415

(CHEMBL4063104)Show SMILES O[C@@H]1CCN(C1)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C17H16FN3O4S2/c18-13-7-10-3-5-19-17(23)12(10)8-14(13)20-27(24,25)16-2-1-15(26-16)21-6-4-11(22)9-21/h1-3,5,7-8,11,20,22H,4,6,9H2,(H,19,23)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 73 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AICARFT in human MDA-MB-231 cells assessed as increase in ZMP levels using regular folate media after 16 hrs by LC-MS method |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243415

(CHEMBL4063104)Show SMILES O[C@@H]1CCN(C1)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C17H16FN3O4S2/c18-13-7-10-3-5-19-17(23)12(10)8-14(13)20-27(24,25)16-2-1-15(26-16)21-6-4-11(22)9-21/h1-3,5,7-8,11,20,22H,4,6,9H2,(H,19,23)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AICARFT in human NCI-H460 cells assessed as increase in ZMP levels using low folate media after 16 hrs by LC-MS method |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243441

(CHEMBL4076500)Show SMILES OC1CCN(CC1)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F Show InChI InChI=1S/C18H18FN3O4S2/c19-14-9-11-3-6-20-18(24)13(11)10-15(14)21-28(25,26)17-2-1-16(27-17)22-7-4-12(23)5-8-22/h1-3,6,9-10,12,21,23H,4-5,7-8H2,(H,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AICARFT in human NCI-H460 cells assessed as increase in ZMP levels using low folate media after 16 hrs by LC-MS method |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243443

(CHEMBL4070790)Show SMILES O[C@H]1CN(CC1(F)F)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C17H14F3N3O4S2/c18-11-5-9-3-4-21-16(25)10(9)6-12(11)22-29(26,27)15-2-1-14(28-15)23-7-13(24)17(19,20)8-23/h1-6,13,22,24H,7-8H2,(H,21,25)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 31 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AICARFT in human NCI-H460 cells assessed as increase in ZMP levels using low folate media after 16 hrs by LC-MS method |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243462

(CHEMBL4083899)Show SMILES CC1(C)CC(=CNC1=O)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |c:4| Show InChI InChI=1S/C20H18FN3O4S2/c1-20(2)9-12(10-23-19(20)26)16-3-4-17(29-16)30(27,28)24-15-8-13-11(7-14(15)21)5-6-22-18(13)25/h3-8,10,24H,9H2,1-2H3,(H,22,25)(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AICARFT in human NCI-H460 cells assessed as increase in ZMP levels using low folate media after 16 hrs by LC-MS method |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243461

(CHEMBL4075503)Show SMILES Fc1cc2cc[nH]c(=O)c2cc1NS(=O)(=O)c1ccc(s1)-c1ccc(=O)[nH]c1 Show InChI InChI=1S/C18H12FN3O4S2/c19-13-7-10-5-6-20-18(24)12(10)8-14(13)22-28(25,26)17-4-2-15(27-17)11-1-3-16(23)21-9-11/h1-9,22H,(H,20,24)(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 62 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AICARFT in human NCI-H460 cells assessed as increase in ZMP levels using low folate media after 16 hrs by LC-MS method |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243487

(CHEMBL4091668)Show SMILES O[C@@H]1CN(CC1(F)F)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C17H14F3N3O4S2/c18-11-5-9-3-4-21-16(25)10(9)6-12(11)22-29(26,27)15-2-1-14(28-15)23-7-13(24)17(19,20)8-23/h1-6,13,22,24H,7-8H2,(H,21,25)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AICARFT in human NCI-H460 cells assessed as increase in ZMP levels using low folate media after 16 hrs by LC-MS method |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243442

(CHEMBL4099409)Show SMILES O[C@H]1CN(C[C@H]1F)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C17H15F2N3O4S2/c18-11-5-9-3-4-20-17(24)10(9)6-13(11)21-28(25,26)16-2-1-15(27-16)22-7-12(19)14(23)8-22/h1-6,12,14,21,23H,7-8H2,(H,20,24)/t12-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AICARFT in human NCI-H460 cells assessed as increase in ZMP levels using low folate media after 16 hrs by LC-MS method |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243486

(CHEMBL4081385)Show SMILES O[C@@H]1CN(C[C@@H]1F)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C17H15F2N3O4S2/c18-11-5-9-3-4-20-17(24)10(9)6-13(11)21-28(25,26)16-2-1-15(27-16)22-7-12(19)14(23)8-22/h1-6,12,14,21,23H,7-8H2,(H,20,24)/t12-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AICARFT in human NCI-H460 cells assessed as increase in ZMP levels using low folate media after 16 hrs by LC-MS method |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243415

(CHEMBL4063104)Show SMILES O[C@@H]1CCN(C1)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C17H16FN3O4S2/c18-13-7-10-3-5-19-17(23)12(10)8-14(13)20-27(24,25)16-2-1-15(26-16)21-6-4-11(22)9-21/h1-3,5,7-8,11,20,22H,4,6,9H2,(H,19,23)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AICARFT in human MDA-MB-231 cells assessed as increase in ZMP levels using low folate media after 16 hrs by LC-MS method |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data