

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Squalene synthase (Rattus norvegicus) | BDBM50031845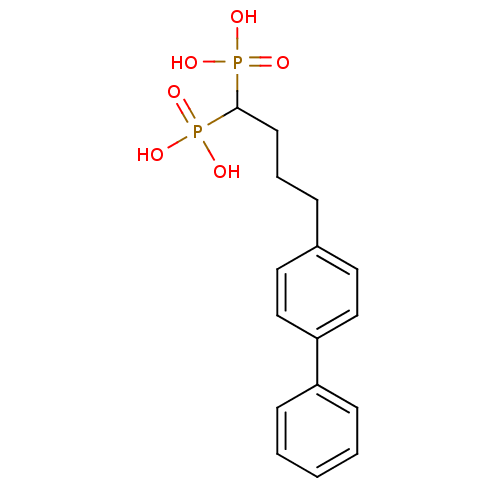 ((4-Biphenyl-4-yl-1-phosphono-butyl)-phosphonic aci...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50049232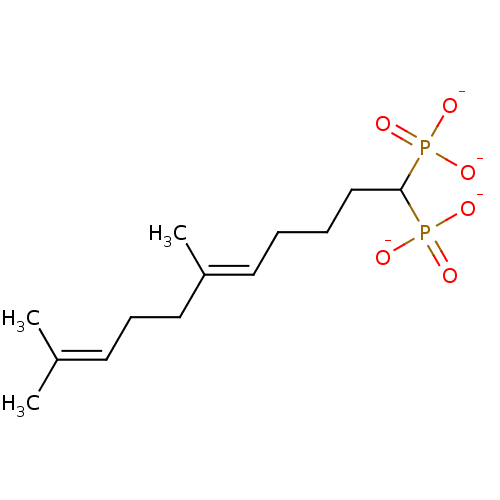 (CHEMBL348349 | Tetrasodium salt of 4-(4'-Methyl-bi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50049227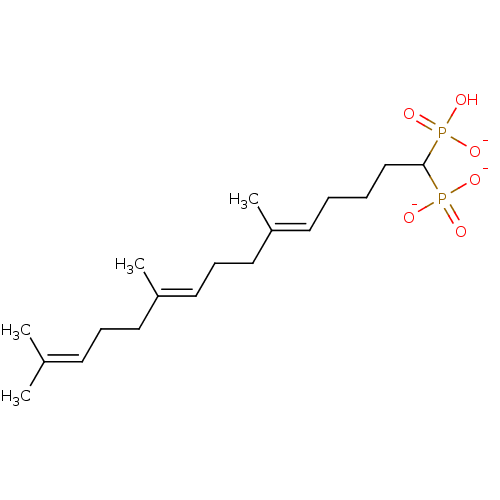 (CHEMBL158707 | Trisodium salt of [1-(Dimethoxy-pho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50049217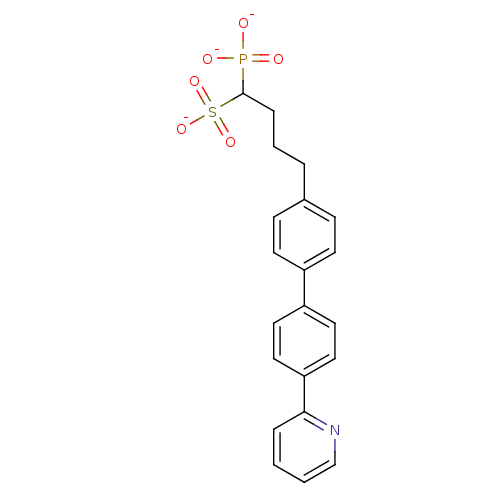 (CHEMBL158517 | Tripotassium salt of 1-Phosphono-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50049233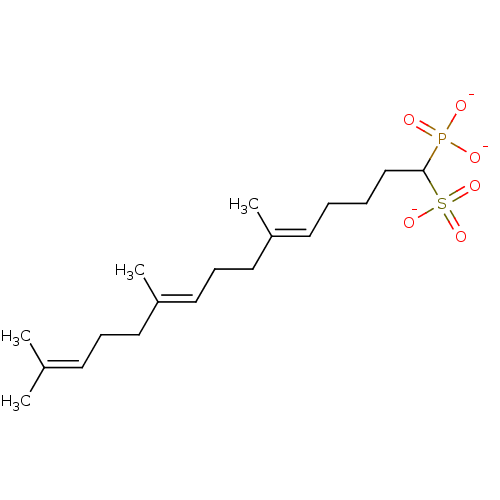 (CHEMBL160240 | Tripotassium salt of 4-Biphenyl-4-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50049230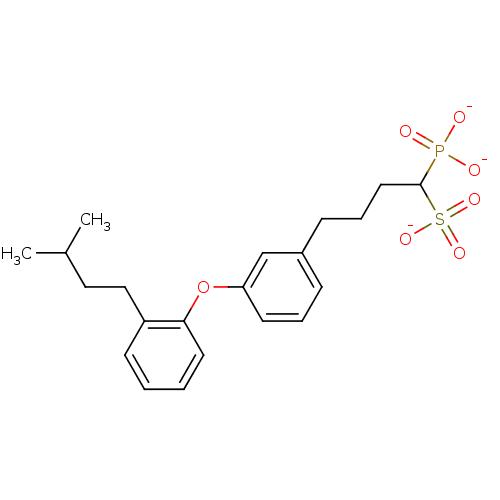 (CHEMBL160267 | Tripotassium salt of 4-{3-[2-(4-Met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50049215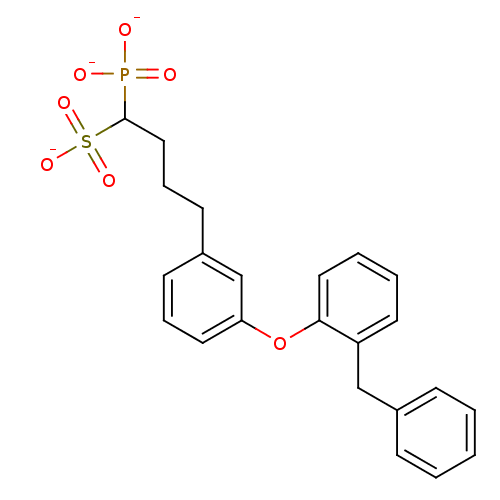 (1-Phosphono-4-[3-(2-benzylphenoxy)phenyl]butylsulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50049228 (CHEMBL158448 | Tripotassium salt of 4-{3-[2-(3-Met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50049223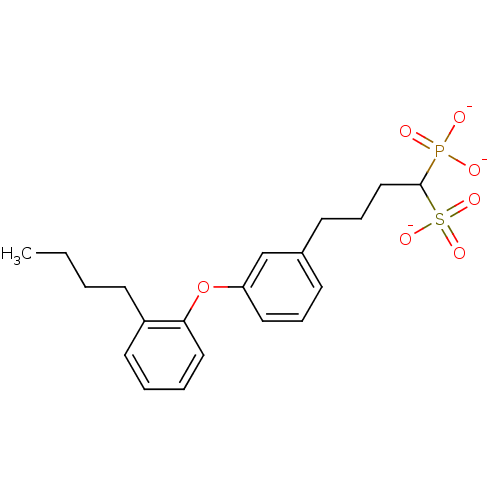 (CHEMBL158851 | Tripotassium salt of4-[3-(2-Butyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50049224 (CHEMBL158862 | Tripotassium salt of 1-Phosphono-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50049221 (CHEMBL158978 | Tripotassium salt of 4-(4'-But-1-en...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50049225 (CHEMBL351866 | Tripotassium salt of 4-(4'-Propyl-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 144 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50049236 ((+/-)-1-Phosphono-4-(3-phenoxyphenyl)butylsulfonic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 169 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50049229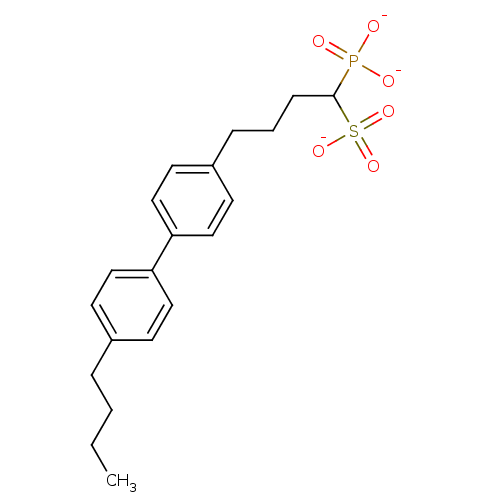 (1-Phosphono-4-[4-(4-butylphenyl)phenyl]butylsulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 192 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50049226 (CHEMBL158527 | Tripotassium salt of 1-Phosphono-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50049220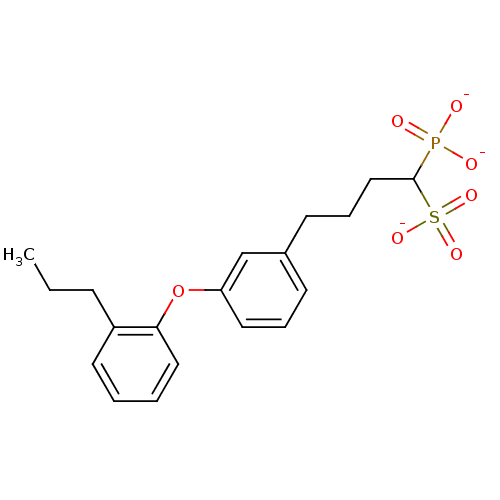 (CHEMBL346762 | Tripotassium salt of 1-Phosphono-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 268 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50049218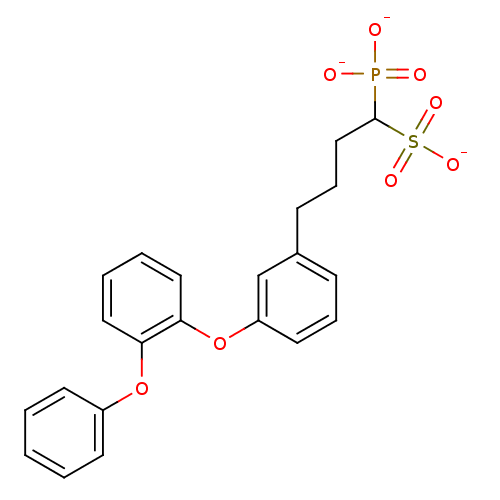 (CHEMBL159021 | Tripotassium salt of 4-[3-(2-Phenox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 286 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50049238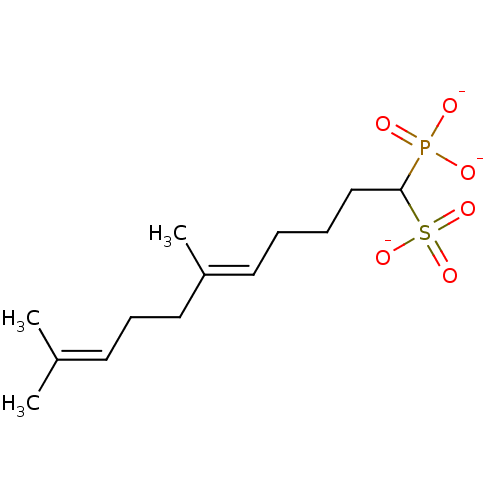 (CHEMBL158154 | trisodium (5E)-6,10-dimethyl-1-phos...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50049231 (CHEMBL347175 | CHEMBL513790 | Trisodium salt of 4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50049216 (CHEMBL160796 | Tripotassium salt of 4-(4'-Ethyl-bi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50049219 (1-Phosphono-4-[4-(4-methylphenyl)phenyl]butylsulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50049235 (CHEMBL158931 | Tripotassium salt of 4-(4'-Pentyl-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50049234 (CHEMBL158667 | Tripotassium salt of 1-Phosphono-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50049237 (CHEMBL347856 | Tripotassium salt of 1-Phosphono-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50049222 (1-Phosphono-4-[3-(4-propylphenoxy)phenyl]butylsulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||