

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cysteine protease ATG4B (Homo sapiens (Human)) | BDBM50604041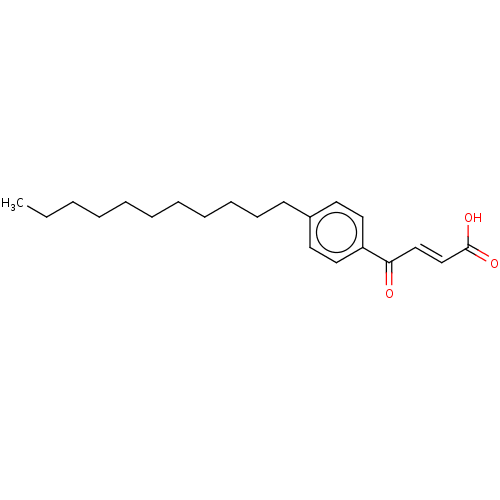 (CHEMBL5200146) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02113 BindingDB Entry DOI: 10.7270/Q2M049JM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease ATG4B (Homo sapiens (Human)) | BDBM50604040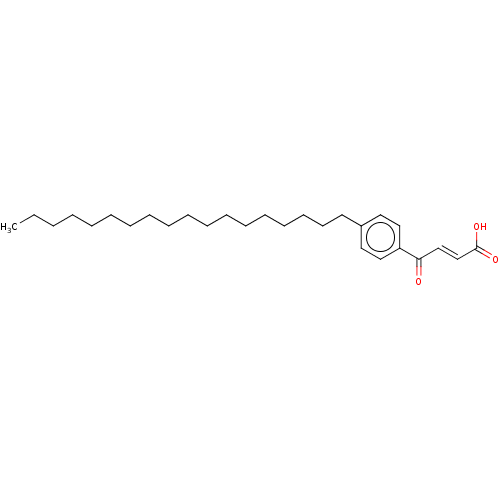 (CHEMBL1358284) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02113 BindingDB Entry DOI: 10.7270/Q2M049JM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50365553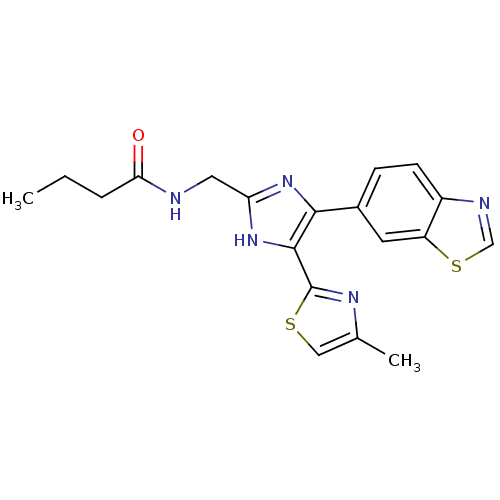 (CHEMBL1957675) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant GST-fused ALK5 using TMB substrate after 30 mins by ELISA | Bioorg Med Chem Lett 22: 2024-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.066 BindingDB Entry DOI: 10.7270/Q2668DPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucuronidase (Homo sapiens (Human)) | BDBM50405568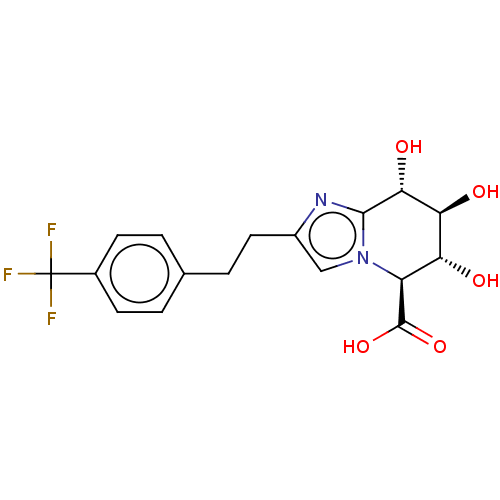 (CHEMBL5273557) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro concentration required to inhibit partially purified dihydropteroate synthase of Escherichia coli by 50% | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM466252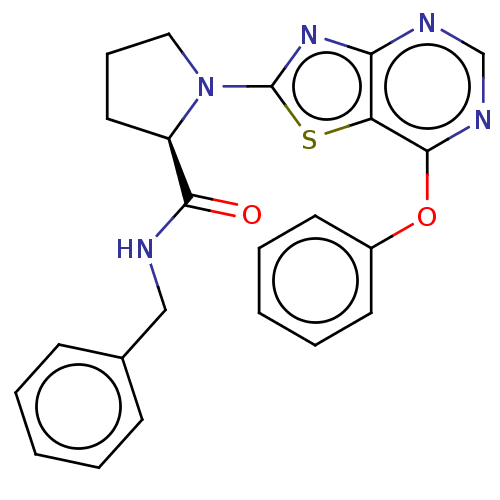 (US10793582, Example 66) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description To a reaction mixture (45 μL) containing 3.0 μmol/L kynurenine, 10 μmol/L pyridoxal phosphate, 2.0 ng/μL human recombinant KAT-II... | US Patent US10793582 (2020) BindingDB Entry DOI: 10.7270/Q27W6G8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM466253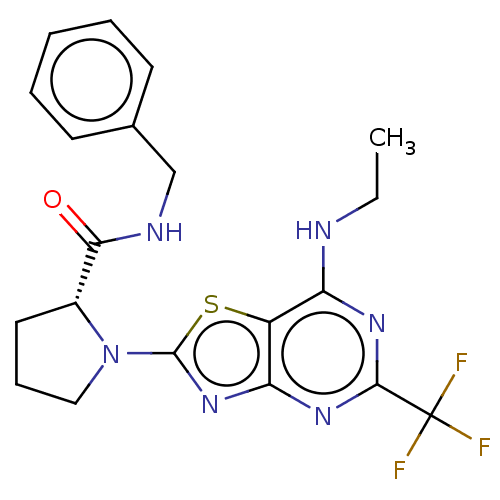 (US10793582, Example 67) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description To a reaction mixture (45 μL) containing 3.0 μmol/L kynurenine, 10 μmol/L pyridoxal phosphate, 2.0 ng/μL human recombinant KAT-II... | US Patent US10793582 (2020) BindingDB Entry DOI: 10.7270/Q27W6G8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM466258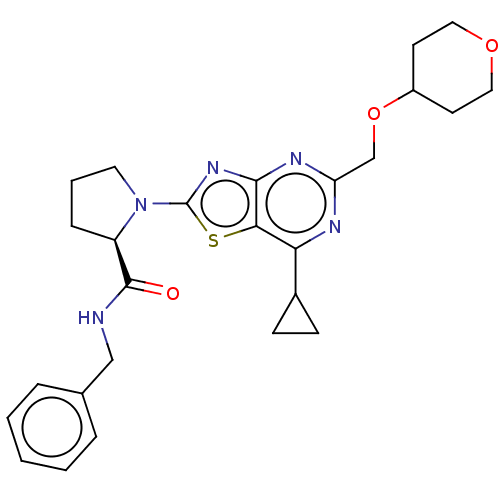 (US10793582, Example 83) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description To a reaction mixture (45 μL) containing 3.0 μmol/L kynurenine, 10 μmol/L pyridoxal phosphate, 2.0 ng/μL human recombinant KAT-II... | US Patent US10793582 (2020) BindingDB Entry DOI: 10.7270/Q27W6G8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543404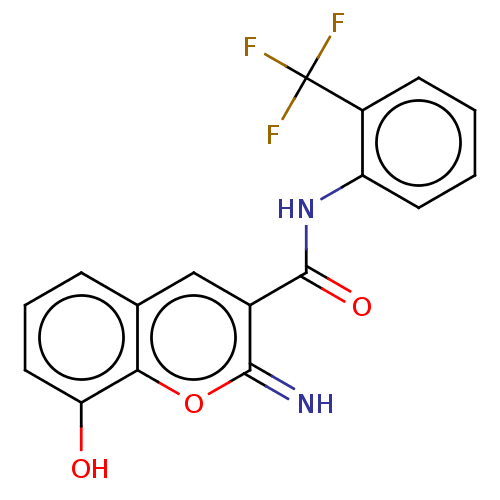 (CHEMBL4640154) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543398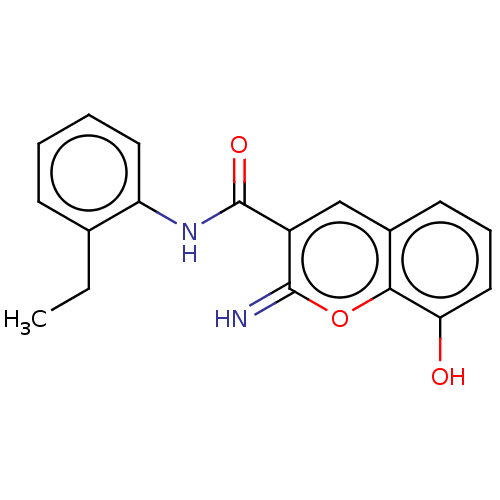 (CHEMBL4642187) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543409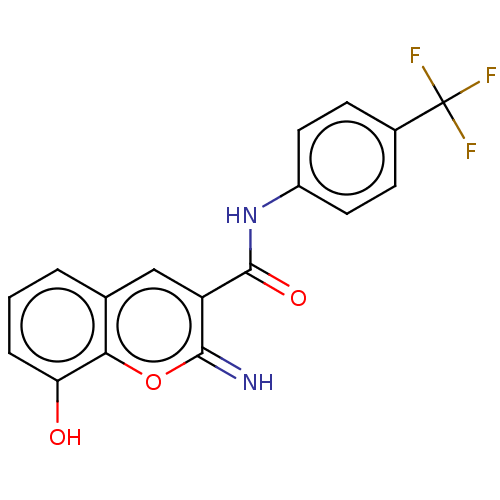 (CHEMBL4642852) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543401 (CHEMBL4644092) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM466232 ((R)—N-benzyl-1-(7-cyclopropyl[1,3]thiazolo[4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description To a reaction mixture (45 μL) containing 3.0 μmol/L kynurenine, 10 μmol/L pyridoxal phosphate, 2.0 ng/μL human recombinant KAT-II... | US Patent US10793582 (2020) BindingDB Entry DOI: 10.7270/Q27W6G8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543399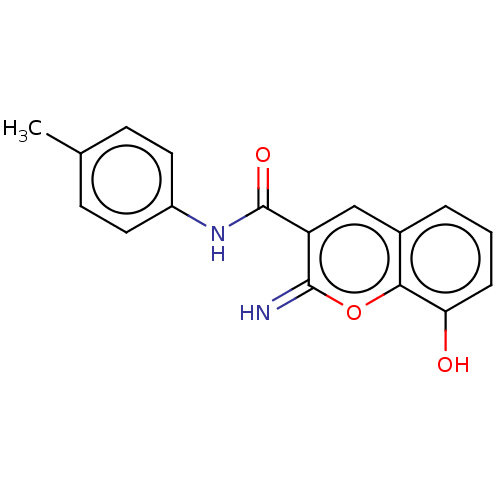 (CHEMBL4648793) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM466226 (US10793582, Example 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description To a reaction mixture (45 μL) containing 3.0 μmol/L kynurenine, 10 μmol/L pyridoxal phosphate, 2.0 ng/μL human recombinant KAT-II... | US Patent US10793582 (2020) BindingDB Entry DOI: 10.7270/Q27W6G8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543402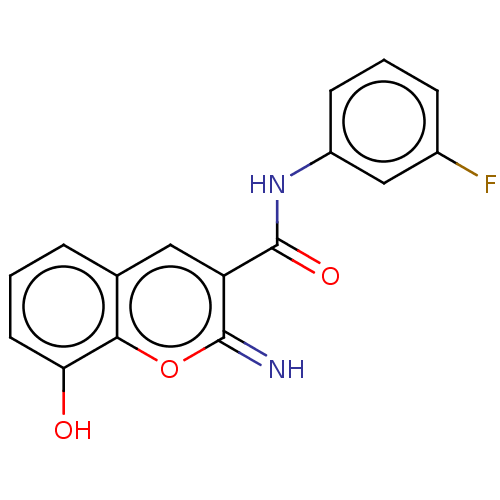 (CHEMBL4634960) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50365559 (CHEMBL1957662) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant GST-fused ALK5 using TMB substrate after 30 mins by ELISA | Bioorg Med Chem Lett 22: 2024-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.066 BindingDB Entry DOI: 10.7270/Q2668DPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543407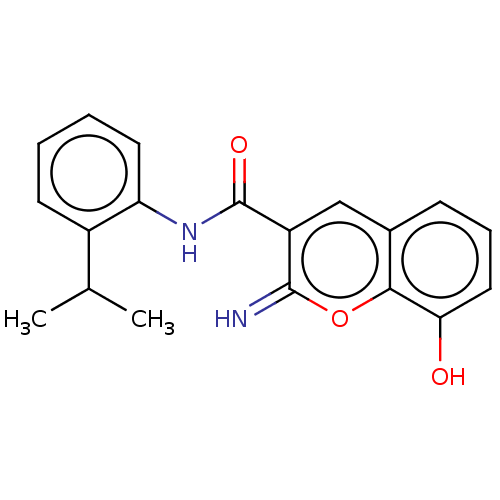 (CHEMBL4641899) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50365553 (CHEMBL1957675) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant ALK5 activity in human A549 cells assessed as TGFbeta-induced smad2/3 phosphorylation after 2 hrs by fluorescence ass... | Bioorg Med Chem Lett 22: 2024-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.066 BindingDB Entry DOI: 10.7270/Q2668DPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM466306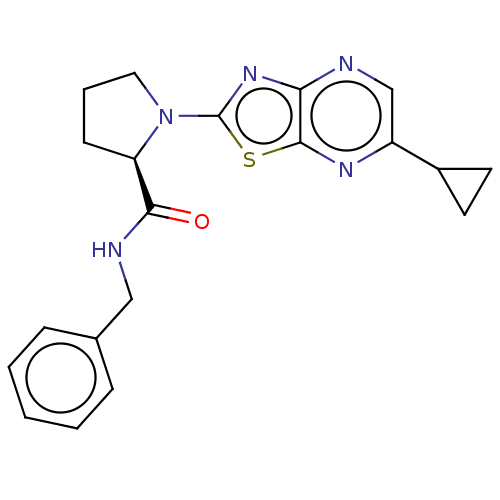 ((R)—N-benzyl-1-(6-cyclopropyl[1,3]thiazolo[4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description To a reaction mixture (45 μL) containing 3.0 μmol/L kynurenine, 10 μmol/L pyridoxal phosphate, 2.0 ng/μL human recombinant KAT-II... | US Patent US10793582 (2020) BindingDB Entry DOI: 10.7270/Q27W6G8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543397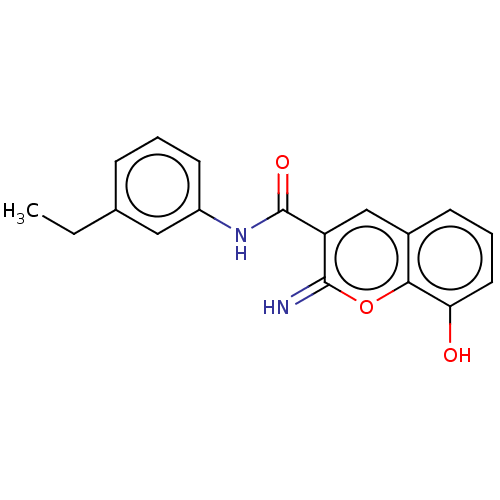 (CHEMBL4646593) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonyl reductase [NADPH] 1 (Homo sapiens (Human)) | BDBM50543400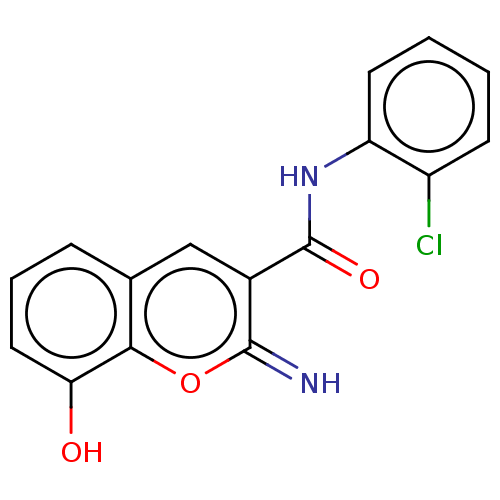 (CHEMBL4640248) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of CBR1 (unknown origin) | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM466295 ((R)—N-benzyl-1-[7-ethyl-6-(1-methyl-1H-pyrazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description To a reaction mixture (45 μL) containing 3.0 μmol/L kynurenine, 10 μmol/L pyridoxal phosphate, 2.0 ng/μL human recombinant KAT-II... | US Patent US10793582 (2020) BindingDB Entry DOI: 10.7270/Q27W6G8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM466246 (US10793582, Example 46) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description To a reaction mixture (45 μL) containing 3.0 μmol/L kynurenine, 10 μmol/L pyridoxal phosphate, 2.0 ng/μL human recombinant KAT-II... | US Patent US10793582 (2020) BindingDB Entry DOI: 10.7270/Q27W6G8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM466225 (US10793582, Example 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description To a reaction mixture (45 μL) containing 3.0 μmol/L kynurenine, 10 μmol/L pyridoxal phosphate, 2.0 ng/μL human recombinant KAT-II... | US Patent US10793582 (2020) BindingDB Entry DOI: 10.7270/Q27W6G8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543416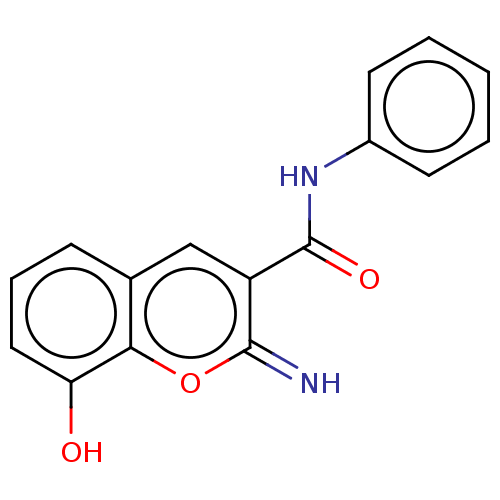 (CHEMBL4639909) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM466230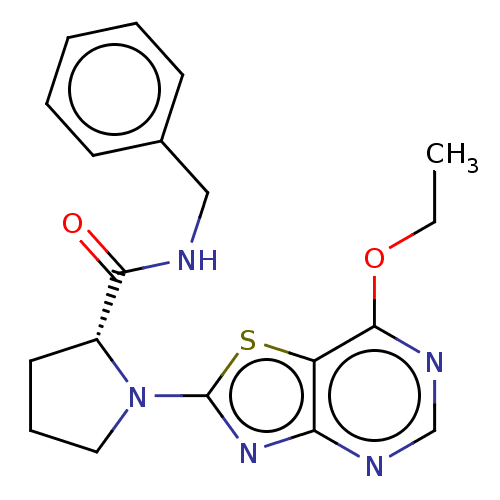 ((R)—N-benzyl-1-(7-ethoxy[1,3]thiazolo[4,5-d]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description To a reaction mixture (45 μL) containing 3.0 μmol/L kynurenine, 10 μmol/L pyridoxal phosphate, 2.0 ng/μL human recombinant KAT-II... | US Patent US10793582 (2020) BindingDB Entry DOI: 10.7270/Q27W6G8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543396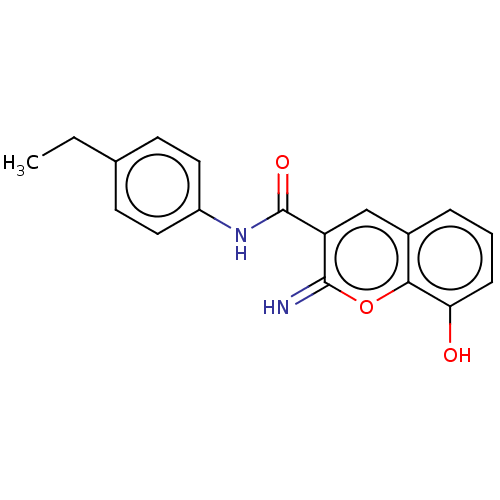 (CHEMBL4641012) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543405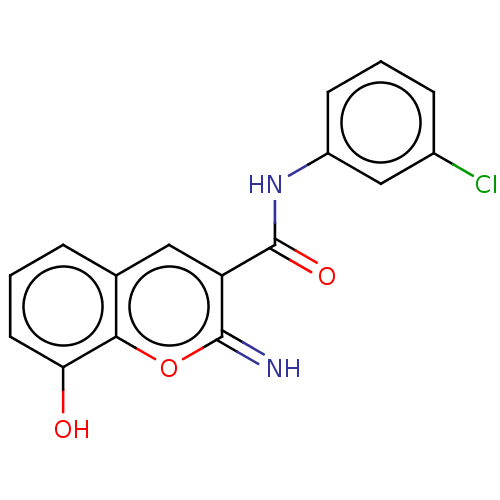 (CHEMBL4639417) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM466261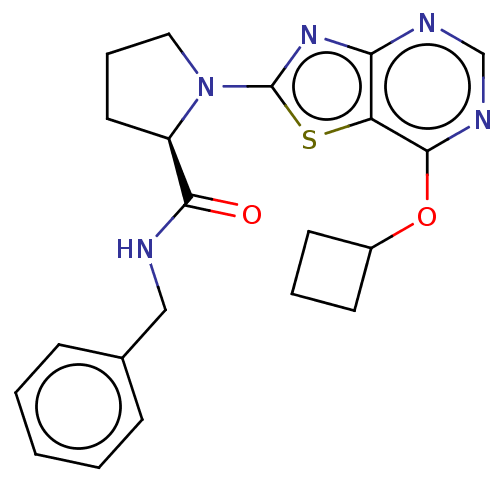 ((R)—N-benzyl-1-[7-(cyclobutyloxy)[1,3]thiazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description To a reaction mixture (45 μL) containing 3.0 μmol/L kynurenine, 10 μmol/L pyridoxal phosphate, 2.0 ng/μL human recombinant KAT-II... | US Patent US10793582 (2020) BindingDB Entry DOI: 10.7270/Q27W6G8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543406 (CHEMBL4637597) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM466223 (US10793582, Example 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description To a reaction mixture (45 μL) containing 3.0 μmol/L kynurenine, 10 μmol/L pyridoxal phosphate, 2.0 ng/μL human recombinant KAT-II... | US Patent US10793582 (2020) BindingDB Entry DOI: 10.7270/Q27W6G8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543411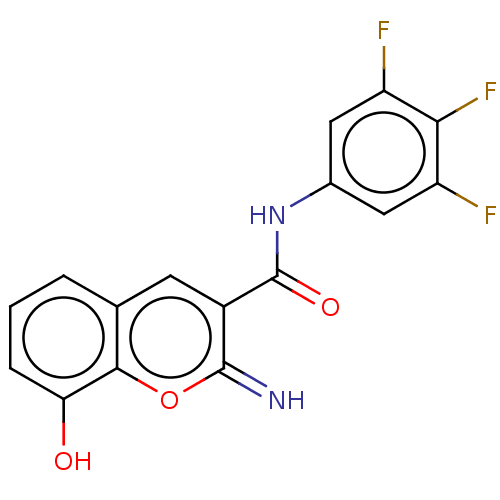 (CHEMBL4634140) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 (Homo sapiens (Human)) | BDBM50604040 (CHEMBL1358284) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02113 BindingDB Entry DOI: 10.7270/Q2M049JM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM466229 (US10793582, Example 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description To a reaction mixture (45 μL) containing 3.0 μmol/L kynurenine, 10 μmol/L pyridoxal phosphate, 2.0 ng/μL human recombinant KAT-II... | US Patent US10793582 (2020) BindingDB Entry DOI: 10.7270/Q27W6G8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543410 (CHEMBL4633866) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543415 (CHEMBL4641799) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543414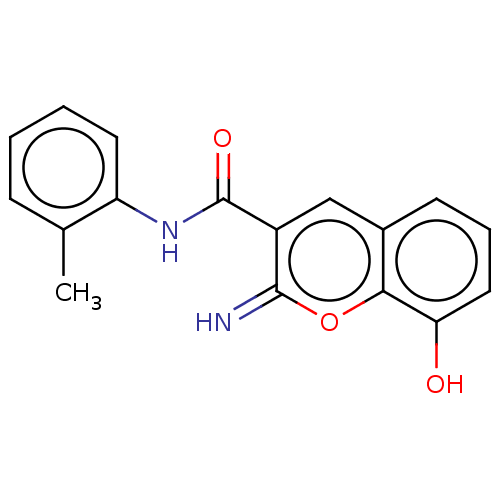 (CHEMBL4636345) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50365555 (CHEMBL1957649) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant GST-fused ALK5 using TMB substrate after 30 mins by ELISA | Bioorg Med Chem Lett 22: 2024-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.066 BindingDB Entry DOI: 10.7270/Q2668DPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543413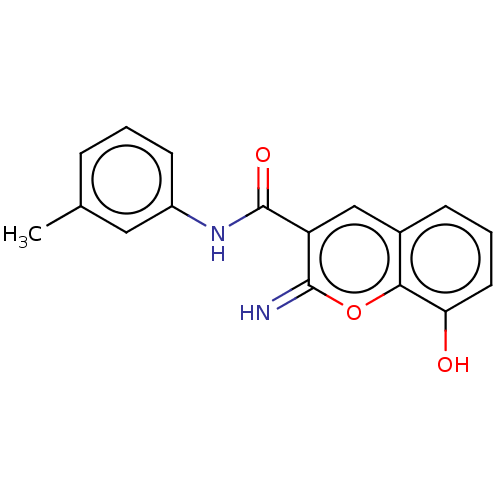 (CHEMBL4649502) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543400 (CHEMBL4640248) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543408 (CHEMBL4640782) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50213370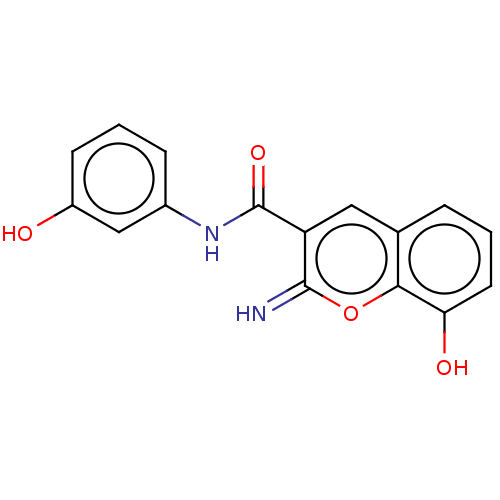 (CHEMBL82085) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50365538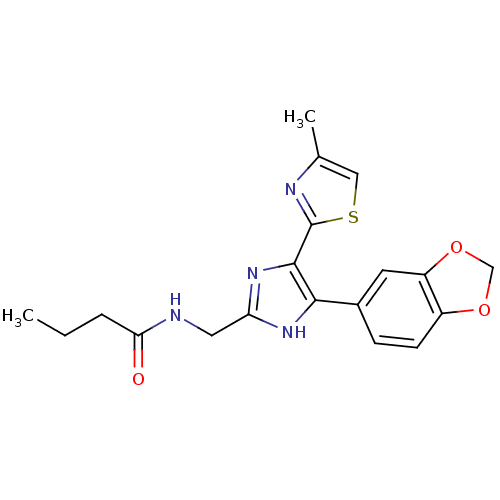 (CHEMBL1957656) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant GST-fused ALK5 using TMB substrate after 30 mins by ELISA | Bioorg Med Chem Lett 22: 2024-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.066 BindingDB Entry DOI: 10.7270/Q2668DPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM466242 (US10793582, Example 36) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description To a reaction mixture (45 μL) containing 3.0 μmol/L kynurenine, 10 μmol/L pyridoxal phosphate, 2.0 ng/μL human recombinant KAT-II... | US Patent US10793582 (2020) BindingDB Entry DOI: 10.7270/Q27W6G8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543403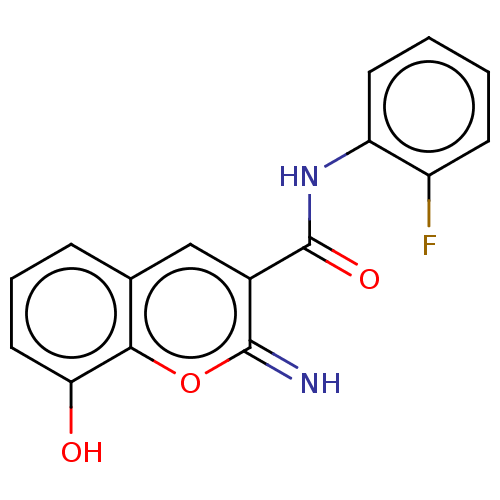 (CHEMBL4642678) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM466228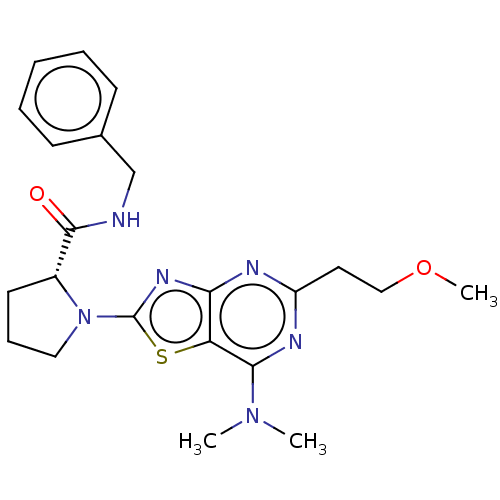 (US10793582, Example 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description To a reaction mixture (45 μL) containing 3.0 μmol/L kynurenine, 10 μmol/L pyridoxal phosphate, 2.0 ng/μL human recombinant KAT-II... | US Patent US10793582 (2020) BindingDB Entry DOI: 10.7270/Q27W6G8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM466237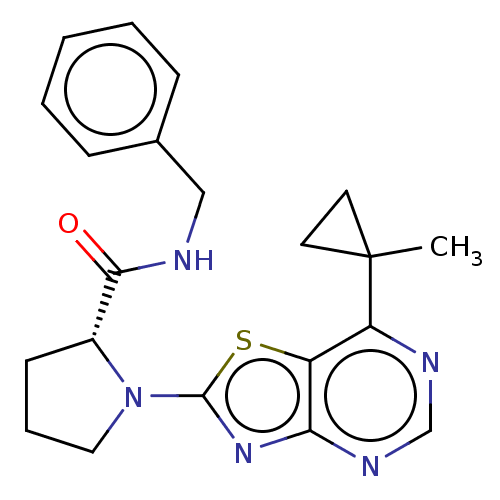 (US10793582, Example 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description To a reaction mixture (45 μL) containing 3.0 μmol/L kynurenine, 10 μmol/L pyridoxal phosphate, 2.0 ng/μL human recombinant KAT-II... | US Patent US10793582 (2020) BindingDB Entry DOI: 10.7270/Q27W6G8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM466274 (US10793582, Example 115) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description To a reaction mixture (45 μL) containing 3.0 μmol/L kynurenine, 10 μmol/L pyridoxal phosphate, 2.0 ng/μL human recombinant KAT-II... | US Patent US10793582 (2020) BindingDB Entry DOI: 10.7270/Q27W6G8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM466224 (US10793582, Example 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description To a reaction mixture (45 μL) containing 3.0 μmol/L kynurenine, 10 μmol/L pyridoxal phosphate, 2.0 ng/μL human recombinant KAT-II... | US Patent US10793582 (2020) BindingDB Entry DOI: 10.7270/Q27W6G8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM466283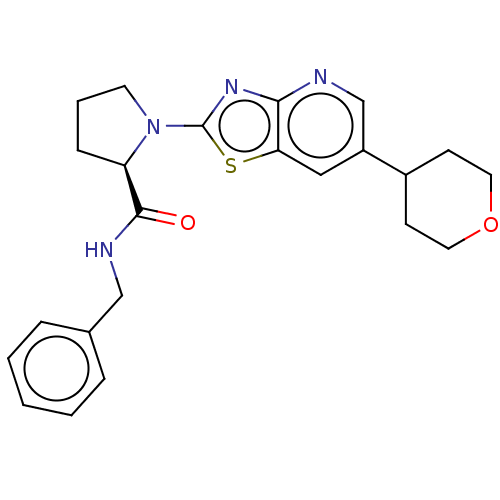 (US10793582, Example 129) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description To a reaction mixture (45 μL) containing 3.0 μmol/L kynurenine, 10 μmol/L pyridoxal phosphate, 2.0 ng/μL human recombinant KAT-II... | US Patent US10793582 (2020) BindingDB Entry DOI: 10.7270/Q27W6G8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 246 total ) | Next | Last >> |