 Found 82 hits with Last Name = 'fukunaga' and Initial = 't'
Found 82 hits with Last Name = 'fukunaga' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Galanin receptor type 1
(RAT) | BDBM50149871

((R)-2-((3-(biphenyl-4-yloxy)-3-(4-fluorophenyl)pro...)Show SMILES CN(CC[C@@H](Oc1ccc(cc1)-c1ccccc1)c1ccc(F)cc1)CC(O)=O |r| Show InChI InChI=1S/C24H24FNO3/c1-26(17-24(27)28)16-15-23(20-7-11-21(25)12-8-20)29-22-13-9-19(10-14-22)18-5-3-2-4-6-18/h2-14,23H,15-17H2,1H3,(H,27,28)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of [3H]glycine uptake at rat glycine transporter 1 expressed in african green monkey COS7 cells |
Bioorg Med Chem Lett 18: 6321-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.104
BindingDB Entry DOI: 10.7270/Q2BZ65WC |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(RAT) | BDBM50256665

((3S,6S,9S,12S)-3-benzyl-9,12-di-sec-butyl-6-((2S,3...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@@H](NC1=O)[C@@H](C)CC)[C@H](C)[C@@H](C)O |r| Show InChI InChI=1S/C27H42N4O5/c1-7-15(3)21-25(34)30-22(16(4)8-2)26(35)31-23(17(5)18(6)32)27(36)28-20(24(33)29-21)14-19-12-10-9-11-13-19/h9-13,15-18,20-23,32H,7-8,14H2,1-6H3,(H,28,36)(H,29,33)(H,30,34)(H,31,35)/t15-,16-,17+,18+,20-,21-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of [3H]glycine uptake at rat glycine transporter 1 expressed in african green monkey COS7 cells |
Bioorg Med Chem Lett 18: 6321-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.104
BindingDB Entry DOI: 10.7270/Q2BZ65WC |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4F2
(Homo sapiens (Human)) | BDBM50591500
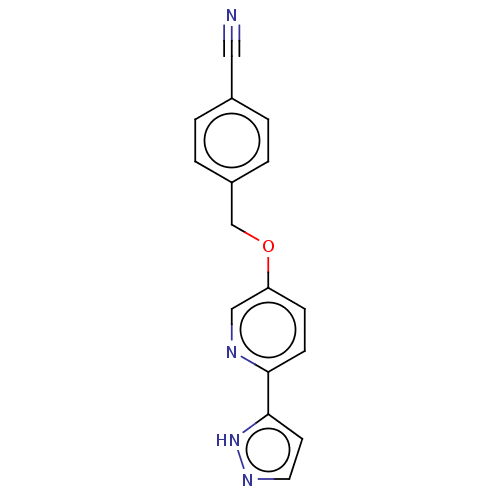
(CHEMBL5195099) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01089
BindingDB Entry DOI: 10.7270/Q2MK6HW6 |
More data for this
Ligand-Target Pair | |
Beta-glucuronidase
(Homo sapiens (Human)) | BDBM50405568
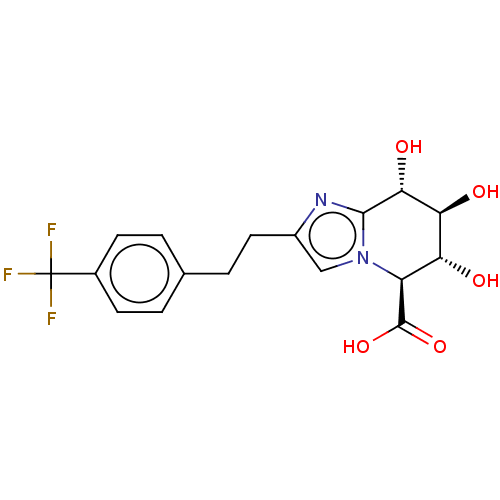
(CHEMBL5273557)Show InChI InChI=1S/C20H20NO/c22-16-4-13-21-14-11-17(12-15-21)9-10-19-7-3-6-18-5-1-2-8-20(18)19/h1-3,5-12,14-15,22H,4,13,16H2/q+1/b10-9+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro concentration required to inhibit partially purified dihydropteroate synthase of Escherichia coli by 50% |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(RAT) | BDBM50256666
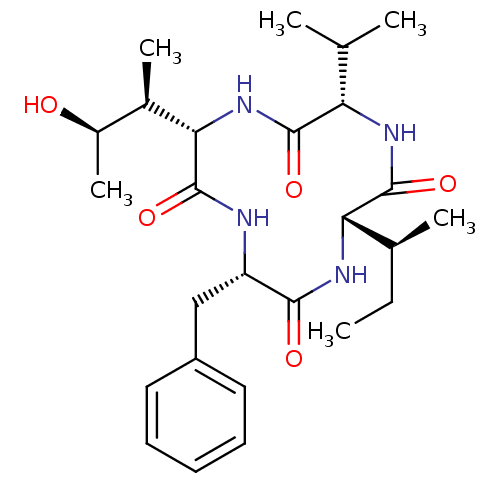
((3S,6S,9S,12S)-3-benzyl-12-sec-butyl-6-((2S,3R)-3-...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@@H](NC1=O)C(C)C)[C@H](C)[C@@H](C)O |r| Show InChI InChI=1S/C26H40N4O5/c1-7-15(4)21-25(34)28-20(14(2)3)24(33)30-22(16(5)17(6)31)26(35)27-19(23(32)29-21)13-18-11-9-8-10-12-18/h8-12,14-17,19-22,31H,7,13H2,1-6H3,(H,27,35)(H,28,34)(H,29,32)(H,30,33)/t15-,16+,17+,19-,20-,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of [3H]glycine uptake at rat glycine transporter 1 expressed in african green monkey COS7 cells |
Bioorg Med Chem Lett 18: 6321-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.104
BindingDB Entry DOI: 10.7270/Q2BZ65WC |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(RAT) | BDBM50256705
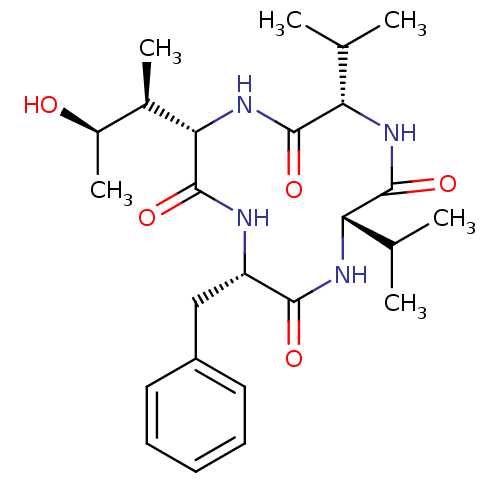
((3S,6S,9S,12S)-3-benzyl-6-((2S,3R)-3-hydroxybutan-...)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@@H](NC1=O)C(C)C)[C@H](C)[C@@H](C)O |r| Show InChI InChI=1S/C25H38N4O5/c1-13(2)19-23(32)28-20(14(3)4)24(33)29-21(15(5)16(6)30)25(34)26-18(22(31)27-19)12-17-10-8-7-9-11-17/h7-11,13-16,18-21,30H,12H2,1-6H3,(H,26,34)(H,27,31)(H,28,32)(H,29,33)/t15-,16-,18+,19+,20+,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of [3H]glycine uptake at rat glycine transporter 1 expressed in african green monkey COS7 cells |
Bioorg Med Chem Lett 18: 6321-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.104
BindingDB Entry DOI: 10.7270/Q2BZ65WC |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(RAT) | BDBM50256704

((3S,6S,9S,12S)-3-benzyl-9-sec-butyl-6-((2S,3R)-3-h...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC1=O)[C@H](C)[C@@H](C)O)C(C)C |r| Show InChI InChI=1S/C26H40N4O5/c1-7-15(4)21-25(34)30-22(16(5)17(6)31)26(35)27-19(13-18-11-9-8-10-12-18)23(32)28-20(14(2)3)24(33)29-21/h8-12,14-17,19-22,31H,7,13H2,1-6H3,(H,27,35)(H,28,32)(H,29,33)(H,30,34)/t15-,16+,17+,19-,20-,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of [3H]glycine uptake at rat glycine transporter 1 expressed in african green monkey COS7 cells |
Bioorg Med Chem Lett 18: 6321-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.104
BindingDB Entry DOI: 10.7270/Q2BZ65WC |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4F2
(Homo sapiens (Human)) | BDBM50591501
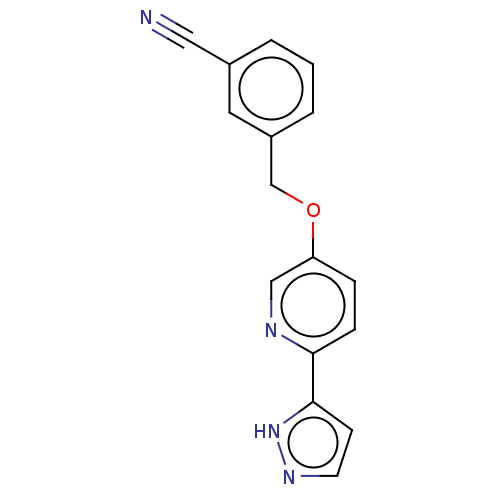
(CHEMBL5183418) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01089
BindingDB Entry DOI: 10.7270/Q2MK6HW6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4F2
(Homo sapiens (Human)) | BDBM50591496

(CHEMBL5204432) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01089
BindingDB Entry DOI: 10.7270/Q2MK6HW6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4F2
(Homo sapiens (Human)) | BDBM50591519

(CHEMBL5174407)Show SMILES CN(C)C(=O)N1CCC(COc2ccc(nc2)-c2ccn[nH]2)CC1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01089
BindingDB Entry DOI: 10.7270/Q2MK6HW6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4A11
(Homo sapiens) | BDBM50591496

(CHEMBL5204432) | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01089
BindingDB Entry DOI: 10.7270/Q2MK6HW6 |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(RAT) | BDBM50256664
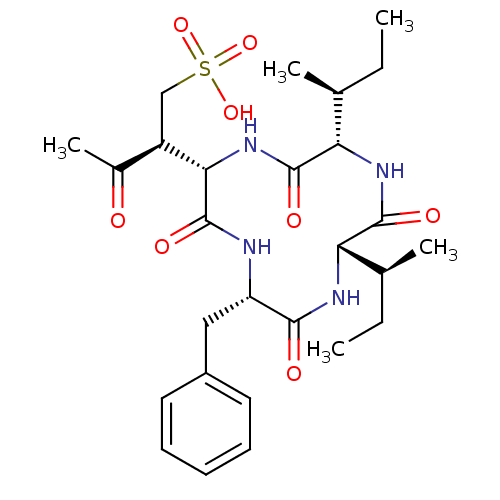
((S)-2-((2S,5S,8S,11S)-5-benzyl-8,11-di-sec-butyl-3...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@@H](NC1=O)[C@@H](C)CC)[C@H](CS(O)(=O)=O)C(C)=O |r| Show InChI InChI=1S/C27H40N4O8S/c1-6-15(3)21-25(34)30-22(16(4)7-2)26(35)31-23(19(17(5)32)14-40(37,38)39)27(36)28-20(24(33)29-21)13-18-11-9-8-10-12-18/h8-12,15-16,19-23H,6-7,13-14H2,1-5H3,(H,28,36)(H,29,33)(H,30,34)(H,31,35)(H,37,38,39)/t15-,16-,19+,20-,21-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of [3H]glycine uptake at rat glycine transporter 1 expressed in african green monkey COS7 cells |
Bioorg Med Chem Lett 18: 6321-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.104
BindingDB Entry DOI: 10.7270/Q2BZ65WC |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4F2
(Homo sapiens (Human)) | BDBM50591499
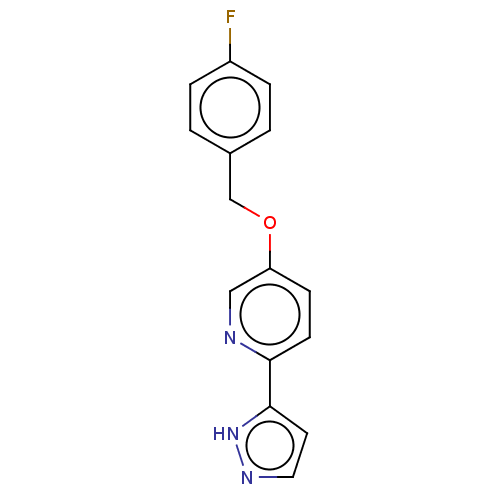
(CHEMBL5173608) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01089
BindingDB Entry DOI: 10.7270/Q2MK6HW6 |
More data for this
Ligand-Target Pair | |
Galanin receptor type 2
(RAT) | BDBM50256745

((2S)-2-amino-3-((2-(benzyloxy)phenyl)(3-fluorophen...)Show SMILES N[C@@H](COC(c1cccc(F)c1)c1ccccc1OCc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C23H22FNO4/c24-18-10-6-9-17(13-18)22(29-15-20(25)23(26)27)19-11-4-5-12-21(19)28-14-16-7-2-1-3-8-16/h1-13,20,22H,14-15,25H2,(H,26,27)/t20-,22?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of [3H]glycine uptake at rat glycine transporter 2 expressed in african green monkey COS7 cells |
Bioorg Med Chem Lett 18: 6321-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.104
BindingDB Entry DOI: 10.7270/Q2BZ65WC |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4A11
(Homo sapiens) | BDBM50591520

(CHEMBL5169535)Show SMILES CN(C)S(=O)(=O)N1CCC[C@H](COc2ccc(nc2)-c2ccn[nH]2)C1 |r| | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01089
BindingDB Entry DOI: 10.7270/Q2MK6HW6 |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(RAT) | BDBM50256706

((3S,6S,9S,12S)-3-benzyl-6,9,12-tri-sec-butyl-1,4,7...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@@H](NC1=O)[C@@H](C)CC)[C@@H](C)CC |r| Show InChI InChI=1S/C27H42N4O4/c1-7-16(4)21-25(33)28-20(15-19-13-11-10-12-14-19)24(32)29-22(17(5)8-2)26(34)31-23(18(6)9-3)27(35)30-21/h10-14,16-18,20-23H,7-9,15H2,1-6H3,(H,28,33)(H,29,32)(H,30,35)(H,31,34)/t16-,17-,18-,20-,21-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of [3H]glycine uptake at rat glycine transporter 1 expressed in african green monkey COS7 cells |
Bioorg Med Chem Lett 18: 6321-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.104
BindingDB Entry DOI: 10.7270/Q2BZ65WC |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4F2
(Homo sapiens (Human)) | BDBM50591520

(CHEMBL5169535)Show SMILES CN(C)S(=O)(=O)N1CCC[C@H](COc2ccc(nc2)-c2ccn[nH]2)C1 |r| | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01089
BindingDB Entry DOI: 10.7270/Q2MK6HW6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4A11
(Homo sapiens) | BDBM50591505

(CHEMBL5199278)Show SMILES CS(=O)(=O)N1CCC[C@@H](COc2ccc(nc2)-c2ccn[nH]2)C1 |r| | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01089
BindingDB Entry DOI: 10.7270/Q2MK6HW6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4F2
(Homo sapiens (Human)) | BDBM50591510

(CHEMBL5206230) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01089
BindingDB Entry DOI: 10.7270/Q2MK6HW6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4A11
(Homo sapiens) | BDBM50591506

(CHEMBL5189943)Show SMILES CS(=O)(=O)N1CCC[C@H](COc2ccc(nc2)-c2ccn[nH]2)C1 |r| | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01089
BindingDB Entry DOI: 10.7270/Q2MK6HW6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4F2
(Homo sapiens (Human)) | BDBM50591497

(CHEMBL5192170) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01089
BindingDB Entry DOI: 10.7270/Q2MK6HW6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4A11
(Homo sapiens) | BDBM50591502

(CHEMBL5205521)Show SMILES CS(=O)(=O)c1cccc(COc2ccc(nc2)-c2ccn[nH]2)c1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01089
BindingDB Entry DOI: 10.7270/Q2MK6HW6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4F2
(Homo sapiens (Human)) | BDBM50591518

(CHEMBL5172100) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01089
BindingDB Entry DOI: 10.7270/Q2MK6HW6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4F2
(Homo sapiens (Human)) | BDBM50591498

(CHEMBL5180285) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01089
BindingDB Entry DOI: 10.7270/Q2MK6HW6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4F2
(Homo sapiens (Human)) | BDBM50591504

(CHEMBL5208981)Show SMILES CS(=O)(=O)c1cc(O)cc(COc2ccc(nc2)-c2ccn[nH]2)c1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01089
BindingDB Entry DOI: 10.7270/Q2MK6HW6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4A11
(Homo sapiens) | BDBM50591508
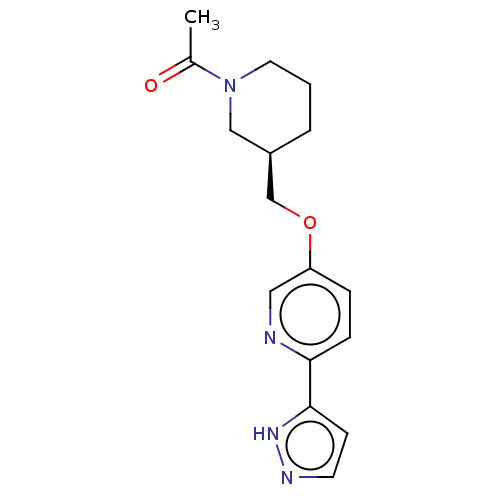
(CHEMBL5192567)Show SMILES CC(=O)N1CCC[C@@H](COc2ccc(nc2)-c2ccn[nH]2)C1 |r| | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01089
BindingDB Entry DOI: 10.7270/Q2MK6HW6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4F2
(Homo sapiens (Human)) | BDBM50591517

(CHEMBL5183091) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01089
BindingDB Entry DOI: 10.7270/Q2MK6HW6 |
More data for this
Ligand-Target Pair | |
Galanin receptor type 2
(RAT) | BDBM50256746

((3S,6S,9S,15aR)-9-sec-butyl-6-((1-methoxy-1H-indol...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2cn(OC)c3ccccc23)NC(=O)[C@H](CCCCC(C)=O)NC(=O)[C@H]2CCCCN2C1=O |r| Show InChI InChI=1S/C32H45N5O6/c1-5-20(2)28-32(42)36-17-11-10-16-27(36)31(41)33-24(14-8-6-12-21(3)38)29(39)34-25(30(40)35-28)18-22-19-37(43-4)26-15-9-7-13-23(22)26/h7,9,13,15,19-20,24-25,27-28H,5-6,8,10-12,14,16-18H2,1-4H3,(H,33,41)(H,34,39)(H,35,40)/t20-,24-,25-,27+,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 87.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of [3H]glycine uptake at rat glycine transporter 2 expressed in african green monkey COS7 cells |
Bioorg Med Chem Lett 18: 6321-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.104
BindingDB Entry DOI: 10.7270/Q2BZ65WC |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(RAT) | BDBM50256746

((3S,6S,9S,15aR)-9-sec-butyl-6-((1-methoxy-1H-indol...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2cn(OC)c3ccccc23)NC(=O)[C@H](CCCCC(C)=O)NC(=O)[C@H]2CCCCN2C1=O |r| Show InChI InChI=1S/C32H45N5O6/c1-5-20(2)28-32(42)36-17-11-10-16-27(36)31(41)33-24(14-8-6-12-21(3)38)29(39)34-25(30(40)35-28)18-22-19-37(43-4)26-15-9-7-13-23(22)26/h7,9,13,15,19-20,24-25,27-28H,5-6,8,10-12,14,16-18H2,1-4H3,(H,33,41)(H,34,39)(H,35,40)/t20-,24-,25-,27+,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 94.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of [3H]glycine uptake at rat glycine transporter 1 expressed in african green monkey COS7 cells |
Bioorg Med Chem Lett 18: 6321-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.104
BindingDB Entry DOI: 10.7270/Q2BZ65WC |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4F2
(Homo sapiens (Human)) | BDBM50591503
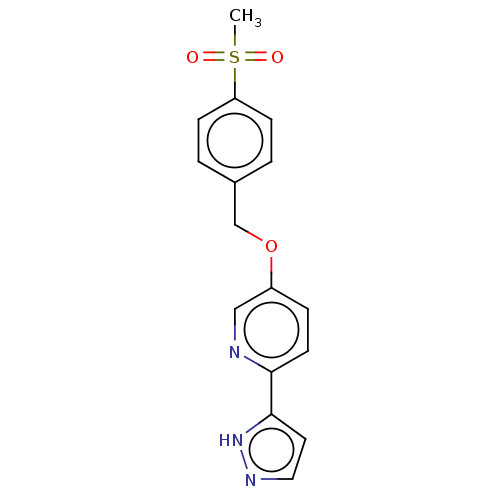
(CHEMBL5195431)Show SMILES CS(=O)(=O)c1ccc(COc2ccc(nc2)-c2ccn[nH]2)cc1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01089
BindingDB Entry DOI: 10.7270/Q2MK6HW6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4F2
(Homo sapiens (Human)) | BDBM50591516
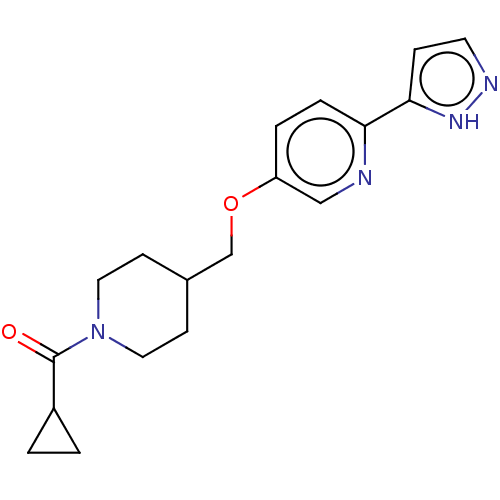
(CHEMBL5199197)Show SMILES O=C(C1CC1)N1CCC(COc2ccc(nc2)-c2ccn[nH]2)CC1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01089
BindingDB Entry DOI: 10.7270/Q2MK6HW6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4A11
(Homo sapiens) | BDBM50591510

(CHEMBL5206230) | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01089
BindingDB Entry DOI: 10.7270/Q2MK6HW6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4A11
(Homo sapiens) | BDBM50591497

(CHEMBL5192170) | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01089
BindingDB Entry DOI: 10.7270/Q2MK6HW6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4F2
(Homo sapiens (Human)) | BDBM50591514
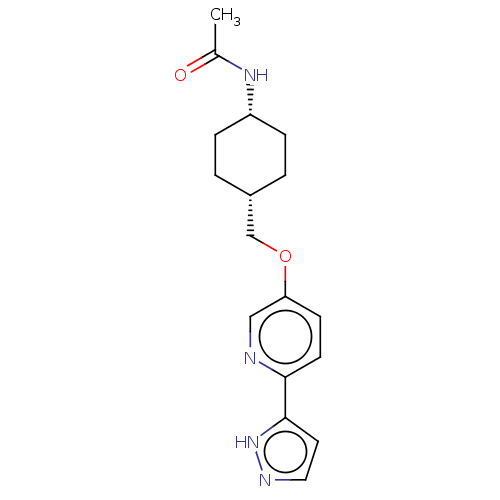
(CHEMBL5184859)Show SMILES CC(=O)N[C@@H]1CC[C@H](COc2ccc(nc2)-c2ccn[nH]2)CC1 |r,wU:7.7,4.3,(-8.55,-3.62,;-7.22,-2.85,;-7.22,-1.31,;-5.88,-3.62,;-4.55,-2.85,;-3.22,-3.62,;-1.89,-2.86,;-1.89,-1.32,;-.55,-.55,;.78,-1.32,;2.12,-.55,;3.45,-1.31,;4.78,-.54,;4.78,1,;3.45,1.77,;2.12,1,;6.11,1.77,;7.52,1.14,;8.55,2.29,;7.78,3.62,;6.27,3.3,;-3.22,-.54,;-4.55,-1.32,)| | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01089
BindingDB Entry DOI: 10.7270/Q2MK6HW6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4F2
(Homo sapiens (Human)) | BDBM50591509
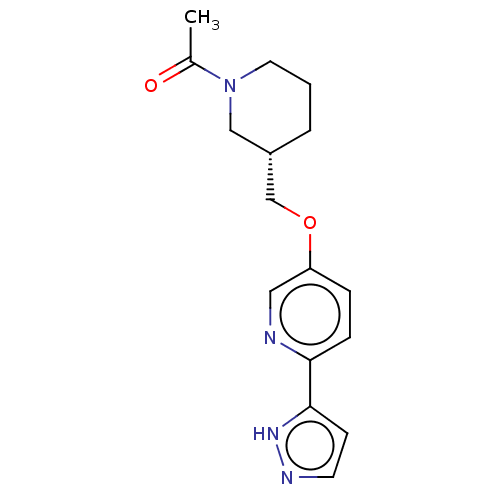
(CHEMBL5191778)Show SMILES CC(=O)N1CCC[C@H](COc2ccc(nc2)-c2ccn[nH]2)C1 |r| | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01089
BindingDB Entry DOI: 10.7270/Q2MK6HW6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4A11
(Homo sapiens) | BDBM50591504

(CHEMBL5208981)Show SMILES CS(=O)(=O)c1cc(O)cc(COc2ccc(nc2)-c2ccn[nH]2)c1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01089
BindingDB Entry DOI: 10.7270/Q2MK6HW6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4A11
(Homo sapiens) | BDBM50591509

(CHEMBL5191778)Show SMILES CC(=O)N1CCC[C@H](COc2ccc(nc2)-c2ccn[nH]2)C1 |r| | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01089
BindingDB Entry DOI: 10.7270/Q2MK6HW6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4F2
(Homo sapiens (Human)) | BDBM50591515
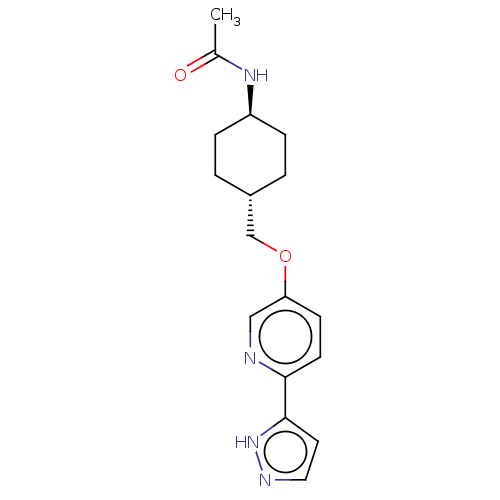
(CHEMBL5203135)Show SMILES CC(=O)N[C@H]1CC[C@H](COc2ccc(nc2)-c2ccn[nH]2)CC1 |r,wU:7.7,wD:4.3,(-8.55,-3.62,;-7.22,-2.85,;-7.22,-1.31,;-5.88,-3.62,;-4.55,-2.85,;-3.22,-3.62,;-1.89,-2.86,;-1.89,-1.32,;-.55,-.55,;.78,-1.32,;2.12,-.55,;3.45,-1.31,;4.78,-.54,;4.78,1,;3.45,1.77,;2.12,1,;6.11,1.77,;7.52,1.14,;8.55,2.29,;7.78,3.62,;6.27,3.3,;-3.22,-.54,;-4.55,-1.32,)| | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01089
BindingDB Entry DOI: 10.7270/Q2MK6HW6 |
More data for this
Ligand-Target Pair | |
Galanin receptor type 2
(RAT) | BDBM50256706

((3S,6S,9S,12S)-3-benzyl-6,9,12-tri-sec-butyl-1,4,7...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@@H](NC1=O)[C@@H](C)CC)[C@@H](C)CC |r| Show InChI InChI=1S/C27H42N4O4/c1-7-16(4)21-25(33)28-20(15-19-13-11-10-12-14-19)24(32)29-22(17(5)8-2)26(34)31-23(18(6)9-3)27(35)30-21/h10-14,16-18,20-23H,7-9,15H2,1-6H3,(H,28,33)(H,29,32)(H,30,35)(H,31,34)/t16-,17-,18-,20-,21-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 226 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of [3H]glycine uptake at rat glycine transporter 2 expressed in african green monkey COS7 cells |
Bioorg Med Chem Lett 18: 6321-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.104
BindingDB Entry DOI: 10.7270/Q2BZ65WC |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4F2
(Homo sapiens (Human)) | BDBM50591502

(CHEMBL5205521)Show SMILES CS(=O)(=O)c1cccc(COc2ccc(nc2)-c2ccn[nH]2)c1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01089
BindingDB Entry DOI: 10.7270/Q2MK6HW6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4A11
(Homo sapiens) | BDBM50591498

(CHEMBL5180285) | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01089
BindingDB Entry DOI: 10.7270/Q2MK6HW6 |
More data for this
Ligand-Target Pair | |
Galanin receptor type 2
(RAT) | BDBM50256665

((3S,6S,9S,12S)-3-benzyl-9,12-di-sec-butyl-6-((2S,3...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@@H](NC1=O)[C@@H](C)CC)[C@H](C)[C@@H](C)O |r| Show InChI InChI=1S/C27H42N4O5/c1-7-15(3)21-25(34)30-22(16(4)8-2)26(35)31-23(17(5)18(6)32)27(36)28-20(24(33)29-21)14-19-12-10-9-11-13-19/h9-13,15-18,20-23,32H,7-8,14H2,1-6H3,(H,28,36)(H,29,33)(H,30,34)(H,31,35)/t15-,16-,17+,18+,20-,21-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 278 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of [3H]glycine uptake at rat glycine transporter 2 expressed in african green monkey COS7 cells |
Bioorg Med Chem Lett 18: 6321-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.104
BindingDB Entry DOI: 10.7270/Q2BZ65WC |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4A11
(Homo sapiens) | BDBM50591517

(CHEMBL5183091) | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01089
BindingDB Entry DOI: 10.7270/Q2MK6HW6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4A11
(Homo sapiens) | BDBM50591499

(CHEMBL5173608) | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01089
BindingDB Entry DOI: 10.7270/Q2MK6HW6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4A11
(Homo sapiens) | BDBM50591501

(CHEMBL5183418) | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01089
BindingDB Entry DOI: 10.7270/Q2MK6HW6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4A11
(Homo sapiens) | BDBM50591514

(CHEMBL5184859)Show SMILES CC(=O)N[C@@H]1CC[C@H](COc2ccc(nc2)-c2ccn[nH]2)CC1 |r,wU:7.7,4.3,(-8.55,-3.62,;-7.22,-2.85,;-7.22,-1.31,;-5.88,-3.62,;-4.55,-2.85,;-3.22,-3.62,;-1.89,-2.86,;-1.89,-1.32,;-.55,-.55,;.78,-1.32,;2.12,-.55,;3.45,-1.31,;4.78,-.54,;4.78,1,;3.45,1.77,;2.12,1,;6.11,1.77,;7.52,1.14,;8.55,2.29,;7.78,3.62,;6.27,3.3,;-3.22,-.54,;-4.55,-1.32,)| | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01089
BindingDB Entry DOI: 10.7270/Q2MK6HW6 |
More data for this
Ligand-Target Pair | |
Galanin receptor type 2
(RAT) | BDBM50256704

((3S,6S,9S,12S)-3-benzyl-9-sec-butyl-6-((2S,3R)-3-h...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC1=O)[C@H](C)[C@@H](C)O)C(C)C |r| Show InChI InChI=1S/C26H40N4O5/c1-7-15(4)21-25(34)30-22(16(5)17(6)31)26(35)27-19(13-18-11-9-8-10-12-18)23(32)28-20(14(2)3)24(33)29-21/h8-12,14-17,19-22,31H,7,13H2,1-6H3,(H,27,35)(H,28,32)(H,29,33)(H,30,34)/t15-,16+,17+,19-,20-,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of [3H]glycine uptake at rat glycine transporter 2 expressed in african green monkey COS7 cells |
Bioorg Med Chem Lett 18: 6321-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.104
BindingDB Entry DOI: 10.7270/Q2BZ65WC |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4F2
(Homo sapiens (Human)) | BDBM50591495
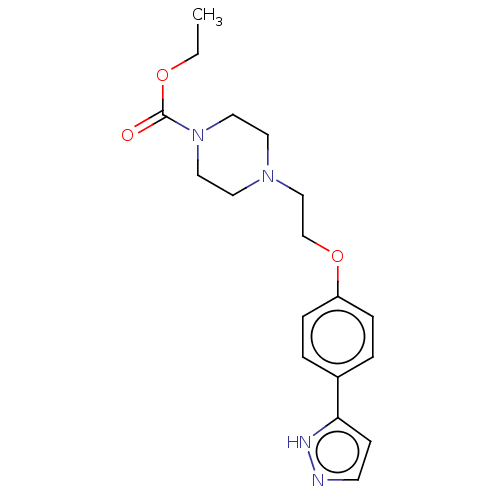
(CHEMBL5181522) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01089
BindingDB Entry DOI: 10.7270/Q2MK6HW6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4A11
(Homo sapiens) | BDBM50591516

(CHEMBL5199197)Show SMILES O=C(C1CC1)N1CCC(COc2ccc(nc2)-c2ccn[nH]2)CC1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01089
BindingDB Entry DOI: 10.7270/Q2MK6HW6 |
More data for this
Ligand-Target Pair | |
Galanin receptor type 2
(RAT) | BDBM50256705

((3S,6S,9S,12S)-3-benzyl-6-((2S,3R)-3-hydroxybutan-...)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@@H](NC1=O)C(C)C)[C@H](C)[C@@H](C)O |r| Show InChI InChI=1S/C25H38N4O5/c1-13(2)19-23(32)28-20(14(3)4)24(33)29-21(15(5)16(6)30)25(34)26-18(22(31)27-19)12-17-10-8-7-9-11-17/h7-11,13-16,18-21,30H,12H2,1-6H3,(H,26,34)(H,27,31)(H,28,32)(H,29,33)/t15-,16-,18+,19+,20+,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 542 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of [3H]glycine uptake at rat glycine transporter 2 expressed in african green monkey COS7 cells |
Bioorg Med Chem Lett 18: 6321-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.104
BindingDB Entry DOI: 10.7270/Q2BZ65WC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data