

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50218673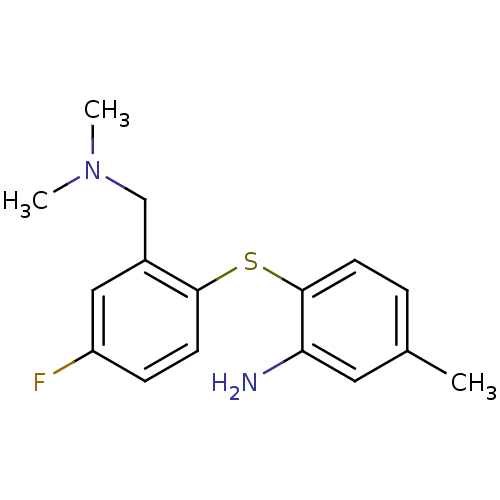 (2-(2-((dimethylamino)methyl)-4-fluorophenylthio)-5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from SERT in rat brain | Bioorg Med Chem Lett 17: 4991-5 (2007) Article DOI: 10.1016/j.bmcl.2007.02.082 BindingDB Entry DOI: 10.7270/Q2B27TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50218674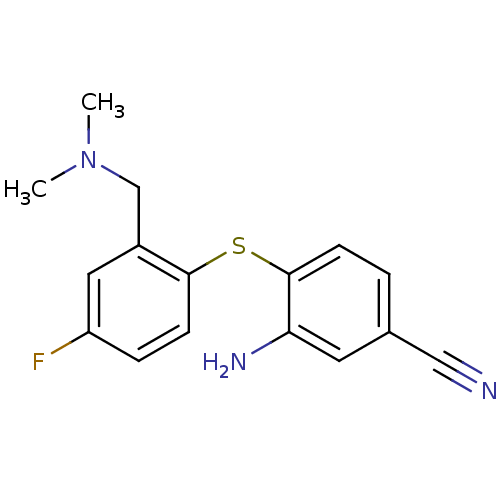 (3-amino-4-(2-((dimethylamino)methyl)-4-fluoropheny...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT in rat brain | Bioorg Med Chem Lett 17: 4991-5 (2007) Article DOI: 10.1016/j.bmcl.2007.02.082 BindingDB Entry DOI: 10.7270/Q2B27TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50218670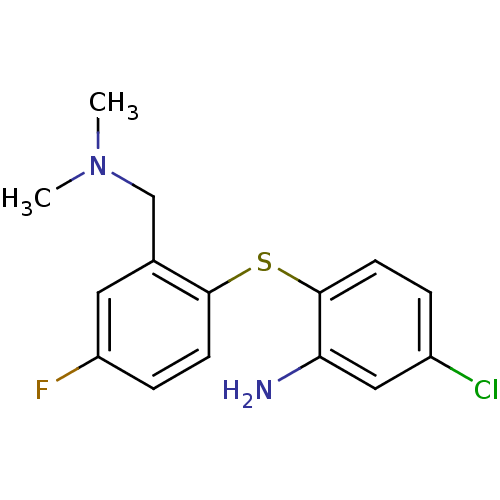 (5-chloro-2-(2-((dimethylamino)methyl)-4-fluorophen...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from SERT in rat brain | Bioorg Med Chem Lett 17: 4991-5 (2007) Article DOI: 10.1016/j.bmcl.2007.02.082 BindingDB Entry DOI: 10.7270/Q2B27TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM22416 ((3S,4R)-3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-f...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from human SERT expressed in HEK293 cells | Bioorg Med Chem Lett 18: 4727-30 (2008) Article DOI: 10.1016/j.bmcl.2008.06.077 BindingDB Entry DOI: 10.7270/Q2M61K2K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM22416 ((3S,4R)-3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-f...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from human SERT expressed in HEK293 cells | Bioorg Med Chem Lett 18: 4727-30 (2008) Article DOI: 10.1016/j.bmcl.2008.06.077 BindingDB Entry DOI: 10.7270/Q2M61K2K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50110788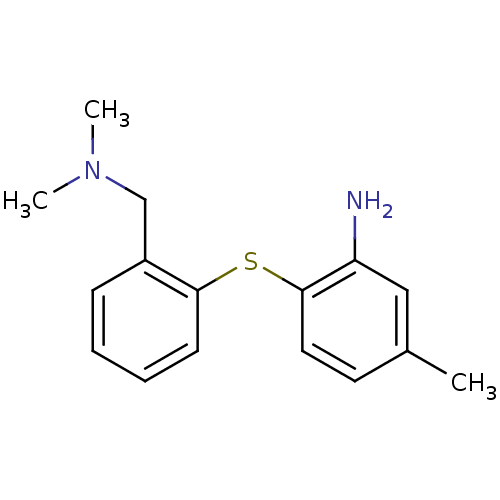 (2-(2-((dimethylamino)methyl)phenylthio)-5-methylan...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from SERT in rat brain | Bioorg Med Chem Lett 17: 4991-5 (2007) Article DOI: 10.1016/j.bmcl.2007.02.082 BindingDB Entry DOI: 10.7270/Q2B27TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50110788 (2-(2-((dimethylamino)methyl)phenylthio)-5-methylan...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from human SERT expressed in HEK293 cells | Bioorg Med Chem Lett 18: 4727-30 (2008) Article DOI: 10.1016/j.bmcl.2008.06.077 BindingDB Entry DOI: 10.7270/Q2M61K2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50110788 (2-(2-((dimethylamino)methyl)phenylthio)-5-methylan...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.03 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from human SERT expressed in HEK293 cells | Bioorg Med Chem Lett 18: 4727-30 (2008) Article DOI: 10.1016/j.bmcl.2008.06.077 BindingDB Entry DOI: 10.7270/Q2M61K2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50218671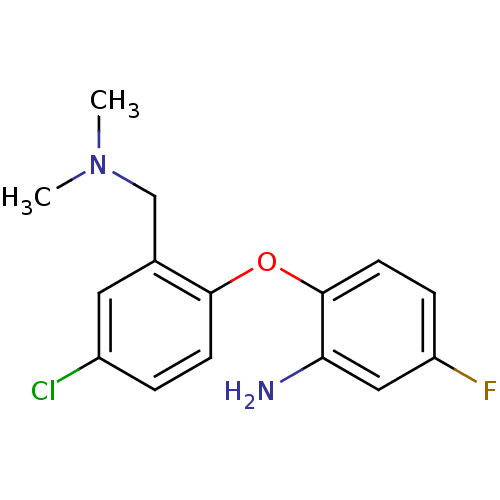 (2-(4-chloro-2-((dimethylamino)methyl)phenoxy)-5-fl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.06 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT in rat brain | Bioorg Med Chem Lett 17: 4991-5 (2007) Article DOI: 10.1016/j.bmcl.2007.02.082 BindingDB Entry DOI: 10.7270/Q2B27TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50218675 (3-amino-4-(2-((dimethylamino)methyl)-4-fluoropheno...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from SERT in rat brain | Bioorg Med Chem Lett 17: 4991-5 (2007) Article DOI: 10.1016/j.bmcl.2007.02.082 BindingDB Entry DOI: 10.7270/Q2B27TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50102006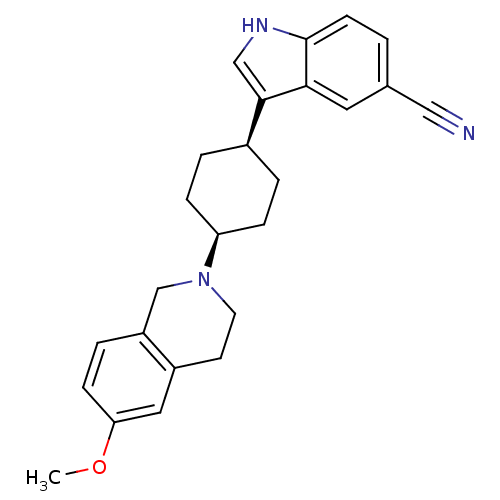 (3-[4-(6-Methoxy-3,4-dihydro-1H-isoquinolin-2-yl)-c...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from human SERT expressed in HEK293 cells | Bioorg Med Chem Lett 18: 4727-30 (2008) Article DOI: 10.1016/j.bmcl.2008.06.077 BindingDB Entry DOI: 10.7270/Q2M61K2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM25870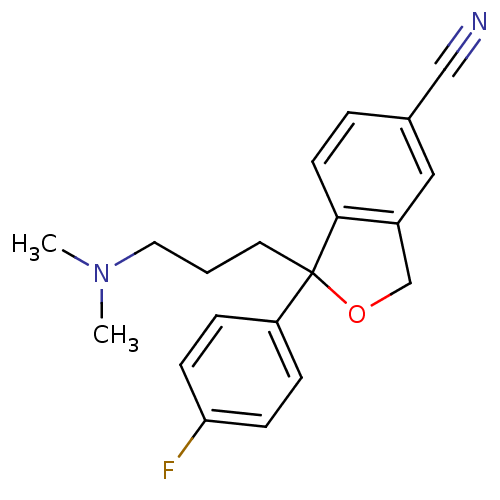 (1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 4.38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from human SERT expressed in HEK293 cells | Bioorg Med Chem Lett 18: 4727-30 (2008) Article DOI: 10.1016/j.bmcl.2008.06.077 BindingDB Entry DOI: 10.7270/Q2M61K2K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50102010 (3-[4-(3,4-Dihydro-1H-isoquinolin-2-yl)-cyclohexyl]...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from human SERT expressed in HEK293 cells | Bioorg Med Chem Lett 18: 4727-30 (2008) Article DOI: 10.1016/j.bmcl.2008.06.077 BindingDB Entry DOI: 10.7270/Q2M61K2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50262513 (CHEMBL449277 | cis-3-(4-(6-(3-fluoropropoxy)-3,4-d...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from human SERT expressed in HEK293 cells | Bioorg Med Chem Lett 18: 4727-30 (2008) Article DOI: 10.1016/j.bmcl.2008.06.077 BindingDB Entry DOI: 10.7270/Q2M61K2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50218672 (2-(2-((dimethylamino)methyl)-4-fluorophenoxy)-5-me...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from SERT in rat brain | Bioorg Med Chem Lett 17: 4991-5 (2007) Article DOI: 10.1016/j.bmcl.2007.02.082 BindingDB Entry DOI: 10.7270/Q2B27TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50218676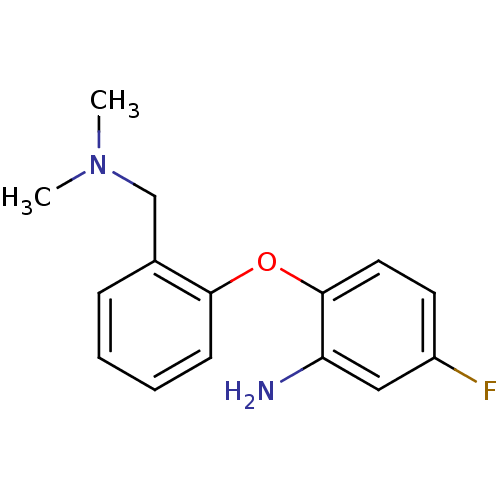 (2-(2-((dimethylamino)methyl)phenoxy)-5-fluorobenze...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from SERT in rat brain | Bioorg Med Chem Lett 17: 4991-5 (2007) Article DOI: 10.1016/j.bmcl.2007.02.082 BindingDB Entry DOI: 10.7270/Q2B27TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM25870 (1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 32.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from human SERT expressed in HEK293 cells | Bioorg Med Chem Lett 18: 4727-30 (2008) Article DOI: 10.1016/j.bmcl.2008.06.077 BindingDB Entry DOI: 10.7270/Q2M61K2K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50102008 (3-[4-(6-Methoxy-3,4-dihydro-1H-isoquinolin-2-yl)-c...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 73.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from human SERT expressed in HEK293 cells | Bioorg Med Chem Lett 18: 4727-30 (2008) Article DOI: 10.1016/j.bmcl.2008.06.077 BindingDB Entry DOI: 10.7270/Q2M61K2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50102019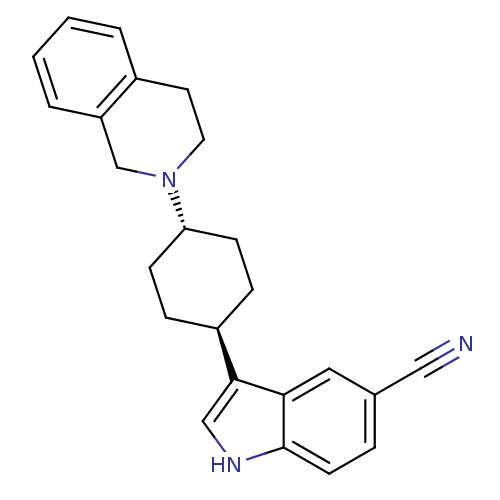 (3-[4-(3,4-Dihydro-1H-isoquinolin-2-yl)-cyclohexyl]...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 90.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from human SERT expressed in HEK293 cells | Bioorg Med Chem Lett 18: 4727-30 (2008) Article DOI: 10.1016/j.bmcl.2008.06.077 BindingDB Entry DOI: 10.7270/Q2M61K2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50102008 (3-[4-(6-Methoxy-3,4-dihydro-1H-isoquinolin-2-yl)-c...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 96.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from human NET expressed in HEK293 cells | Bioorg Med Chem Lett 18: 4727-30 (2008) Article DOI: 10.1016/j.bmcl.2008.06.077 BindingDB Entry DOI: 10.7270/Q2M61K2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50262514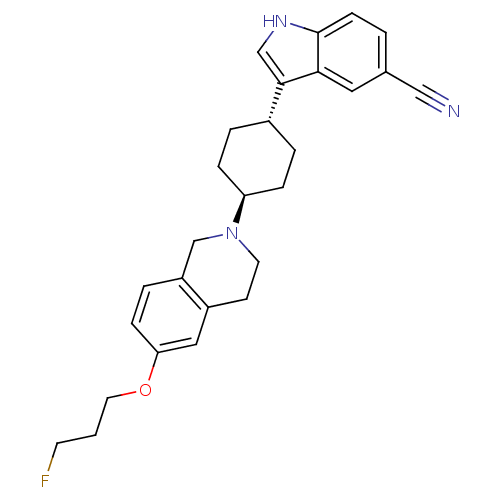 (CHEMBL477587 | trans-3-(4-(6-(3-fluoropropoxy)-3,4...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from human NET expressed in HEK293 cells | Bioorg Med Chem Lett 18: 4727-30 (2008) Article DOI: 10.1016/j.bmcl.2008.06.077 BindingDB Entry DOI: 10.7270/Q2M61K2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50102006 (3-[4-(6-Methoxy-3,4-dihydro-1H-isoquinolin-2-yl)-c...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 165 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from human SERT expressed in HEK293 cells | Bioorg Med Chem Lett 18: 4727-30 (2008) Article DOI: 10.1016/j.bmcl.2008.06.077 BindingDB Entry DOI: 10.7270/Q2M61K2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50102019 (3-[4-(3,4-Dihydro-1H-isoquinolin-2-yl)-cyclohexyl]...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 184 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from human NET expressed in HEK293 cells | Bioorg Med Chem Lett 18: 4727-30 (2008) Article DOI: 10.1016/j.bmcl.2008.06.077 BindingDB Entry DOI: 10.7270/Q2M61K2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50102010 (3-[4-(3,4-Dihydro-1H-isoquinolin-2-yl)-cyclohexyl]...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 189 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from human SERT expressed in HEK293 cells | Bioorg Med Chem Lett 18: 4727-30 (2008) Article DOI: 10.1016/j.bmcl.2008.06.077 BindingDB Entry DOI: 10.7270/Q2M61K2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50102008 (3-[4-(6-Methoxy-3,4-dihydro-1H-isoquinolin-2-yl)-c...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 267 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor in rat cortical membrane | Bioorg Med Chem Lett 18: 4727-30 (2008) Article DOI: 10.1016/j.bmcl.2008.06.077 BindingDB Entry DOI: 10.7270/Q2M61K2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50102019 (3-[4-(3,4-Dihydro-1H-isoquinolin-2-yl)-cyclohexyl]...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 286 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from human SERT expressed in HEK293 cells | Bioorg Med Chem Lett 18: 4727-30 (2008) Article DOI: 10.1016/j.bmcl.2008.06.077 BindingDB Entry DOI: 10.7270/Q2M61K2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50102019 (3-[4-(3,4-Dihydro-1H-isoquinolin-2-yl)-cyclohexyl]...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 309 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from human DAT expressed in HEK293 cells | Bioorg Med Chem Lett 18: 4727-30 (2008) Article DOI: 10.1016/j.bmcl.2008.06.077 BindingDB Entry DOI: 10.7270/Q2M61K2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50262514 (CHEMBL477587 | trans-3-(4-(6-(3-fluoropropoxy)-3,4...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 327 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from human SERT expressed in HEK293 cells | Bioorg Med Chem Lett 18: 4727-30 (2008) Article DOI: 10.1016/j.bmcl.2008.06.077 BindingDB Entry DOI: 10.7270/Q2M61K2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50262514 (CHEMBL477587 | trans-3-(4-(6-(3-fluoropropoxy)-3,4...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 343 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from human DAT expressed in HEK293 cells | Bioorg Med Chem Lett 18: 4727-30 (2008) Article DOI: 10.1016/j.bmcl.2008.06.077 BindingDB Entry DOI: 10.7270/Q2M61K2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50262513 (CHEMBL449277 | cis-3-(4-(6-(3-fluoropropoxy)-3,4-d...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 366 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from human DAT expressed in HEK293 cells | Bioorg Med Chem Lett 18: 4727-30 (2008) Article DOI: 10.1016/j.bmcl.2008.06.077 BindingDB Entry DOI: 10.7270/Q2M61K2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50102006 (3-[4-(6-Methoxy-3,4-dihydro-1H-isoquinolin-2-yl)-c...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from human DAT expressed in HEK293 cells | Bioorg Med Chem Lett 18: 4727-30 (2008) Article DOI: 10.1016/j.bmcl.2008.06.077 BindingDB Entry DOI: 10.7270/Q2M61K2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50102010 (3-[4-(3,4-Dihydro-1H-isoquinolin-2-yl)-cyclohexyl]...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 424 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from human DAT expressed in HEK293 cells | Bioorg Med Chem Lett 18: 4727-30 (2008) Article DOI: 10.1016/j.bmcl.2008.06.077 BindingDB Entry DOI: 10.7270/Q2M61K2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50102008 (3-[4-(6-Methoxy-3,4-dihydro-1H-isoquinolin-2-yl)-c...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 462 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from human DAT expressed in HEK293 cells | Bioorg Med Chem Lett 18: 4727-30 (2008) Article DOI: 10.1016/j.bmcl.2008.06.077 BindingDB Entry DOI: 10.7270/Q2M61K2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transporter (Rattus norvegicus) | BDBM50218672 (2-(2-((dimethylamino)methyl)-4-fluorophenoxy)-5-me...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 518 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from NET in rat brain | Bioorg Med Chem Lett 17: 4991-5 (2007) Article DOI: 10.1016/j.bmcl.2007.02.082 BindingDB Entry DOI: 10.7270/Q2B27TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50102008 (3-[4-(6-Methoxy-3,4-dihydro-1H-isoquinolin-2-yl)-c...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 518 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from human SERT expressed in HEK293 cells | Bioorg Med Chem Lett 18: 4727-30 (2008) Article DOI: 10.1016/j.bmcl.2008.06.077 BindingDB Entry DOI: 10.7270/Q2M61K2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50262513 (CHEMBL449277 | cis-3-(4-(6-(3-fluoropropoxy)-3,4-d...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 772 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from human NET expressed in HEK293 cells | Bioorg Med Chem Lett 18: 4727-30 (2008) Article DOI: 10.1016/j.bmcl.2008.06.077 BindingDB Entry DOI: 10.7270/Q2M61K2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50262513 (CHEMBL449277 | cis-3-(4-(6-(3-fluoropropoxy)-3,4-d...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 914 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from human SERT expressed in HEK293 cells | Bioorg Med Chem Lett 18: 4727-30 (2008) Article DOI: 10.1016/j.bmcl.2008.06.077 BindingDB Entry DOI: 10.7270/Q2M61K2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transporter (Rattus norvegicus) | BDBM50218670 (5-chloro-2-(2-((dimethylamino)methyl)-4-fluorophen...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 919 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from NET in rat brain | Bioorg Med Chem Lett 17: 4991-5 (2007) Article DOI: 10.1016/j.bmcl.2007.02.082 BindingDB Entry DOI: 10.7270/Q2B27TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50102010 (3-[4-(3,4-Dihydro-1H-isoquinolin-2-yl)-cyclohexyl]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor in rat cortical membrane | Bioorg Med Chem Lett 18: 4727-30 (2008) Article DOI: 10.1016/j.bmcl.2008.06.077 BindingDB Entry DOI: 10.7270/Q2M61K2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50102006 (3-[4-(6-Methoxy-3,4-dihydro-1H-isoquinolin-2-yl)-c...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from human NET expressed in HEK293 cells | Bioorg Med Chem Lett 18: 4727-30 (2008) Article DOI: 10.1016/j.bmcl.2008.06.077 BindingDB Entry DOI: 10.7270/Q2M61K2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transporter (Rattus norvegicus) | BDBM50218673 (2-(2-((dimethylamino)methyl)-4-fluorophenylthio)-5...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from NET in rat brain | Bioorg Med Chem Lett 17: 4991-5 (2007) Article DOI: 10.1016/j.bmcl.2007.02.082 BindingDB Entry DOI: 10.7270/Q2B27TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transporter (Rattus norvegicus) | BDBM50218676 (2-(2-((dimethylamino)methyl)phenoxy)-5-fluorobenze...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from NET in rat brain | Bioorg Med Chem Lett 17: 4991-5 (2007) Article DOI: 10.1016/j.bmcl.2007.02.082 BindingDB Entry DOI: 10.7270/Q2B27TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50102010 (3-[4-(3,4-Dihydro-1H-isoquinolin-2-yl)-cyclohexyl]...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from human NET expressed in HEK293 cells | Bioorg Med Chem Lett 18: 4727-30 (2008) Article DOI: 10.1016/j.bmcl.2008.06.077 BindingDB Entry DOI: 10.7270/Q2M61K2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50218670 (5-chloro-2-(2-((dimethylamino)methyl)-4-fluorophen...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from DAT in rat brain | Bioorg Med Chem Lett 17: 4991-5 (2007) Article DOI: 10.1016/j.bmcl.2007.02.082 BindingDB Entry DOI: 10.7270/Q2B27TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50262514 (CHEMBL477587 | trans-3-(4-(6-(3-fluoropropoxy)-3,4...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from human SERT expressed in HEK293 cells | Bioorg Med Chem Lett 18: 4727-30 (2008) Article DOI: 10.1016/j.bmcl.2008.06.077 BindingDB Entry DOI: 10.7270/Q2M61K2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50102019 (3-[4-(3,4-Dihydro-1H-isoquinolin-2-yl)-cyclohexyl]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor in rat cortical membrane | Bioorg Med Chem Lett 18: 4727-30 (2008) Article DOI: 10.1016/j.bmcl.2008.06.077 BindingDB Entry DOI: 10.7270/Q2M61K2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50218671 (2-(4-chloro-2-((dimethylamino)methyl)phenoxy)-5-fl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from DAT in rat brain | Bioorg Med Chem Lett 17: 4991-5 (2007) Article DOI: 10.1016/j.bmcl.2007.02.082 BindingDB Entry DOI: 10.7270/Q2B27TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transporter (Rattus norvegicus) | BDBM50218671 (2-(4-chloro-2-((dimethylamino)methyl)phenoxy)-5-fl...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from NET in rat brain | Bioorg Med Chem Lett 17: 4991-5 (2007) Article DOI: 10.1016/j.bmcl.2007.02.082 BindingDB Entry DOI: 10.7270/Q2B27TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50218672 (2-(2-((dimethylamino)methyl)-4-fluorophenoxy)-5-me...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from DAT in rat brain | Bioorg Med Chem Lett 17: 4991-5 (2007) Article DOI: 10.1016/j.bmcl.2007.02.082 BindingDB Entry DOI: 10.7270/Q2B27TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50262514 (CHEMBL477587 | trans-3-(4-(6-(3-fluoropropoxy)-3,4...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Interdisziplinäre Isotopenforschung Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor in rat cortical membrane | Bioorg Med Chem Lett 18: 4727-30 (2008) Article DOI: 10.1016/j.bmcl.2008.06.077 BindingDB Entry DOI: 10.7270/Q2M61K2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 59 total ) | Next | Last >> |