 Found 44 hits with Last Name = 'furimsky' and Initial = 'a'
Found 44 hits with Last Name = 'furimsky' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50558881
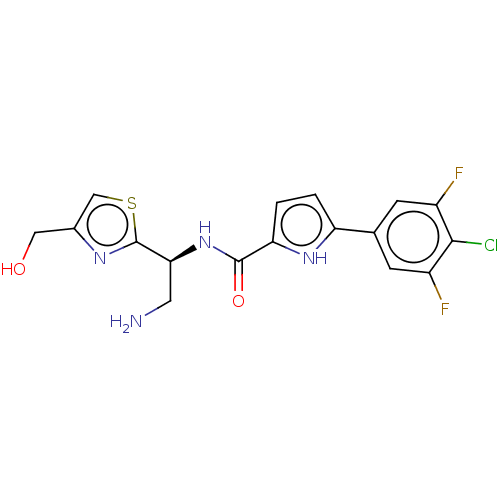
(CHEMBL4761836)Show SMILES NC[C@H](NC(=O)c1ccc([nH]1)-c1cc(F)c(Cl)c(F)c1)c1nc(CO)cs1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG transfected in HEK293 cells assessed as reduction in channel current at -80 mV holding potential by whole-cell patch clamp me... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2018.04.062
BindingDB Entry DOI: 10.7270/Q2183B73 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50357206
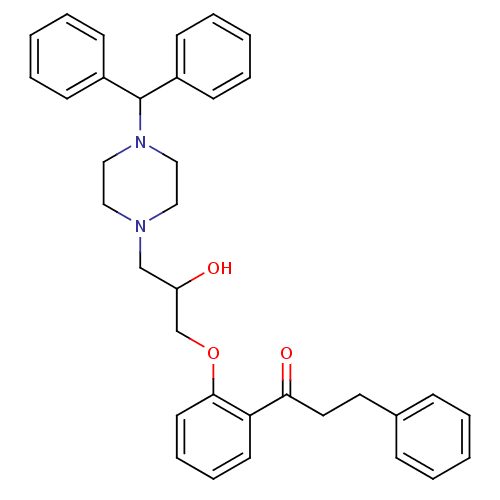
(CHEMBL1915083 | CHEMBL1962378)Show SMILES OC(COc1ccccc1C(=O)CCc1ccccc1)CN1CCN(CC1)C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C35H38N2O3/c38-31(27-40-34-19-11-10-18-32(34)33(39)21-20-28-12-4-1-5-13-28)26-36-22-24-37(25-23-36)35(29-14-6-2-7-15-29)30-16-8-3-9-17-30/h1-19,31,35,38H,20-27H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp assay |
J Med Chem 55: 6087-93 (2012)
Article DOI: 10.1021/jm300286a
BindingDB Entry DOI: 10.7270/Q21Z45HW |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50558880
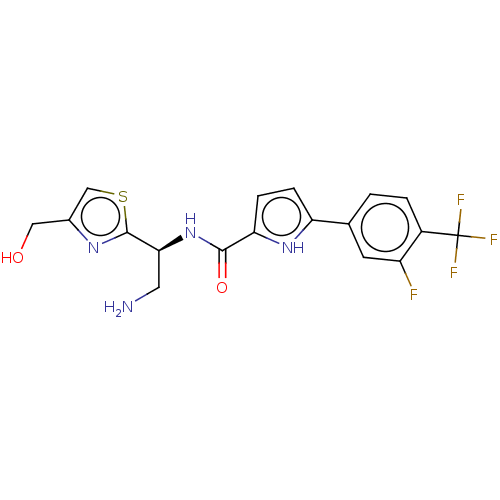
(CHEMBL4533529)Show SMILES NC[C@H](NC(=O)c1ccc([nH]1)-c1ccc(c(F)c1)C(F)(F)F)c1nc(CO)cs1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG transfected in HEK293 cells assessed as reduction in channel current at -80 mV holding potential by whole-cell patch clamp me... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2018.04.062
BindingDB Entry DOI: 10.7270/Q2183B73 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50357204

(CHEMBL1914835 | CHEMBL1963280)Show SMILES OC(COc1ccccc1C(=O)CCc1ccccc1)CN1CCC(CC1)c1ccccc1 Show InChI InChI=1S/C29H33NO3/c31-26(21-30-19-17-25(18-20-30)24-11-5-2-6-12-24)22-33-29-14-8-7-13-27(29)28(32)16-15-23-9-3-1-4-10-23/h1-14,25-26,31H,15-22H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp assay |
J Med Chem 55: 6087-93 (2012)
Article DOI: 10.1021/jm300286a
BindingDB Entry DOI: 10.7270/Q21Z45HW |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50357205

(CHEMBL1914844 | CHEMBL1963594)Show SMILES Cc1ccc(cc1)N1CCN(CC(O)COc2ccccc2C(=O)CCc2ccccc2)CC1 Show InChI InChI=1S/C29H34N2O3/c1-23-11-14-25(15-12-23)31-19-17-30(18-20-31)21-26(32)22-34-29-10-6-5-9-27(29)28(33)16-13-24-7-3-2-4-8-24/h2-12,14-15,26,32H,13,16-22H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp assay |
J Med Chem 55: 6087-93 (2012)
Article DOI: 10.1021/jm300286a
BindingDB Entry DOI: 10.7270/Q21Z45HW |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50357207

(CHEMBL1915364 | CHEMBL1963307)Show SMILES OC(COc1ccc(F)cc1C(=O)CCc1ccccc1)CN1CCN(CC1)C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C35H37FN2O3/c36-30-17-19-34(32(24-30)33(40)18-16-27-10-4-1-5-11-27)41-26-31(39)25-37-20-22-38(23-21-37)35(28-12-6-2-7-13-28)29-14-8-3-9-15-29/h1-15,17,19,24,31,35,39H,16,18,20-23,25-26H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp assay |
J Med Chem 55: 6087-93 (2012)
Article DOI: 10.1021/jm300286a
BindingDB Entry DOI: 10.7270/Q21Z45HW |
More data for this
Ligand-Target Pair | |
ATP-sensitive inward rectifier potassium channel 11
(Homo sapiens (Human)) | BDBM50357205

(CHEMBL1914844 | CHEMBL1963594)Show SMILES Cc1ccc(cc1)N1CCN(CC(O)COc2ccccc2C(=O)CCc2ccccc2)CC1 Show InChI InChI=1S/C29H34N2O3/c1-23-11-14-25(15-12-23)31-19-17-30(18-20-31)21-26(32)22-34-29-10-6-5-9-27(29)28(33)16-13-24-7-3-2-4-8-24/h2-12,14-15,26,32H,13,16-22H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of Kir6.2 by patch clamp assay |
J Med Chem 55: 6087-93 (2012)
Article DOI: 10.1021/jm300286a
BindingDB Entry DOI: 10.7270/Q21Z45HW |
More data for this
Ligand-Target Pair | |
ATP-sensitive inward rectifier potassium channel 11
(Homo sapiens (Human)) | BDBM50357207

(CHEMBL1915364 | CHEMBL1963307)Show SMILES OC(COc1ccc(F)cc1C(=O)CCc1ccccc1)CN1CCN(CC1)C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C35H37FN2O3/c36-30-17-19-34(32(24-30)33(40)18-16-27-10-4-1-5-11-27)41-26-31(39)25-37-20-22-38(23-21-37)35(28-12-6-2-7-13-28)29-14-8-3-9-15-29/h1-15,17,19,24,31,35,39H,16,18,20-23,25-26H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of Kir6.2 by patch clamp assay |
J Med Chem 55: 6087-93 (2012)
Article DOI: 10.1021/jm300286a
BindingDB Entry DOI: 10.7270/Q21Z45HW |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 5 subunit alpha
(Homo sapiens (Human)) | BDBM50357207

(CHEMBL1915364 | CHEMBL1963307)Show SMILES OC(COc1ccc(F)cc1C(=O)CCc1ccccc1)CN1CCN(CC1)C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C35H37FN2O3/c36-30-17-19-34(32(24-30)33(40)18-16-27-10-4-1-5-11-27)41-26-31(39)25-37-20-22-38(23-21-37)35(28-12-6-2-7-13-28)29-14-8-3-9-15-29/h1-15,17,19,24,31,35,39H,16,18,20-23,25-26H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of Nav1.5 assessed as tonic inhibition by patch clamp assay |
J Med Chem 55: 6087-93 (2012)
Article DOI: 10.1021/jm300286a
BindingDB Entry DOI: 10.7270/Q21Z45HW |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 5 subunit alpha
(Homo sapiens (Human)) | BDBM50357204

(CHEMBL1914835 | CHEMBL1963280)Show SMILES OC(COc1ccccc1C(=O)CCc1ccccc1)CN1CCC(CC1)c1ccccc1 Show InChI InChI=1S/C29H33NO3/c31-26(21-30-19-17-25(18-20-30)24-11-5-2-6-12-24)22-33-29-14-8-7-13-27(29)28(32)16-15-23-9-3-1-4-10-23/h1-14,25-26,31H,15-22H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of Nav1.5 assessed as tonic inhibition by patch clamp assay |
J Med Chem 55: 6087-93 (2012)
Article DOI: 10.1021/jm300286a
BindingDB Entry DOI: 10.7270/Q21Z45HW |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 5 subunit alpha
(Homo sapiens (Human)) | BDBM50357205

(CHEMBL1914844 | CHEMBL1963594)Show SMILES Cc1ccc(cc1)N1CCN(CC(O)COc2ccccc2C(=O)CCc2ccccc2)CC1 Show InChI InChI=1S/C29H34N2O3/c1-23-11-14-25(15-12-23)31-19-17-30(18-20-31)21-26(32)22-34-29-10-6-5-9-27(29)28(33)16-13-24-7-3-2-4-8-24/h2-12,14-15,26,32H,13,16-22H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of Nav1.5 assessed as tonic inhibition by patch clamp assay |
J Med Chem 55: 6087-93 (2012)
Article DOI: 10.1021/jm300286a
BindingDB Entry DOI: 10.7270/Q21Z45HW |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 5 subunit alpha
(Homo sapiens (Human)) | BDBM50357207

(CHEMBL1915364 | CHEMBL1963307)Show SMILES OC(COc1ccc(F)cc1C(=O)CCc1ccccc1)CN1CCN(CC1)C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C35H37FN2O3/c36-30-17-19-34(32(24-30)33(40)18-16-27-10-4-1-5-11-27)41-26-31(39)25-37-20-22-38(23-21-37)35(28-12-6-2-7-13-28)29-14-8-3-9-15-29/h1-15,17,19,24,31,35,39H,16,18,20-23,25-26H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of Nav1.5 assessed as phasic inhibition by patch clamp assay |
J Med Chem 55: 6087-93 (2012)
Article DOI: 10.1021/jm300286a
BindingDB Entry DOI: 10.7270/Q21Z45HW |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 5 subunit alpha
(Homo sapiens (Human)) | BDBM50357204

(CHEMBL1914835 | CHEMBL1963280)Show SMILES OC(COc1ccccc1C(=O)CCc1ccccc1)CN1CCC(CC1)c1ccccc1 Show InChI InChI=1S/C29H33NO3/c31-26(21-30-19-17-25(18-20-30)24-11-5-2-6-12-24)22-33-29-14-8-7-13-27(29)28(32)16-15-23-9-3-1-4-10-23/h1-14,25-26,31H,15-22H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of Nav1.5 assessed as phasic inhibition by patch clamp assay |
J Med Chem 55: 6087-93 (2012)
Article DOI: 10.1021/jm300286a
BindingDB Entry DOI: 10.7270/Q21Z45HW |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 5 subunit alpha
(Homo sapiens (Human)) | BDBM50357205

(CHEMBL1914844 | CHEMBL1963594)Show SMILES Cc1ccc(cc1)N1CCN(CC(O)COc2ccccc2C(=O)CCc2ccccc2)CC1 Show InChI InChI=1S/C29H34N2O3/c1-23-11-14-25(15-12-23)31-19-17-30(18-20-31)21-26(32)22-34-29-10-6-5-9-27(29)28(33)16-13-24-7-3-2-4-8-24/h2-12,14-15,26,32H,13,16-22H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of Nav1.5 assessed as phasic inhibition by patch clamp assay |
J Med Chem 55: 6087-93 (2012)
Article DOI: 10.1021/jm300286a
BindingDB Entry DOI: 10.7270/Q21Z45HW |
More data for this
Ligand-Target Pair | |
ATP-sensitive inward rectifier potassium channel 11
(Homo sapiens (Human)) | BDBM50357204

(CHEMBL1914835 | CHEMBL1963280)Show SMILES OC(COc1ccccc1C(=O)CCc1ccccc1)CN1CCC(CC1)c1ccccc1 Show InChI InChI=1S/C29H33NO3/c31-26(21-30-19-17-25(18-20-30)24-11-5-2-6-12-24)22-33-29-14-8-7-13-27(29)28(32)16-15-23-9-3-1-4-10-23/h1-14,25-26,31H,15-22H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of Kir6.2 by patch clamp assay |
J Med Chem 55: 6087-93 (2012)
Article DOI: 10.1021/jm300286a
BindingDB Entry DOI: 10.7270/Q21Z45HW |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50236759
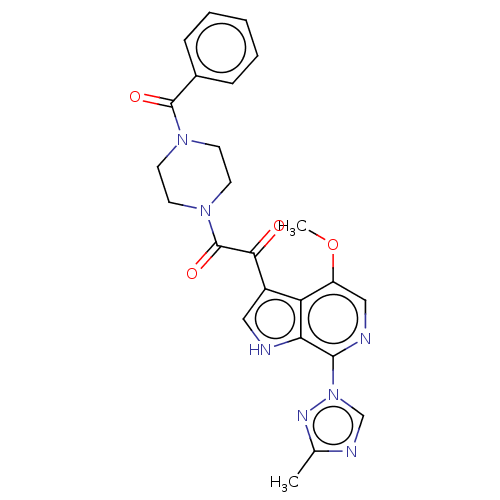
(BMS-626529 | Temsavir)Show SMILES COc1cnc(-n2cnc(C)n2)c2[nH]cc(C(=O)C(=O)N3CCN(CC3)C(=O)c3ccccc3)c12 Show InChI InChI=1S/C24H23N7O4/c1-15-27-14-31(28-15)22-20-19(18(35-2)13-26-22)17(12-25-20)21(32)24(34)30-10-8-29(9-11-30)23(33)16-6-4-3-5-7-16/h3-7,12-14,25H,8-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG transfected in HEK293 cells assessed as reduction in channel current at -80 mV holding potential by whole-cell patch clamp me... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2018.04.062
BindingDB Entry DOI: 10.7270/Q2183B73 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50558880

(CHEMBL4533529)Show SMILES NC[C@H](NC(=O)c1ccc([nH]1)-c1ccc(c(F)c1)C(F)(F)F)c1nc(CO)cs1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human liver microsome CYP2B6 using bupropion as substrate incubated for 20 mins by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2018.04.062
BindingDB Entry DOI: 10.7270/Q2183B73 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50558881

(CHEMBL4761836)Show SMILES NC[C@H](NC(=O)c1ccc([nH]1)-c1cc(F)c(Cl)c(F)c1)c1nc(CO)cs1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human liver microsome CYP3A4 using midazolam as substrate incubated for 20 mins by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2018.04.062
BindingDB Entry DOI: 10.7270/Q2183B73 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50558880

(CHEMBL4533529)Show SMILES NC[C@H](NC(=O)c1ccc([nH]1)-c1ccc(c(F)c1)C(F)(F)F)c1nc(CO)cs1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human liver microsome CYP3A4 using midazolam as substrate incubated for 20 mins by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2018.04.062
BindingDB Entry DOI: 10.7270/Q2183B73 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50236759

(BMS-626529 | Temsavir)Show SMILES COc1cnc(-n2cnc(C)n2)c2[nH]cc(C(=O)C(=O)N3CCN(CC3)C(=O)c3ccccc3)c12 Show InChI InChI=1S/C24H23N7O4/c1-15-27-14-31(28-15)22-20-19(18(35-2)13-26-22)17(12-25-20)21(32)24(34)30-10-8-29(9-11-30)23(33)16-6-4-3-5-7-16/h3-7,12-14,25H,8-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human liver microsome CYP3A4 using midazolam as substrate incubated for 20 mins by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2018.04.062
BindingDB Entry DOI: 10.7270/Q2183B73 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50236759

(BMS-626529 | Temsavir)Show SMILES COc1cnc(-n2cnc(C)n2)c2[nH]cc(C(=O)C(=O)N3CCN(CC3)C(=O)c3ccccc3)c12 Show InChI InChI=1S/C24H23N7O4/c1-15-27-14-31(28-15)22-20-19(18(35-2)13-26-22)17(12-25-20)21(32)24(34)30-10-8-29(9-11-30)23(33)16-6-4-3-5-7-16/h3-7,12-14,25H,8-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human liver microsome CYP1A2 using phenacetin as substrate incubated for 20 mins by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2018.04.062
BindingDB Entry DOI: 10.7270/Q2183B73 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50558880

(CHEMBL4533529)Show SMILES NC[C@H](NC(=O)c1ccc([nH]1)-c1ccc(c(F)c1)C(F)(F)F)c1nc(CO)cs1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human liver microsome CYP1A2 using phenacetin as substrate incubated for 20 mins by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2018.04.062
BindingDB Entry DOI: 10.7270/Q2183B73 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50558881

(CHEMBL4761836)Show SMILES NC[C@H](NC(=O)c1ccc([nH]1)-c1cc(F)c(Cl)c(F)c1)c1nc(CO)cs1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human liver microsome CYP1A2 using phenacetin as substrate incubated for 20 mins by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2018.04.062
BindingDB Entry DOI: 10.7270/Q2183B73 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50236759

(BMS-626529 | Temsavir)Show SMILES COc1cnc(-n2cnc(C)n2)c2[nH]cc(C(=O)C(=O)N3CCN(CC3)C(=O)c3ccccc3)c12 Show InChI InChI=1S/C24H23N7O4/c1-15-27-14-31(28-15)22-20-19(18(35-2)13-26-22)17(12-25-20)21(32)24(34)30-10-8-29(9-11-30)23(33)16-6-4-3-5-7-16/h3-7,12-14,25H,8-11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human liver microsome CYP2B6 using bupropion as substrate incubated for 20 mins by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2018.04.062
BindingDB Entry DOI: 10.7270/Q2183B73 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50558881

(CHEMBL4761836)Show SMILES NC[C@H](NC(=O)c1ccc([nH]1)-c1cc(F)c(Cl)c(F)c1)c1nc(CO)cs1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human liver microsome CYP2B6 using bupropion as substrate incubated for 20 mins by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2018.04.062
BindingDB Entry DOI: 10.7270/Q2183B73 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50236759

(BMS-626529 | Temsavir)Show SMILES COc1cnc(-n2cnc(C)n2)c2[nH]cc(C(=O)C(=O)N3CCN(CC3)C(=O)c3ccccc3)c12 Show InChI InChI=1S/C24H23N7O4/c1-15-27-14-31(28-15)22-20-19(18(35-2)13-26-22)17(12-25-20)21(32)24(34)30-10-8-29(9-11-30)23(33)16-6-4-3-5-7-16/h3-7,12-14,25H,8-11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human liver microsome CYP2C8 using paclitaxel as substrate incubated for 20 mins by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2018.04.062
BindingDB Entry DOI: 10.7270/Q2183B73 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50558880

(CHEMBL4533529)Show SMILES NC[C@H](NC(=O)c1ccc([nH]1)-c1ccc(c(F)c1)C(F)(F)F)c1nc(CO)cs1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human liver microsome CYP2C8 using paclitaxel as substrate incubated for 20 mins by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2018.04.062
BindingDB Entry DOI: 10.7270/Q2183B73 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50558881

(CHEMBL4761836)Show SMILES NC[C@H](NC(=O)c1ccc([nH]1)-c1cc(F)c(Cl)c(F)c1)c1nc(CO)cs1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human liver microsome CYP2C8 using paclitaxel as substrate incubated for 20 mins by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2018.04.062
BindingDB Entry DOI: 10.7270/Q2183B73 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50236759

(BMS-626529 | Temsavir)Show SMILES COc1cnc(-n2cnc(C)n2)c2[nH]cc(C(=O)C(=O)N3CCN(CC3)C(=O)c3ccccc3)c12 Show InChI InChI=1S/C24H23N7O4/c1-15-27-14-31(28-15)22-20-19(18(35-2)13-26-22)17(12-25-20)21(32)24(34)30-10-8-29(9-11-30)23(33)16-6-4-3-5-7-16/h3-7,12-14,25H,8-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human liver microsome CYP2C9 using diclofenac as substrate incubated for 20 mins by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2018.04.062
BindingDB Entry DOI: 10.7270/Q2183B73 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50558880

(CHEMBL4533529)Show SMILES NC[C@H](NC(=O)c1ccc([nH]1)-c1ccc(c(F)c1)C(F)(F)F)c1nc(CO)cs1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human liver microsome CYP2C9 using diclofenac as substrate incubated for 20 mins by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2018.04.062
BindingDB Entry DOI: 10.7270/Q2183B73 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50558881

(CHEMBL4761836)Show SMILES NC[C@H](NC(=O)c1ccc([nH]1)-c1cc(F)c(Cl)c(F)c1)c1nc(CO)cs1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human liver microsome CYP2C9 using diclofenac as substrate incubated for 20 mins by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2018.04.062
BindingDB Entry DOI: 10.7270/Q2183B73 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50236759

(BMS-626529 | Temsavir)Show SMILES COc1cnc(-n2cnc(C)n2)c2[nH]cc(C(=O)C(=O)N3CCN(CC3)C(=O)c3ccccc3)c12 Show InChI InChI=1S/C24H23N7O4/c1-15-27-14-31(28-15)22-20-19(18(35-2)13-26-22)17(12-25-20)21(32)24(34)30-10-8-29(9-11-30)23(33)16-6-4-3-5-7-16/h3-7,12-14,25H,8-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human liver microsome CYP2C19 using mephenytoin as substrate incubated for 20 mins by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2018.04.062
BindingDB Entry DOI: 10.7270/Q2183B73 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50558880

(CHEMBL4533529)Show SMILES NC[C@H](NC(=O)c1ccc([nH]1)-c1ccc(c(F)c1)C(F)(F)F)c1nc(CO)cs1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human liver microsome CYP2C19 using mephenytoin as substrate incubated for 20 mins by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2018.04.062
BindingDB Entry DOI: 10.7270/Q2183B73 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50558881

(CHEMBL4761836)Show SMILES NC[C@H](NC(=O)c1ccc([nH]1)-c1cc(F)c(Cl)c(F)c1)c1nc(CO)cs1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human liver microsome CYP2C19 using mephenytoin as substrate incubated for 20 mins by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2018.04.062
BindingDB Entry DOI: 10.7270/Q2183B73 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50236759

(BMS-626529 | Temsavir)Show SMILES COc1cnc(-n2cnc(C)n2)c2[nH]cc(C(=O)C(=O)N3CCN(CC3)C(=O)c3ccccc3)c12 Show InChI InChI=1S/C24H23N7O4/c1-15-27-14-31(28-15)22-20-19(18(35-2)13-26-22)17(12-25-20)21(32)24(34)30-10-8-29(9-11-30)23(33)16-6-4-3-5-7-16/h3-7,12-14,25H,8-11H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human liver microsome CYP2D6 using bufuralol as substrate incubated for 20 mins by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2018.04.062
BindingDB Entry DOI: 10.7270/Q2183B73 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50558880

(CHEMBL4533529)Show SMILES NC[C@H](NC(=O)c1ccc([nH]1)-c1ccc(c(F)c1)C(F)(F)F)c1nc(CO)cs1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human liver microsome CYP2D6 using bufuralol as substrate incubated for 20 mins by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2018.04.062
BindingDB Entry DOI: 10.7270/Q2183B73 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50558881

(CHEMBL4761836)Show SMILES NC[C@H](NC(=O)c1ccc([nH]1)-c1cc(F)c(Cl)c(F)c1)c1nc(CO)cs1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human liver microsome CYP2D6 using bufuralol as substrate incubated for 20 mins by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2018.04.062
BindingDB Entry DOI: 10.7270/Q2183B73 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50236759

(BMS-626529 | Temsavir)Show SMILES COc1cnc(-n2cnc(C)n2)c2[nH]cc(C(=O)C(=O)N3CCN(CC3)C(=O)c3ccccc3)c12 Show InChI InChI=1S/C24H23N7O4/c1-15-27-14-31(28-15)22-20-19(18(35-2)13-26-22)17(12-25-20)21(32)24(34)30-10-8-29(9-11-30)23(33)16-6-4-3-5-7-16/h3-7,12-14,25H,8-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human liver microsome CYP3A4 using testosterone as substrate incubated for 20 mins by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2018.04.062
BindingDB Entry DOI: 10.7270/Q2183B73 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50558880

(CHEMBL4533529)Show SMILES NC[C@H](NC(=O)c1ccc([nH]1)-c1ccc(c(F)c1)C(F)(F)F)c1nc(CO)cs1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human liver microsome CYP3A4 using testosterone as substrate incubated for 20 mins by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2018.04.062
BindingDB Entry DOI: 10.7270/Q2183B73 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50558881

(CHEMBL4761836)Show SMILES NC[C@H](NC(=O)c1ccc([nH]1)-c1cc(F)c(Cl)c(F)c1)c1nc(CO)cs1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human liver microsome CYP3A4 using testosterone as substrate incubated for 20 mins by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2018.04.062
BindingDB Entry DOI: 10.7270/Q2183B73 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Plasmodium falciparum) | BDBM50396100
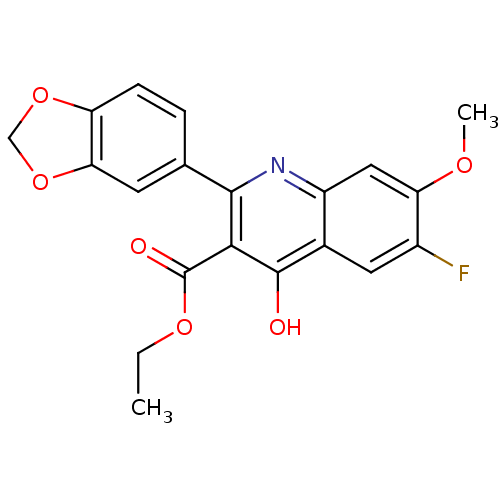
(CHEMBL2170881)Show SMILES CCOC(=O)c1c(O)c2cc(F)c(OC)cc2nc1-c1ccc2OCOc2c1 Show InChI InChI=1S/C20H16FNO6/c1-3-26-20(24)17-18(10-4-5-14-16(6-10)28-9-27-14)22-13-8-15(25-2)12(21)7-11(13)19(17)23/h4-8H,3,9H2,1-2H3,(H,22,23) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum DHOD after 5 to 10 mins by spectrophotometry |
J Med Chem 55: 4205-19 (2012)
Article DOI: 10.1021/jm201642z
BindingDB Entry DOI: 10.7270/Q28S4R13 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Plasmodium falciparum) | BDBM50396102

(CHEMBL2170887)Show SMILES CCOC(=O)c1c(O)c2cc(F)c(OC)cc2nc1-c1cccc(Cl)c1 Show InChI InChI=1S/C19H15ClFNO4/c1-3-26-19(24)16-17(10-5-4-6-11(20)7-10)22-14-9-15(25-2)13(21)8-12(14)18(16)23/h4-9H,3H2,1-2H3,(H,22,23) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum DHOD after 5 to 10 mins by spectrophotometry |
J Med Chem 55: 4205-19 (2012)
Article DOI: 10.1021/jm201642z
BindingDB Entry DOI: 10.7270/Q28S4R13 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Plasmodium falciparum) | BDBM50396101

(CHEMBL2170884)Show SMILES CCOC(=O)c1c(O)c2cc(F)c(OC)cc2nc1-c1cccc(Oc2ccccc2)c1 Show InChI InChI=1S/C25H20FNO5/c1-3-31-25(29)22-23(27-20-14-21(30-2)19(26)13-18(20)24(22)28)15-8-7-11-17(12-15)32-16-9-5-4-6-10-16/h4-14H,3H2,1-2H3,(H,27,28) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum DHOD after 5 to 10 mins by spectrophotometry |
J Med Chem 55: 4205-19 (2012)
Article DOI: 10.1021/jm201642z
BindingDB Entry DOI: 10.7270/Q28S4R13 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Plasmodium falciparum) | BDBM50396103
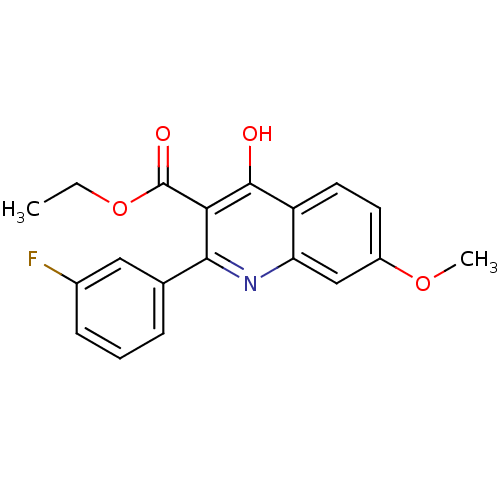
(CHEMBL1093727)Show InChI InChI=1S/C19H16FNO4/c1-3-25-19(23)16-17(11-5-4-6-12(20)9-11)21-15-10-13(24-2)7-8-14(15)18(16)22/h4-10H,3H2,1-2H3,(H,21,22) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum DHOD after 5 to 10 mins by spectrophotometry |
J Med Chem 55: 4205-19 (2012)
Article DOI: 10.1021/jm201642z
BindingDB Entry DOI: 10.7270/Q28S4R13 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data