 Found 63 hits with Last Name = 'furugohri' and Initial = 't'
Found 63 hits with Last Name = 'furugohri' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neprilysin
(Homo sapiens (Human)) | BDBM50287299
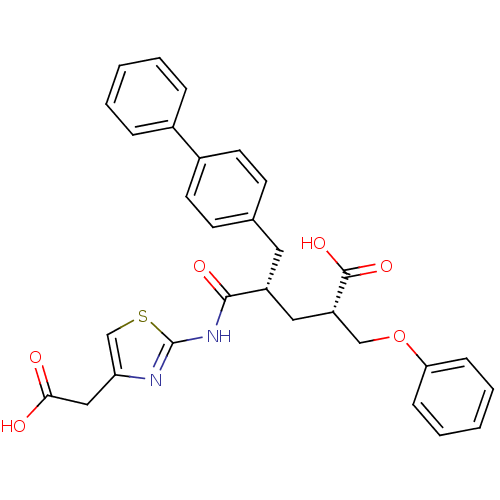
((2S,4S)-5-Biphenyl-4-yl-4-(4-carboxymethyl-thiazol...)Show SMILES OC(=O)Cc1csc(NC(=O)[C@@H](C[C@@H](COc2ccccc2)C(O)=O)Cc2ccc(cc2)-c2ccccc2)n1 Show InChI InChI=1S/C30H28N2O6S/c33-27(34)17-25-19-39-30(31-25)32-28(35)23(16-24(29(36)37)18-38-26-9-5-2-6-10-26)15-20-11-13-22(14-12-20)21-7-3-1-4-8-21/h1-14,19,23-24H,15-18H2,(H,33,34)(H,36,37)(H,31,32,35)/t23-,24+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Neutral Endopeptidase |
Bioorg Med Chem Lett 6: 1487-1490 (1996)
Article DOI: 10.1016/S0960-894X(96)00255-7
BindingDB Entry DOI: 10.7270/Q2B27VSQ |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50286907
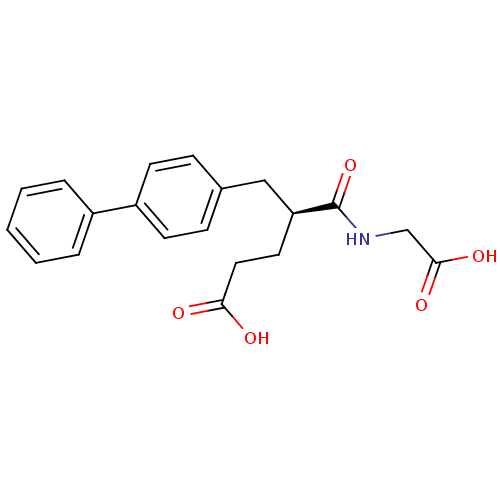
((S)-5-Biphenyl-4-yl-4-(carboxymethyl-carbamoyl)-pe...)Show SMILES OC(=O)CC[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)NCC(O)=O Show InChI InChI=1S/C20H21NO5/c22-18(23)11-10-17(20(26)21-13-19(24)25)12-14-6-8-16(9-7-14)15-4-2-1-3-5-15/h1-9,17H,10-13H2,(H,21,26)(H,22,23)(H,24,25)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of neutral endopeptidase from rat kidney. |
Bioorg Med Chem Lett 6: 65-70 (1996)
Article DOI: 10.1016/0960-894X(95)00551-4
BindingDB Entry DOI: 10.7270/Q2BZ66J9 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50286905
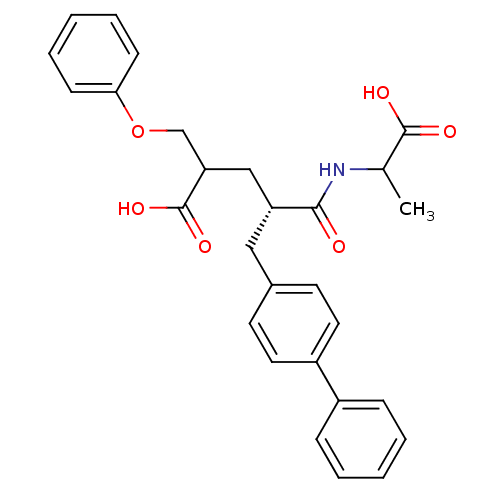
((S)-5-Biphenyl-4-yl-4-(1-carboxy-ethylcarbamoyl)-2...)Show SMILES CC(NC(=O)[C@@H](CC(COc1ccccc1)C(O)=O)Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C28H29NO6/c1-19(27(31)32)29-26(30)23(17-24(28(33)34)18-35-25-10-6-3-7-11-25)16-20-12-14-22(15-13-20)21-8-4-2-5-9-21/h2-15,19,23-24H,16-18H2,1H3,(H,29,30)(H,31,32)(H,33,34)/t19?,23-,24?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of neutral endopeptidase from rat kidney. |
Bioorg Med Chem Lett 6: 65-70 (1996)
Article DOI: 10.1016/0960-894X(95)00551-4
BindingDB Entry DOI: 10.7270/Q2BZ66J9 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50286905

((S)-5-Biphenyl-4-yl-4-(1-carboxy-ethylcarbamoyl)-2...)Show SMILES CC(NC(=O)[C@@H](CC(COc1ccccc1)C(O)=O)Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C28H29NO6/c1-19(27(31)32)29-26(30)23(17-24(28(33)34)18-35-25-10-6-3-7-11-25)16-20-12-14-22(15-13-20)21-8-4-2-5-9-21/h2-15,19,23-24H,16-18H2,1H3,(H,29,30)(H,31,32)(H,33,34)/t19?,23-,24?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of neutral endopeptidase from rat kidney. |
Bioorg Med Chem Lett 6: 65-70 (1996)
Article DOI: 10.1016/0960-894X(95)00551-4
BindingDB Entry DOI: 10.7270/Q2BZ66J9 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50286906
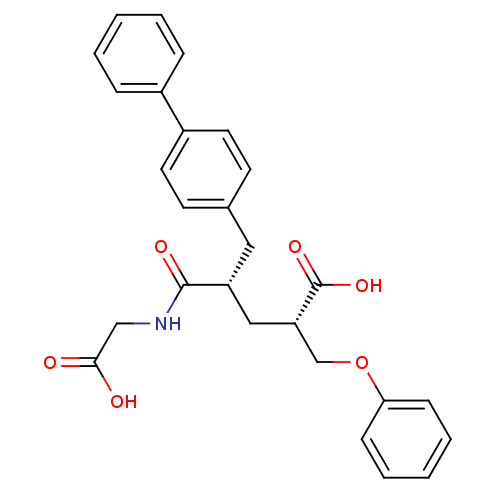
((2S,4S)-5-Biphenyl-4-yl-4-(carboxymethyl-carbamoyl...)Show SMILES OC(=O)CNC(=O)[C@@H](C[C@@H](COc1ccccc1)C(O)=O)Cc1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C27H27NO6/c29-25(30)17-28-26(31)22(16-23(27(32)33)18-34-24-9-5-2-6-10-24)15-19-11-13-21(14-12-19)20-7-3-1-4-8-20/h1-14,22-23H,15-18H2,(H,28,31)(H,29,30)(H,32,33)/t22-,23+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Neutral Endopeptidase |
Bioorg Med Chem Lett 6: 1487-1490 (1996)
Article DOI: 10.1016/S0960-894X(96)00255-7
BindingDB Entry DOI: 10.7270/Q2B27VSQ |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50286906

((2S,4S)-5-Biphenyl-4-yl-4-(carboxymethyl-carbamoyl...)Show SMILES OC(=O)CNC(=O)[C@@H](C[C@@H](COc1ccccc1)C(O)=O)Cc1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C27H27NO6/c29-25(30)17-28-26(31)22(16-23(27(32)33)18-34-24-9-5-2-6-10-24)15-19-11-13-21(14-12-19)20-7-3-1-4-8-20/h1-14,22-23H,15-18H2,(H,28,31)(H,29,30)(H,32,33)/t22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of neutral endopeptidase from rat kidney. |
Bioorg Med Chem Lett 6: 65-70 (1996)
Article DOI: 10.1016/0960-894X(95)00551-4
BindingDB Entry DOI: 10.7270/Q2BZ66J9 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50286906

((2S,4S)-5-Biphenyl-4-yl-4-(carboxymethyl-carbamoyl...)Show SMILES OC(=O)CNC(=O)[C@@H](C[C@@H](COc1ccccc1)C(O)=O)Cc1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C27H27NO6/c29-25(30)17-28-26(31)22(16-23(27(32)33)18-34-24-9-5-2-6-10-24)15-19-11-13-21(14-12-19)20-7-3-1-4-8-20/h1-14,22-23H,15-18H2,(H,28,31)(H,29,30)(H,32,33)/t22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Vascular endothelial growth factor receptor 2 (KDR) |
Bioorg Med Chem Lett 6: 65-70 (1996)
Article DOI: 10.1016/0960-894X(95)00551-4
BindingDB Entry DOI: 10.7270/Q2BZ66J9 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50287301

((2R,4S)-5-Biphenyl-4-yl-4-(4-carboxymethyl-thiazol...)Show SMILES OC(=O)Cc1csc(NC(=O)[C@@H](C[C@H](COc2ccccc2)C(O)=O)Cc2ccc(cc2)-c2ccccc2)n1 Show InChI InChI=1S/C30H28N2O6S/c33-27(34)17-25-19-39-30(31-25)32-28(35)23(16-24(29(36)37)18-38-26-9-5-2-6-10-26)15-20-11-13-22(14-12-20)21-7-3-1-4-8-21/h1-14,19,23-24H,15-18H2,(H,33,34)(H,36,37)(H,31,32,35)/t23-,24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Neutral Endopeptidase |
Bioorg Med Chem Lett 6: 1487-1490 (1996)
Article DOI: 10.1016/S0960-894X(96)00255-7
BindingDB Entry DOI: 10.7270/Q2B27VSQ |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50286908
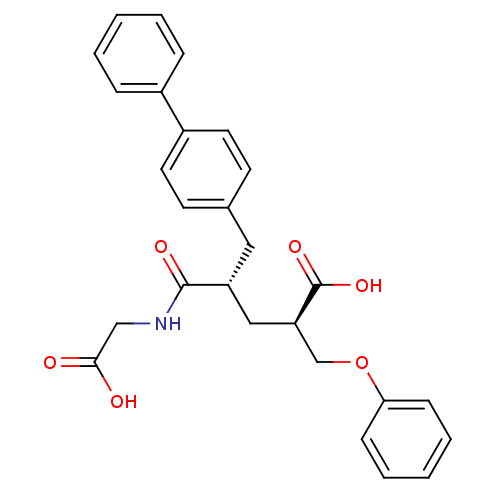
((2R,4S)-5-Biphenyl-4-yl-4-(carboxymethyl-carbamoyl...)Show SMILES OC(=O)CNC(=O)[C@@H](C[C@H](COc1ccccc1)C(O)=O)Cc1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C27H27NO6/c29-25(30)17-28-26(31)22(16-23(27(32)33)18-34-24-9-5-2-6-10-24)15-19-11-13-21(14-12-19)20-7-3-1-4-8-20/h1-14,22-23H,15-18H2,(H,28,31)(H,29,30)(H,32,33)/t22-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of neutral endopeptidase from rat kidney. |
Bioorg Med Chem Lett 6: 65-70 (1996)
Article DOI: 10.1016/0960-894X(95)00551-4
BindingDB Entry DOI: 10.7270/Q2BZ66J9 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50286908

((2R,4S)-5-Biphenyl-4-yl-4-(carboxymethyl-carbamoyl...)Show SMILES OC(=O)CNC(=O)[C@@H](C[C@H](COc1ccccc1)C(O)=O)Cc1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C27H27NO6/c29-25(30)17-28-26(31)22(16-23(27(32)33)18-34-24-9-5-2-6-10-24)15-19-11-13-21(14-12-19)20-7-3-1-4-8-20/h1-14,22-23H,15-18H2,(H,28,31)(H,29,30)(H,32,33)/t22-,23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Neutral Endopeptidase |
Bioorg Med Chem Lett 6: 1487-1490 (1996)
Article DOI: 10.1016/S0960-894X(96)00255-7
BindingDB Entry DOI: 10.7270/Q2B27VSQ |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50286906

((2S,4S)-5-Biphenyl-4-yl-4-(carboxymethyl-carbamoyl...)Show SMILES OC(=O)CNC(=O)[C@@H](C[C@@H](COc1ccccc1)C(O)=O)Cc1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C27H27NO6/c29-25(30)17-28-26(31)22(16-23(27(32)33)18-34-24-9-5-2-6-10-24)15-19-11-13-21(14-12-19)20-7-3-1-4-8-20/h1-14,22-23H,15-18H2,(H,28,31)(H,29,30)(H,32,33)/t22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of neutral endopeptidase from rat kidney. |
Bioorg Med Chem Lett 6: 65-70 (1996)
Article DOI: 10.1016/0960-894X(95)00551-4
BindingDB Entry DOI: 10.7270/Q2BZ66J9 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50287304
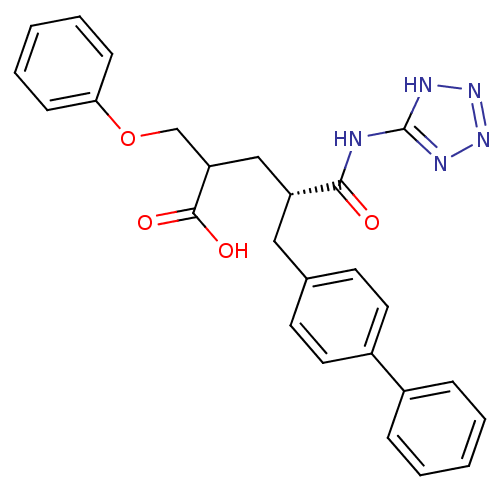
((S)-5-Biphenyl-4-yl-2-phenoxymethyl-4-(1H-tetrazol...)Show SMILES OC(=O)C(COc1ccccc1)C[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)Nc1nnn[nH]1 Show InChI InChI=1S/C26H25N5O4/c32-24(27-26-28-30-31-29-26)21(16-22(25(33)34)17-35-23-9-5-2-6-10-23)15-18-11-13-20(14-12-18)19-7-3-1-4-8-19/h1-14,21-22H,15-17H2,(H,33,34)(H2,27,28,29,30,31,32)/t21-,22?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Neutral Endopeptidase |
Bioorg Med Chem Lett 6: 1487-1490 (1996)
Article DOI: 10.1016/S0960-894X(96)00255-7
BindingDB Entry DOI: 10.7270/Q2B27VSQ |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM21641

(2-(2-benzyl-3-sulfanylpropanamido)acetic acid | CH...)Show InChI InChI=1S/C12H15NO3S/c14-11(15)7-13-12(16)10(8-17)6-9-4-2-1-3-5-9/h1-5,10,17H,6-8H2,(H,13,16)(H,14,15) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Neutral Endopeptidase |
Bioorg Med Chem Lett 6: 1487-1490 (1996)
Article DOI: 10.1016/S0960-894X(96)00255-7
BindingDB Entry DOI: 10.7270/Q2B27VSQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM21641

(2-(2-benzyl-3-sulfanylpropanamido)acetic acid | CH...)Show InChI InChI=1S/C12H15NO3S/c14-11(15)7-13-12(16)10(8-17)6-9-4-2-1-3-5-9/h1-5,10,17H,6-8H2,(H,13,16)(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of neutral endopeptidase from rat kidney. |
Bioorg Med Chem Lett 6: 65-70 (1996)
Article DOI: 10.1016/0960-894X(95)00551-4
BindingDB Entry DOI: 10.7270/Q2BZ66J9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50286909
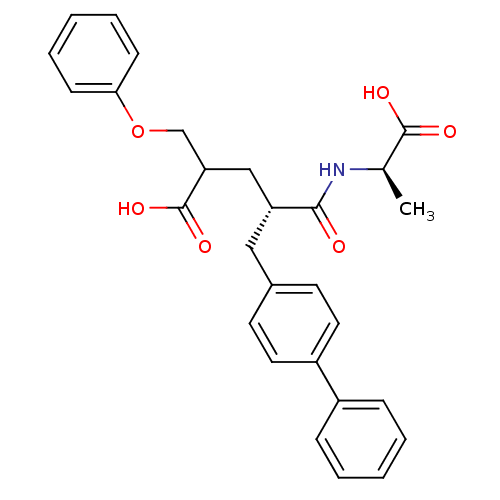
((S)-5-Biphenyl-4-yl-4-((R)-1-carboxy-ethylcarbamoy...)Show SMILES C[C@@H](NC(=O)[C@@H](CC(COc1ccccc1)C(O)=O)Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C28H29NO6/c1-19(27(31)32)29-26(30)23(17-24(28(33)34)18-35-25-10-6-3-7-11-25)16-20-12-14-22(15-13-20)21-8-4-2-5-9-21/h2-15,19,23-24H,16-18H2,1H3,(H,29,30)(H,31,32)(H,33,34)/t19-,23-,24?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of neutral endopeptidase from rat kidney. |
Bioorg Med Chem Lett 6: 65-70 (1996)
Article DOI: 10.1016/0960-894X(95)00551-4
BindingDB Entry DOI: 10.7270/Q2BZ66J9 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50286909

((S)-5-Biphenyl-4-yl-4-((R)-1-carboxy-ethylcarbamoy...)Show SMILES C[C@@H](NC(=O)[C@@H](CC(COc1ccccc1)C(O)=O)Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C28H29NO6/c1-19(27(31)32)29-26(30)23(17-24(28(33)34)18-35-25-10-6-3-7-11-25)16-20-12-14-22(15-13-20)21-8-4-2-5-9-21/h2-15,19,23-24H,16-18H2,1H3,(H,29,30)(H,31,32)(H,33,34)/t19-,23-,24?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of neutral endopeptidase from rat kidney. |
Bioorg Med Chem Lett 6: 65-70 (1996)
Article DOI: 10.1016/0960-894X(95)00551-4
BindingDB Entry DOI: 10.7270/Q2BZ66J9 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50287300
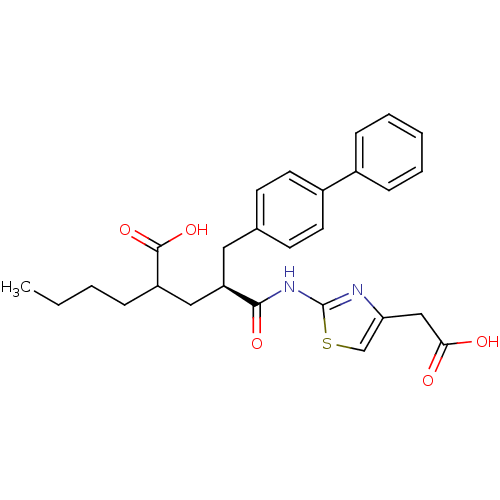
(2-[(S)-3-Biphenyl-4-yl-2-(4-carboxymethyl-thiazol-...)Show SMILES CCCCC(C[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)Nc1nc(CC(O)=O)cs1)C(O)=O Show InChI InChI=1S/C27H30N2O5S/c1-2-3-7-21(26(33)34)15-22(25(32)29-27-28-23(17-35-27)16-24(30)31)14-18-10-12-20(13-11-18)19-8-5-4-6-9-19/h4-6,8-13,17,21-22H,2-3,7,14-16H2,1H3,(H,30,31)(H,33,34)(H,28,29,32)/t21?,22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Neutral Endopeptidase |
Bioorg Med Chem Lett 6: 1487-1490 (1996)
Article DOI: 10.1016/S0960-894X(96)00255-7
BindingDB Entry DOI: 10.7270/Q2B27VSQ |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50287300

(2-[(S)-3-Biphenyl-4-yl-2-(4-carboxymethyl-thiazol-...)Show SMILES CCCCC(C[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)Nc1nc(CC(O)=O)cs1)C(O)=O Show InChI InChI=1S/C27H30N2O5S/c1-2-3-7-21(26(33)34)15-22(25(32)29-27-28-23(17-35-27)16-24(30)31)14-18-10-12-20(13-11-18)19-8-5-4-6-9-19/h4-6,8-13,17,21-22H,2-3,7,14-16H2,1H3,(H,30,31)(H,33,34)(H,28,29,32)/t21?,22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Neutral Endopeptidase |
Bioorg Med Chem Lett 6: 1487-1490 (1996)
Article DOI: 10.1016/S0960-894X(96)00255-7
BindingDB Entry DOI: 10.7270/Q2B27VSQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50214997
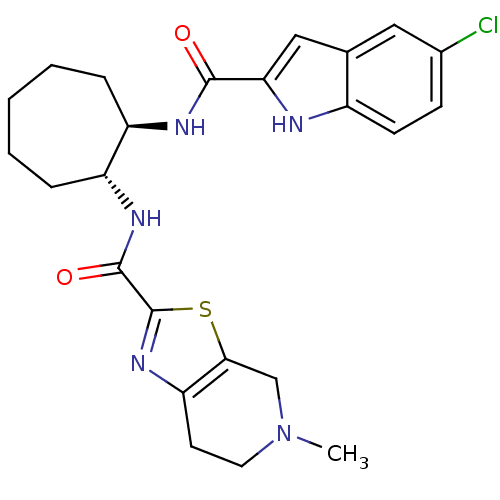
(CHEMBL439391 | trans-N-(2-(5-chloro-1H-indole-2-ca...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N[C@@H]1CCCCC[C@H]1NC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C24H28ClN5O2S/c1-30-10-9-19-21(13-30)33-24(29-19)23(32)28-18-6-4-2-3-5-17(18)27-22(31)20-12-14-11-15(25)7-8-16(14)26-20/h7-8,11-12,17-18,26H,2-6,9-10,13H2,1H3,(H,27,31)(H,28,32)/t17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a after 10 seconds |
Bioorg Med Chem Lett 17: 4683-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.068
BindingDB Entry DOI: 10.7270/Q2183660 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50214983
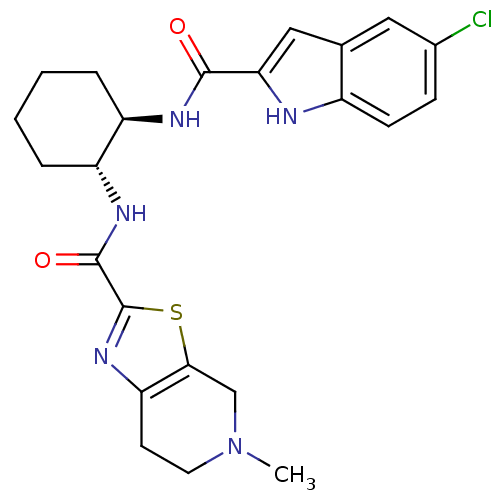
(CHEMBL391805 | N-((1R,2R)-2-(5-CHLORO-1H-INDOLE-2-...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N[C@@H]1CCCC[C@H]1NC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C23H26ClN5O2S/c1-29-9-8-18-20(12-29)32-23(28-18)22(31)27-17-5-3-2-4-16(17)26-21(30)19-11-13-10-14(24)6-7-15(13)25-19/h6-7,10-11,16-17,25H,2-5,8-9,12H2,1H3,(H,26,30)(H,27,31)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a after 10 seconds |
Bioorg Med Chem Lett 17: 4683-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.068
BindingDB Entry DOI: 10.7270/Q2183660 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50214995
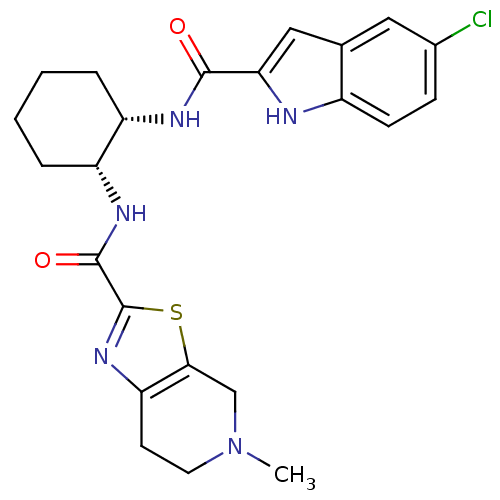
(CHEMBL245678 | N-((1R,2S)-2-(5-CHLORO-1H-INDOLE-2-...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N[C@@H]1CCCC[C@@H]1NC(=O)c1cc2cc(Cl)ccc2[nH]1 |r| Show InChI InChI=1S/C23H26ClN5O2S/c1-29-9-8-18-20(12-29)32-23(28-18)22(31)27-17-5-3-2-4-16(17)26-21(30)19-11-13-10-14(24)6-7-15(13)25-19/h6-7,10-11,16-17,25H,2-5,8-9,12H2,1H3,(H,26,30)(H,27,31)/t16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a after 10 seconds |
Bioorg Med Chem Lett 17: 4683-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.068
BindingDB Entry DOI: 10.7270/Q2183660 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50214995

(CHEMBL245678 | N-((1R,2S)-2-(5-CHLORO-1H-INDOLE-2-...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N[C@@H]1CCCC[C@@H]1NC(=O)c1cc2cc(Cl)ccc2[nH]1 |r| Show InChI InChI=1S/C23H26ClN5O2S/c1-29-9-8-18-20(12-29)32-23(28-18)22(31)27-17-5-3-2-4-16(17)26-21(30)19-11-13-10-14(24)6-7-15(13)25-19/h6-7,10-11,16-17,25H,2-5,8-9,12H2,1H3,(H,26,30)(H,27,31)/t16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a after 10 seconds |
Bioorg Med Chem Lett 17: 4683-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.068
BindingDB Entry DOI: 10.7270/Q2183660 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neprilysin
(Homo sapiens (Human)) | BDBM50287297

((S)-5-Biphenyl-4-yl-2-phenoxymethyl-4-[(1H-tetrazo...)Show SMILES OC(=O)C(COc1ccccc1)C[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)NCc1nnn[nH]1 Show InChI InChI=1S/C27H27N5O4/c33-26(28-17-25-29-31-32-30-25)22(16-23(27(34)35)18-36-24-9-5-2-6-10-24)15-19-11-13-21(14-12-19)20-7-3-1-4-8-20/h1-14,22-23H,15-18H2,(H,28,33)(H,34,35)(H,29,30,31,32)/t22-,23?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Neutral Endopeptidase |
Bioorg Med Chem Lett 6: 1487-1490 (1996)
Article DOI: 10.1016/S0960-894X(96)00255-7
BindingDB Entry DOI: 10.7270/Q2B27VSQ |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50287302

((S)-5-Biphenyl-4-yl-4-(4-carboxymethyl-thiazol-2-y...)Show SMILES CC(C[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)Nc1nc(CC(O)=O)cs1)C(O)=O Show InChI InChI=1S/C24H24N2O5S/c1-15(23(30)31)11-19(22(29)26-24-25-20(14-32-24)13-21(27)28)12-16-7-9-18(10-8-16)17-5-3-2-4-6-17/h2-10,14-15,19H,11-13H2,1H3,(H,27,28)(H,30,31)(H,25,26,29)/t15?,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Neutral Endopeptidase |
Bioorg Med Chem Lett 6: 1487-1490 (1996)
Article DOI: 10.1016/S0960-894X(96)00255-7
BindingDB Entry DOI: 10.7270/Q2B27VSQ |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50403562
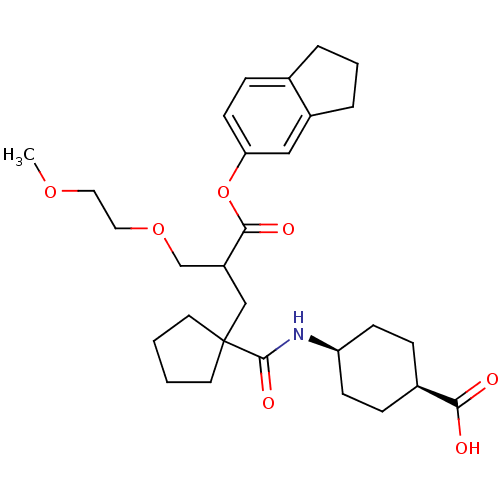
(CHEMBL2062138 | UK-69578)Show SMILES COCCOCC(CC1(CCCC1)C(=O)N[C@H]1CC[C@H](CC1)C(O)=O)C(=O)Oc1ccc2CCCc2c1 |wU:19.23,16.16,(-7.72,2.65,;-6.63,1.56,;-5.54,2.65,;-4.45,1.54,;-3.34,2.63,;-2.23,1.53,;-2.23,-.01,;-.89,-.72,;.43,.09,;1.68,.98,;1.19,2.46,;-.35,2.44,;-.82,.98,;1.77,-.64,;1.84,-2.18,;3.09,.18,;4.46,-.55,;5.74,.28,;7.1,-.46,;7.16,-2,;5.85,-2.8,;4.5,-2.08,;8.51,-2.73,;8.56,-4.27,;9.82,-1.9,;-3.55,-.81,;-3.5,-2.35,;-4.9,-.07,;-6.21,-.91,;-7.57,-.17,;-8.88,-.99,;-8.84,-2.54,;-9.94,-3.63,;-9.26,-5.01,;-7.72,-4.79,;-7.46,-3.25,;-6.16,-2.45,)| Show InChI InChI=1S/C29H41NO7/c1-35-15-16-36-19-23(27(33)37-25-12-9-20-5-4-6-22(20)17-25)18-29(13-2-3-14-29)28(34)30-24-10-7-21(8-11-24)26(31)32/h9,12,17,21,23-24H,2-8,10-11,13-16,18-19H2,1H3,(H,30,34)(H,31,32)/t21-,23?,24+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Neutral Endopeptidase |
Bioorg Med Chem Lett 6: 1487-1490 (1996)
Article DOI: 10.1016/S0960-894X(96)00255-7
BindingDB Entry DOI: 10.7270/Q2B27VSQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50214988

(CHEMBL393873 | trans-N-(2-(5-chloro-1H-indole-2-ca...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N[C@@H]1CCC[C@H]1NC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C22H24ClN5O2S/c1-28-8-7-17-19(11-28)31-22(27-17)21(30)26-16-4-2-3-15(16)25-20(29)18-10-12-9-13(23)5-6-14(12)24-18/h5-6,9-10,15-16,24H,2-4,7-8,11H2,1H3,(H,25,29)(H,26,30)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a after 10 seconds |
Bioorg Med Chem Lett 17: 4683-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.068
BindingDB Entry DOI: 10.7270/Q2183660 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50287303
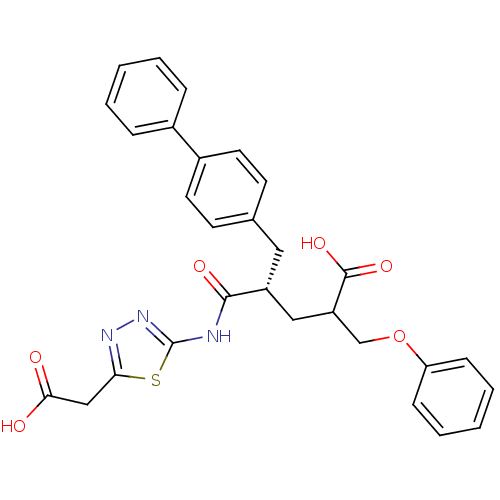
((S)-5-Biphenyl-4-yl-4-(5-carboxymethyl-[1,3,4]thia...)Show SMILES OC(=O)Cc1nnc(NC(=O)[C@@H](CC(COc2ccccc2)C(O)=O)Cc2ccc(cc2)-c2ccccc2)s1 Show InChI InChI=1S/C29H27N3O6S/c33-26(34)17-25-31-32-29(39-25)30-27(35)22(16-23(28(36)37)18-38-24-9-5-2-6-10-24)15-19-11-13-21(14-12-19)20-7-3-1-4-8-20/h1-14,22-23H,15-18H2,(H,33,34)(H,36,37)(H,30,32,35)/t22-,23?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Neutral Endopeptidase |
Bioorg Med Chem Lett 6: 1487-1490 (1996)
Article DOI: 10.1016/S0960-894X(96)00255-7
BindingDB Entry DOI: 10.7270/Q2B27VSQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50214986
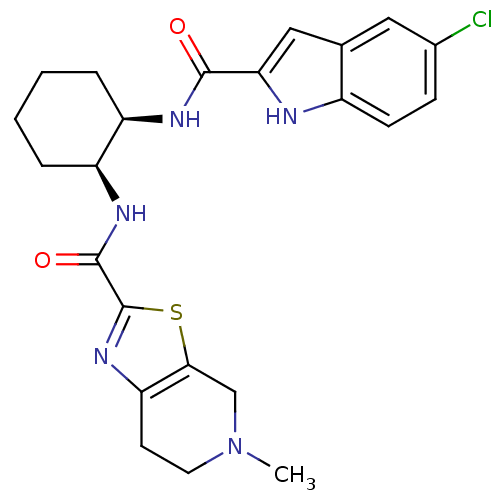
(CHEMBL247732 | N-((1S,2R)-2-(5-chloro-1H-indole-2-...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N[C@H]1CCCC[C@H]1NC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C23H26ClN5O2S/c1-29-9-8-18-20(12-29)32-23(28-18)22(31)27-17-5-3-2-4-16(17)26-21(30)19-11-13-10-14(24)6-7-15(13)25-19/h6-7,10-11,16-17,25H,2-5,8-9,12H2,1H3,(H,26,30)(H,27,31)/t16-,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 2a after 10 seconds |
Bioorg Med Chem Lett 17: 4683-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.068
BindingDB Entry DOI: 10.7270/Q2183660 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50286910
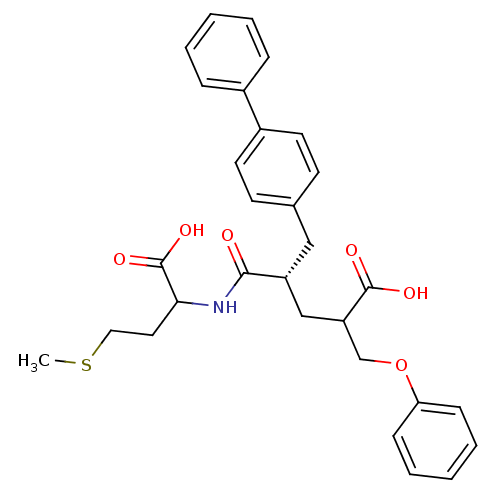
((S)-5-Biphenyl-4-yl-4-(1-carboxy-3-methylsulfanyl-...)Show SMILES CSCCC(NC(=O)[C@@H](CC(COc1ccccc1)C(O)=O)Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C30H33NO6S/c1-38-17-16-27(30(35)36)31-28(32)24(19-25(29(33)34)20-37-26-10-6-3-7-11-26)18-21-12-14-23(15-13-21)22-8-4-2-5-9-22/h2-15,24-25,27H,16-20H2,1H3,(H,31,32)(H,33,34)(H,35,36)/t24-,25?,27?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of neutral endopeptidase from rat kidney. |
Bioorg Med Chem Lett 6: 65-70 (1996)
Article DOI: 10.1016/0960-894X(95)00551-4
BindingDB Entry DOI: 10.7270/Q2BZ66J9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50214985
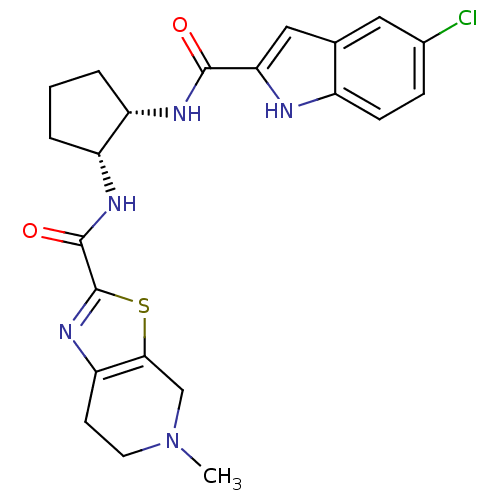
(CHEMBL391804 | cis-N-(2-(5-chloro-1H-indole-2-carb...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N[C@@H]1CCC[C@@H]1NC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C22H24ClN5O2S/c1-28-8-7-17-19(11-28)31-22(27-17)21(30)26-16-4-2-3-15(16)25-20(29)18-10-12-9-13(23)5-6-14(12)24-18/h5-6,9-10,15-16,24H,2-4,7-8,11H2,1H3,(H,25,29)(H,26,30)/t15-,16+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a after 10 seconds |
Bioorg Med Chem Lett 17: 4683-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.068
BindingDB Entry DOI: 10.7270/Q2183660 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50287305

((S)-5-Biphenyl-4-yl-4-(4-carboxymethyl-thiazol-2-y...)Show SMILES OC(=O)CC[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)Nc1nc(CC(O)=O)cs1 Show InChI InChI=1S/C23H22N2O5S/c26-20(27)11-10-18(22(30)25-23-24-19(14-31-23)13-21(28)29)12-15-6-8-17(9-7-15)16-4-2-1-3-5-16/h1-9,14,18H,10-13H2,(H,26,27)(H,28,29)(H,24,25,30)/t18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Neutral Endopeptidase |
Bioorg Med Chem Lett 6: 1487-1490 (1996)
Article DOI: 10.1016/S0960-894X(96)00255-7
BindingDB Entry DOI: 10.7270/Q2B27VSQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50214987
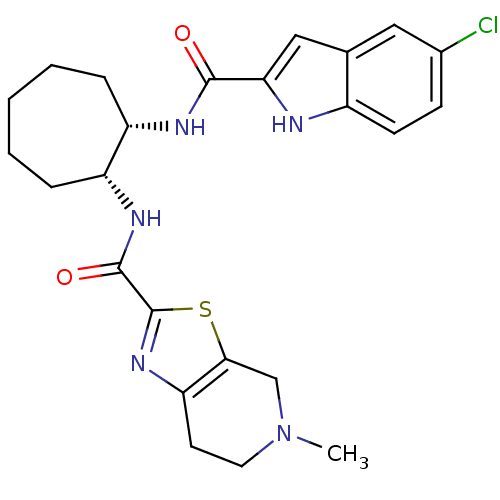
(CHEMBL245679 | cis-N-(2-(5-chloro-1H-indole-2-carb...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N[C@@H]1CCCCC[C@@H]1NC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C24H28ClN5O2S/c1-30-10-9-19-21(13-30)33-24(29-19)23(32)28-18-6-4-2-3-5-17(18)27-22(31)20-12-14-11-15(25)7-8-16(14)26-20/h7-8,11-12,17-18,26H,2-6,9-10,13H2,1H3,(H,27,31)(H,28,32)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 175 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a after 10 seconds |
Bioorg Med Chem Lett 17: 4683-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.068
BindingDB Entry DOI: 10.7270/Q2183660 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50214998
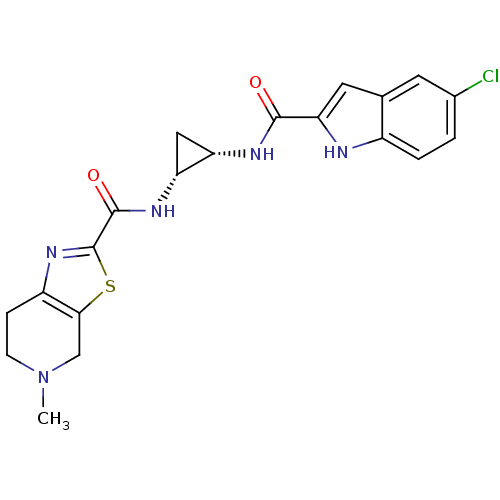
(CHEMBL391593 | cis-N-(2-(5-chloro-1H-indole-2-carb...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N[C@@H]1C[C@@H]1NC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C20H20ClN5O2S/c1-26-5-4-13-17(9-26)29-20(25-13)19(28)24-15-8-14(15)23-18(27)16-7-10-6-11(21)2-3-12(10)22-16/h2-3,6-7,14-15,22H,4-5,8-9H2,1H3,(H,23,27)(H,24,28)/t14-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 187 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a after 10 seconds |
Bioorg Med Chem Lett 17: 4683-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.068
BindingDB Entry DOI: 10.7270/Q2183660 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50287298

((S)-5-Biphenyl-4-yl-4-(4-carboxymethyl-1H-imidazol...)Show SMILES OC(=O)Cc1cnc(NC(=O)[C@@H](CC(COc2ccccc2)C(O)=O)Cc2ccc(cc2)-c2ccccc2)[nH]1 Show InChI InChI=1S/C30H29N3O6/c34-27(35)17-25-18-31-30(32-25)33-28(36)23(16-24(29(37)38)19-39-26-9-5-2-6-10-26)15-20-11-13-22(14-12-20)21-7-3-1-4-8-21/h1-14,18,23-24H,15-17,19H2,(H,34,35)(H,37,38)(H2,31,32,33,36)/t23-,24?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 218 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Neutral Endopeptidase |
Bioorg Med Chem Lett 6: 1487-1490 (1996)
Article DOI: 10.1016/S0960-894X(96)00255-7
BindingDB Entry DOI: 10.7270/Q2B27VSQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50214999

(CHEMBL247525 | cis-N-(2-(5-chloro-1H-indole-2-carb...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N[C@@H]1CC[C@@H]1NC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C21H22ClN5O2S/c1-27-7-6-16-18(10-27)30-21(26-16)20(29)25-15-5-4-14(15)24-19(28)17-9-11-8-12(22)2-3-13(11)23-17/h2-3,8-9,14-15,23H,4-7,10H2,1H3,(H,24,28)(H,25,29)/t14-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 265 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a after 10 seconds |
Bioorg Med Chem Lett 17: 4683-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.068
BindingDB Entry DOI: 10.7270/Q2183660 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50214992
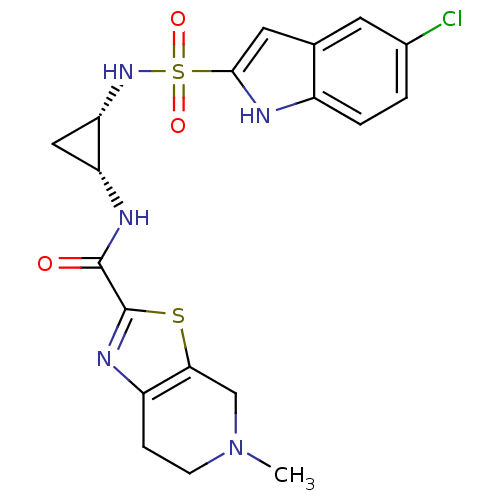
(CHEMBL246915 | cis-N-(2-(5-chloro-1H-indole-2-sulf...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N[C@@H]1C[C@@H]1NS(=O)(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C19H20ClN5O3S2/c1-25-5-4-13-16(9-25)29-19(23-13)18(26)22-14-8-15(14)24-30(27,28)17-7-10-6-11(20)2-3-12(10)21-17/h2-3,6-7,14-15,21,24H,4-5,8-9H2,1H3,(H,22,26)/t14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a after 10 seconds |
Bioorg Med Chem Lett 17: 4683-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.068
BindingDB Entry DOI: 10.7270/Q2183660 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50286917
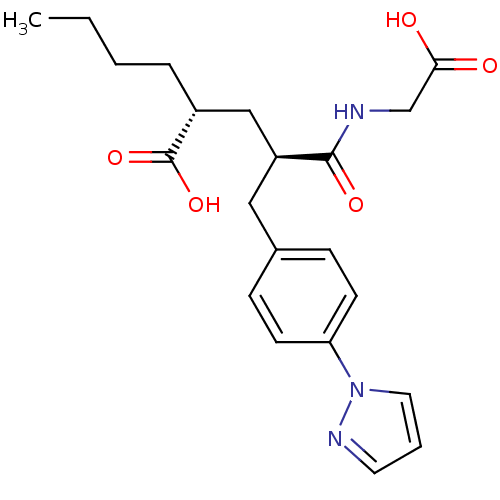
((R)-2-[(R)-2-(Carboxymethyl-carbamoyl)-3-(4-pyrazo...)Show SMILES CCCC[C@H](C[C@H](Cc1ccc(cc1)-n1cccn1)C(=O)NCC(O)=O)C(O)=O Show InChI InChI=1S/C21H27N3O5/c1-2-3-5-16(21(28)29)13-17(20(27)22-14-19(25)26)12-15-6-8-18(9-7-15)24-11-4-10-23-24/h4,6-11,16-17H,2-3,5,12-14H2,1H3,(H,22,27)(H,25,26)(H,28,29)/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 366 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of neutral endopeptidase from rat kidney. |
Bioorg Med Chem Lett 6: 65-70 (1996)
Article DOI: 10.1016/0960-894X(95)00551-4
BindingDB Entry DOI: 10.7270/Q2BZ66J9 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50286915
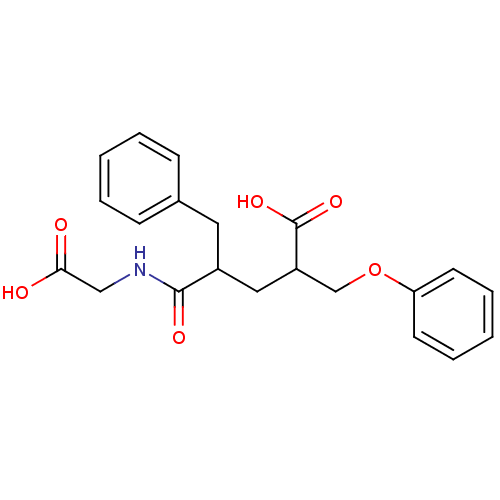
(4-(Carboxymethyl-carbamoyl)-2-phenoxymethyl-5-phen...)Show SMILES OC(=O)CNC(=O)C(CC(COc1ccccc1)C(O)=O)Cc1ccccc1 Show InChI InChI=1S/C21H23NO6/c23-19(24)13-22-20(25)16(11-15-7-3-1-4-8-15)12-17(21(26)27)14-28-18-9-5-2-6-10-18/h1-10,16-17H,11-14H2,(H,22,25)(H,23,24)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 407 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of neutral endopeptidase from rat kidney. |
Bioorg Med Chem Lett 6: 65-70 (1996)
Article DOI: 10.1016/0960-894X(95)00551-4
BindingDB Entry DOI: 10.7270/Q2BZ66J9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50214986

(CHEMBL247732 | N-((1S,2R)-2-(5-chloro-1H-indole-2-...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N[C@H]1CCCC[C@H]1NC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C23H26ClN5O2S/c1-29-9-8-18-20(12-29)32-23(28-18)22(31)27-17-5-3-2-4-16(17)26-21(30)19-11-13-10-14(24)6-7-15(13)25-19/h6-7,10-11,16-17,25H,2-5,8-9,12H2,1H3,(H,26,30)(H,27,31)/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 753 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a after 10 seconds |
Bioorg Med Chem Lett 17: 4683-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.068
BindingDB Entry DOI: 10.7270/Q2183660 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50287296
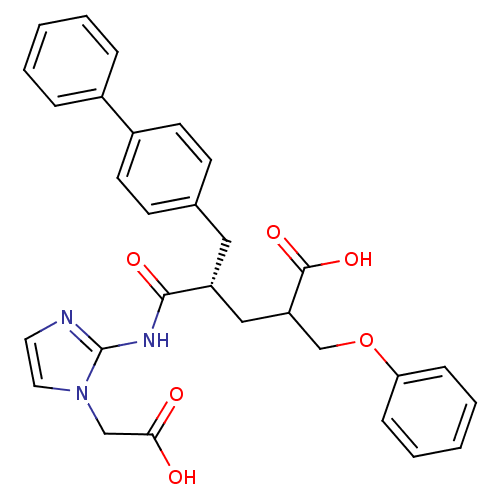
((S)-5-Biphenyl-4-yl-4-(1-carboxymethyl-1H-imidazol...)Show SMILES OC(=O)Cn1ccnc1NC(=O)[C@@H](CC(COc1ccccc1)C(O)=O)Cc1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C30H29N3O6/c34-27(35)19-33-16-15-31-30(33)32-28(36)24(18-25(29(37)38)20-39-26-9-5-2-6-10-26)17-21-11-13-23(14-12-21)22-7-3-1-4-8-22/h1-16,24-25H,17-20H2,(H,34,35)(H,37,38)(H,31,32,36)/t24-,25?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 763 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Neutral Endopeptidase |
Bioorg Med Chem Lett 6: 1487-1490 (1996)
Article DOI: 10.1016/S0960-894X(96)00255-7
BindingDB Entry DOI: 10.7270/Q2B27VSQ |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50286916
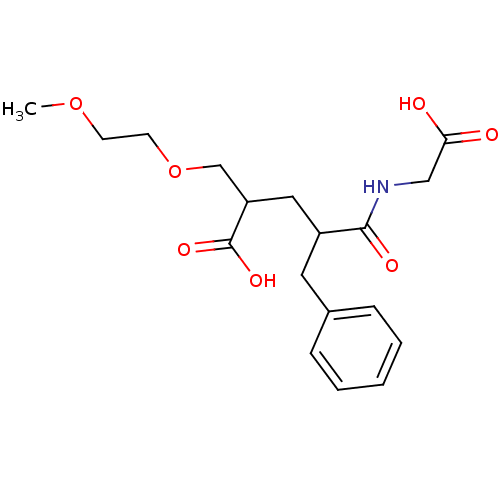
(4-(Carboxymethyl-carbamoyl)-2-(2-methoxy-ethoxymet...)Show InChI InChI=1S/C18H25NO7/c1-25-7-8-26-12-15(18(23)24)10-14(17(22)19-11-16(20)21)9-13-5-3-2-4-6-13/h2-6,14-15H,7-12H2,1H3,(H,19,22)(H,20,21)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 945 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of neutral endopeptidase from rat kidney. |
Bioorg Med Chem Lett 6: 65-70 (1996)
Article DOI: 10.1016/0960-894X(95)00551-4
BindingDB Entry DOI: 10.7270/Q2BZ66J9 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50286915

(4-(Carboxymethyl-carbamoyl)-2-phenoxymethyl-5-phen...)Show SMILES OC(=O)CNC(=O)C(CC(COc1ccccc1)C(O)=O)Cc1ccccc1 Show InChI InChI=1S/C21H23NO6/c23-19(24)13-22-20(25)16(11-15-7-3-1-4-8-15)12-17(21(26)27)14-28-18-9-5-2-6-10-18/h1-10,16-17H,11-14H2,(H,22,25)(H,23,24)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of neutral endopeptidase from rat kidney. |
Bioorg Med Chem Lett 6: 65-70 (1996)
Article DOI: 10.1016/0960-894X(95)00551-4
BindingDB Entry DOI: 10.7270/Q2BZ66J9 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50286914
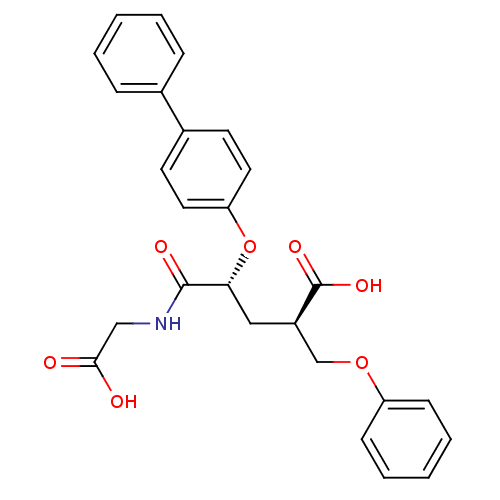
((2R,4R)-4-(Biphenyl-4-yloxy)-4-(carboxymethyl-carb...)Show SMILES OC(=O)CNC(=O)[C@@H](C[C@H](COc1ccccc1)C(O)=O)Oc1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C26H25NO7/c28-24(29)16-27-25(30)23(15-20(26(31)32)17-33-21-9-5-2-6-10-21)34-22-13-11-19(12-14-22)18-7-3-1-4-8-18/h1-14,20,23H,15-17H2,(H,27,30)(H,28,29)(H,31,32)/t20-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of neutral endopeptidase from rat kidney. |
Bioorg Med Chem Lett 6: 65-70 (1996)
Article DOI: 10.1016/0960-894X(95)00551-4
BindingDB Entry DOI: 10.7270/Q2BZ66J9 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50286913

((Z)-2-[(R)-2-(Carboxymethyl-carbamoyl)-3-(4-pyrazo...)Show SMILES CCC\C=C(\C[C@@H](Cc1ccc(cc1)-n1cccn1)C(=O)NCC(O)=O)C(O)=O Show InChI InChI=1S/C21H25N3O5/c1-2-3-5-16(21(28)29)13-17(20(27)22-14-19(25)26)12-15-6-8-18(9-7-15)24-11-4-10-23-24/h4-11,17H,2-3,12-14H2,1H3,(H,22,27)(H,25,26)(H,28,29)/b16-5-/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of neutral endopeptidase from rat kidney. |
Bioorg Med Chem Lett 6: 65-70 (1996)
Article DOI: 10.1016/0960-894X(95)00551-4
BindingDB Entry DOI: 10.7270/Q2BZ66J9 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50286911
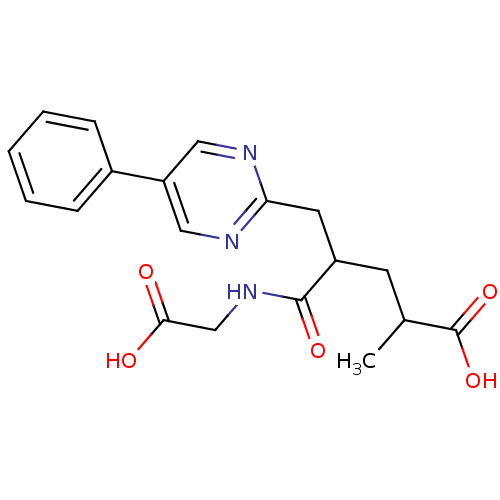
(4-(Carboxymethyl-carbamoyl)-2-methyl-5-(5-phenyl-p...)Show SMILES CC(CC(Cc1ncc(cn1)-c1ccccc1)C(=O)NCC(O)=O)C(O)=O Show InChI InChI=1S/C19H21N3O5/c1-12(19(26)27)7-14(18(25)22-11-17(23)24)8-16-20-9-15(10-21-16)13-5-3-2-4-6-13/h2-6,9-10,12,14H,7-8,11H2,1H3,(H,22,25)(H,23,24)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of neutral endopeptidase from rat kidney. |
Bioorg Med Chem Lett 6: 65-70 (1996)
Article DOI: 10.1016/0960-894X(95)00551-4
BindingDB Entry DOI: 10.7270/Q2BZ66J9 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50286912
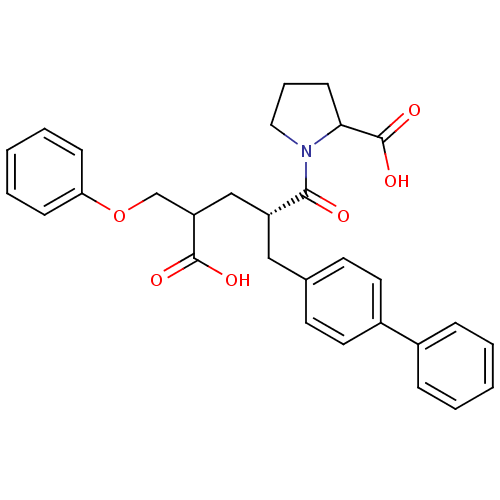
(1-((S)-2-Biphenyl-4-ylmethyl-4-carboxy-5-phenoxy-p...)Show SMILES OC(=O)C(COc1ccccc1)C[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N1CCCC1C(O)=O Show InChI InChI=1S/C30H31NO6/c32-28(31-17-7-12-27(31)30(35)36)24(19-25(29(33)34)20-37-26-10-5-2-6-11-26)18-21-13-15-23(16-14-21)22-8-3-1-4-9-22/h1-6,8-11,13-16,24-25,27H,7,12,17-20H2,(H,33,34)(H,35,36)/t24-,25?,27?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of neutral endopeptidase from rat kidney. |
Bioorg Med Chem Lett 6: 65-70 (1996)
Article DOI: 10.1016/0960-894X(95)00551-4
BindingDB Entry DOI: 10.7270/Q2BZ66J9 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50286912

(1-((S)-2-Biphenyl-4-ylmethyl-4-carboxy-5-phenoxy-p...)Show SMILES OC(=O)C(COc1ccccc1)C[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N1CCCC1C(O)=O Show InChI InChI=1S/C30H31NO6/c32-28(31-17-7-12-27(31)30(35)36)24(19-25(29(33)34)20-37-26-10-5-2-6-11-26)18-21-13-15-23(16-14-21)22-8-3-1-4-9-22/h1-6,8-11,13-16,24-25,27H,7,12,17-20H2,(H,33,34)(H,35,36)/t24-,25?,27?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Vascular endothelial growth factor receptor 2 (KDR) |
Bioorg Med Chem Lett 6: 65-70 (1996)
Article DOI: 10.1016/0960-894X(95)00551-4
BindingDB Entry DOI: 10.7270/Q2BZ66J9 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50286916

(4-(Carboxymethyl-carbamoyl)-2-(2-methoxy-ethoxymet...)Show InChI InChI=1S/C18H25NO7/c1-25-7-8-26-12-15(18(23)24)10-14(17(22)19-11-16(20)21)9-13-5-3-2-4-6-13/h2-6,14-15H,7-12H2,1H3,(H,19,22)(H,20,21)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of neutral endopeptidase from rat kidney. |
Bioorg Med Chem Lett 6: 65-70 (1996)
Article DOI: 10.1016/0960-894X(95)00551-4
BindingDB Entry DOI: 10.7270/Q2BZ66J9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50214993
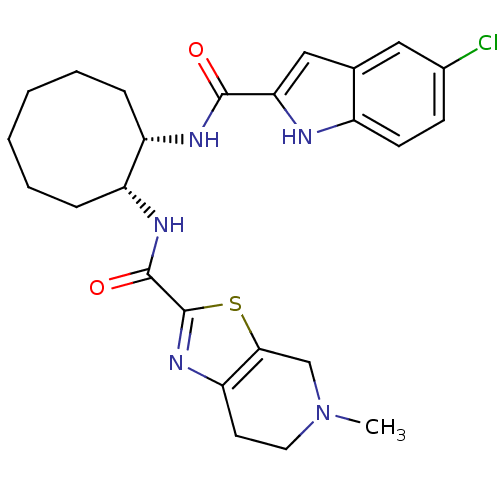
(CHEMBL247535 | cis-N-(2-(5-chloro-1H-indole-2-carb...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N[C@@H]1CCCCCC[C@@H]1NC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C25H30ClN5O2S/c1-31-11-10-20-22(14-31)34-25(30-20)24(33)29-19-7-5-3-2-4-6-18(19)28-23(32)21-13-15-12-16(26)8-9-17(15)27-21/h8-9,12-13,18-19,27H,2-7,10-11,14H2,1H3,(H,28,32)(H,29,33)/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a after 10 seconds |
Bioorg Med Chem Lett 17: 4683-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.068
BindingDB Entry DOI: 10.7270/Q2183660 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50214990
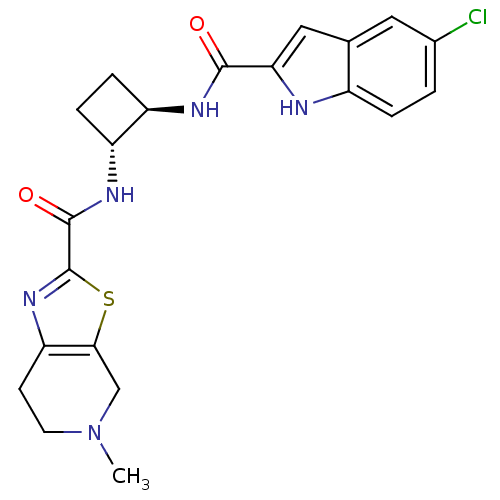
(CHEMBL391594 | trans-N-(2-(5-chloro-1H-indole-2-ca...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N[C@@H]1CC[C@H]1NC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C21H22ClN5O2S/c1-27-7-6-16-18(10-27)30-21(26-16)20(29)25-15-5-4-14(15)24-19(28)17-9-11-8-12(22)2-3-13(11)23-17/h2-3,8-9,14-15,23H,4-7,10H2,1H3,(H,24,28)(H,25,29)/t14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a after 10 seconds |
Bioorg Med Chem Lett 17: 4683-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.068
BindingDB Entry DOI: 10.7270/Q2183660 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data