 Found 44 hits with Last Name = 'furugori' and Initial = 't'
Found 44 hits with Last Name = 'furugori' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50146539
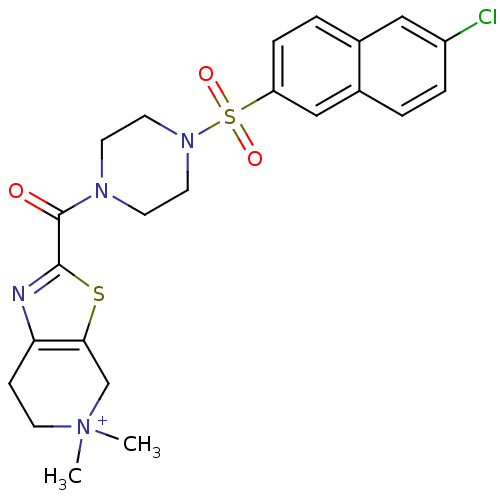
(2-[4-(6-Chloro-naphthalene-2-sulfonyl)-piperazine-...)Show SMILES C[N+]1(C)CCc2nc(sc2C1)C(=O)N1CCN(CC1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C23H26ClN4O3S2/c1-28(2)12-7-20-21(15-28)32-22(25-20)23(29)26-8-10-27(11-9-26)33(30,31)19-6-4-16-13-18(24)5-3-17(16)14-19/h3-6,13-14H,7-12,15H2,1-2H3/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human Coagulation factor X |
Bioorg Med Chem Lett 14: 2935-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.036
BindingDB Entry DOI: 10.7270/Q2VH5N9B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50146534

(CHEMBL100732 | [4-(6-Chloro-naphthalene-2-sulfonyl...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N1CCN(CC1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C22H23ClN4O3S2/c1-25-7-6-19-20(14-25)31-21(24-19)22(28)26-8-10-27(11-9-26)32(29,30)18-5-3-15-12-17(23)4-2-16(15)13-18/h2-5,12-13H,6-11,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Coagulation factor X |
J Med Chem 47: 5167-82 (2004)
Article DOI: 10.1021/jm049884d
BindingDB Entry DOI: 10.7270/Q2V987JK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50146534

(CHEMBL100732 | [4-(6-Chloro-naphthalene-2-sulfonyl...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N1CCN(CC1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C22H23ClN4O3S2/c1-25-7-6-19-20(14-25)31-21(24-19)22(28)26-8-10-27(11-9-26)32(29,30)18-5-3-15-12-17(23)4-2-16(15)13-18/h2-5,12-13H,6-11,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human Coagulation factor X |
Bioorg Med Chem Lett 14: 2935-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.036
BindingDB Entry DOI: 10.7270/Q2VH5N9B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50146538
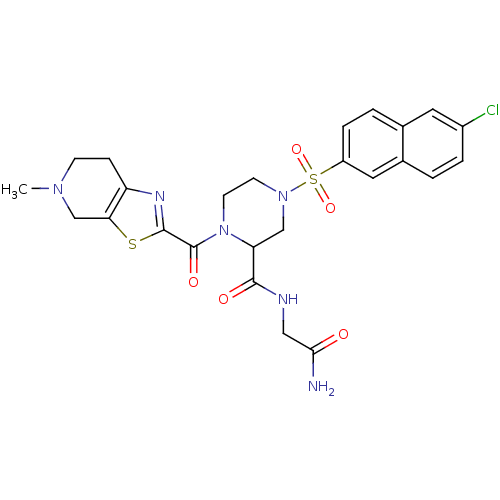
(4-(6-Chloro-naphthalene-2-sulfonyl)-1-(5-methyl-4,...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N1CCN(CC1C(=O)NCC(N)=O)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C25H27ClN6O5S2/c1-30-7-6-19-21(14-30)38-24(29-19)25(35)32-9-8-31(13-20(32)23(34)28-12-22(27)33)39(36,37)18-5-3-15-10-17(26)4-2-16(15)11-18/h2-5,10-11,20H,6-9,12-14H2,1H3,(H2,27,33)(H,28,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human Coagulation factor X |
Bioorg Med Chem Lett 14: 2935-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.036
BindingDB Entry DOI: 10.7270/Q2VH5N9B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50146536
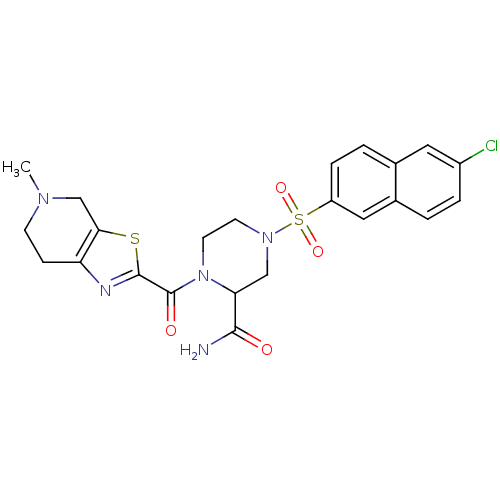
(4-(6-Chloro-naphthalene-2-sulfonyl)-1-(5-methyl-4,...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N1CCN(CC1C(N)=O)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C23H24ClN5O4S2/c1-27-7-6-18-20(13-27)34-22(26-18)23(31)29-9-8-28(12-19(29)21(25)30)35(32,33)17-5-3-14-10-16(24)4-2-15(14)11-17/h2-5,10-11,19H,6-9,12-13H2,1H3,(H2,25,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human Coagulation factor X |
Bioorg Med Chem Lett 14: 2935-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.036
BindingDB Entry DOI: 10.7270/Q2VH5N9B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50146536

(4-(6-Chloro-naphthalene-2-sulfonyl)-1-(5-methyl-4,...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N1CCN(CC1C(N)=O)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C23H24ClN5O4S2/c1-27-7-6-18-20(13-27)34-22(26-18)23(31)29-9-8-28(12-19(29)21(25)30)35(32,33)17-5-3-14-10-16(24)4-2-15(14)11-17/h2-5,10-11,19H,6-9,12-13H2,1H3,(H2,25,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Coagulation factor X |
J Med Chem 47: 5167-82 (2004)
Article DOI: 10.1021/jm049884d
BindingDB Entry DOI: 10.7270/Q2V987JK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50146532
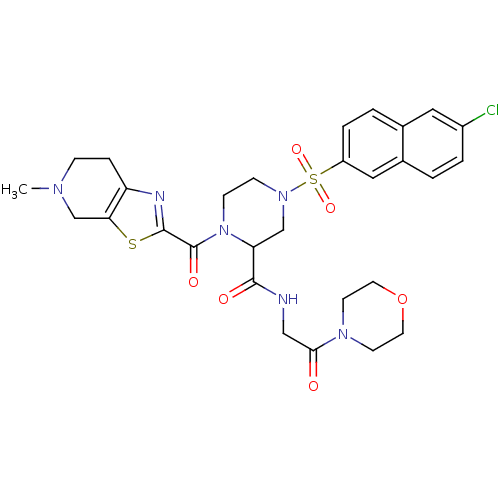
(4-(6-Chloro-naphthalene-2-sulfonyl)-1-(5-methyl-4,...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N1CCN(CC1C(=O)NCC(=O)N1CCOCC1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C29H33ClN6O6S2/c1-33-7-6-23-25(18-33)43-28(32-23)29(39)36-9-8-35(17-24(36)27(38)31-16-26(37)34-10-12-42-13-11-34)44(40,41)22-5-3-19-14-21(30)4-2-20(19)15-22/h2-5,14-15,24H,6-13,16-18H2,1H3,(H,31,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human Coagulation factor X |
Bioorg Med Chem Lett 14: 2935-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.036
BindingDB Entry DOI: 10.7270/Q2VH5N9B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50146531
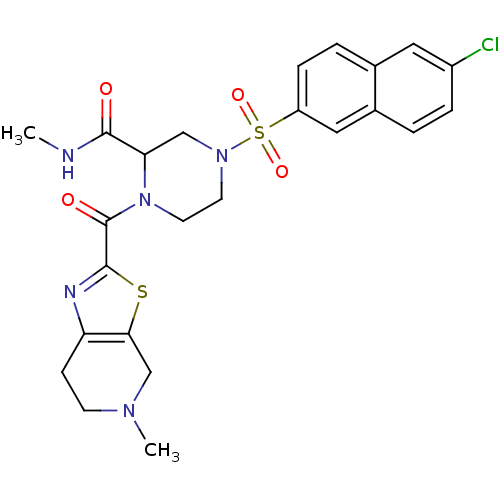
(4-(6-Chloro-naphthalene-2-sulfonyl)-1-(5-methyl-4,...)Show SMILES CNC(=O)C1CN(CCN1C(=O)c1nc2CCN(C)Cc2s1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C24H26ClN5O4S2/c1-26-22(31)20-13-29(36(33,34)18-6-4-15-11-17(25)5-3-16(15)12-18)9-10-30(20)24(32)23-27-19-7-8-28(2)14-21(19)35-23/h3-6,11-12,20H,7-10,13-14H2,1-2H3,(H,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human Coagulation factor X |
Bioorg Med Chem Lett 14: 2935-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.036
BindingDB Entry DOI: 10.7270/Q2VH5N9B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50146537

(4-(6-Chloro-naphthalene-2-sulfonyl)-1-(5-methyl-4,...)Show SMILES CC(C)NC(=O)C1CN(CCN1C(=O)c1nc2CCN(C)Cc2s1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C26H30ClN5O4S2/c1-16(2)28-24(33)22-14-31(38(35,36)20-7-5-17-12-19(27)6-4-18(17)13-20)10-11-32(22)26(34)25-29-21-8-9-30(3)15-23(21)37-25/h4-7,12-13,16,22H,8-11,14-15H2,1-3H3,(H,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human Coagulation factor X |
Bioorg Med Chem Lett 14: 2935-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.036
BindingDB Entry DOI: 10.7270/Q2VH5N9B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50146535
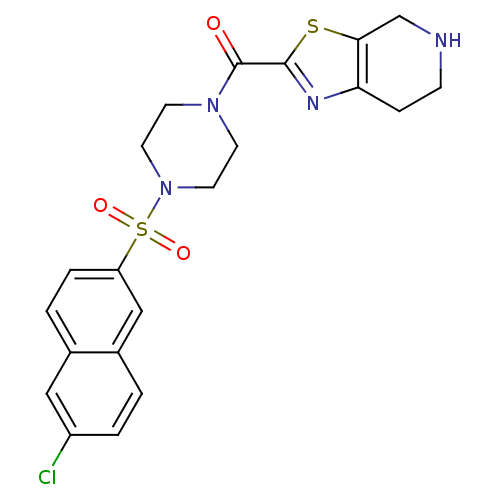
(CHEMBL99483 | [4-(6-Chloro-naphthalene-2-sulfonyl)...)Show SMILES Clc1ccc2cc(ccc2c1)S(=O)(=O)N1CCN(CC1)C(=O)c1nc2CCNCc2s1 Show InChI InChI=1S/C21H21ClN4O3S2/c22-16-3-1-15-12-17(4-2-14(15)11-16)31(28,29)26-9-7-25(8-10-26)21(27)20-24-18-5-6-23-13-19(18)30-20/h1-4,11-12,23H,5-10,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Coagulation factor X |
J Med Chem 47: 5167-82 (2004)
Article DOI: 10.1021/jm049884d
BindingDB Entry DOI: 10.7270/Q2V987JK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50146535

(CHEMBL99483 | [4-(6-Chloro-naphthalene-2-sulfonyl)...)Show SMILES Clc1ccc2cc(ccc2c1)S(=O)(=O)N1CCN(CC1)C(=O)c1nc2CCNCc2s1 Show InChI InChI=1S/C21H21ClN4O3S2/c22-16-3-1-15-12-17(4-2-14(15)11-16)31(28,29)26-9-7-25(8-10-26)21(27)20-24-18-5-6-23-13-19(18)30-20/h1-4,11-12,23H,5-10,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human Coagulation factor X |
Bioorg Med Chem Lett 14: 2935-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.036
BindingDB Entry DOI: 10.7270/Q2VH5N9B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50154263
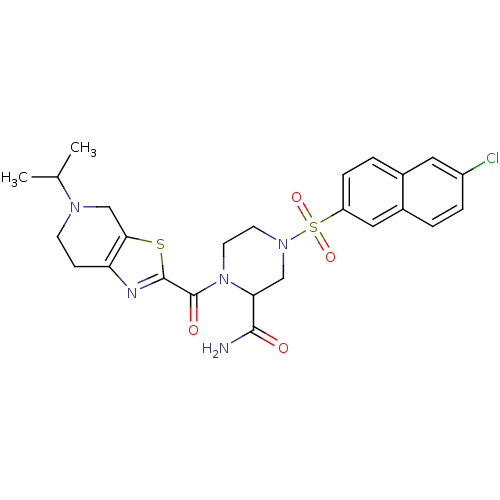
(4-(6-Chloro-naphthalene-2-sulfonyl)-1-(5-isopropyl...)Show SMILES CC(C)N1CCc2nc(sc2C1)C(=O)N1CCN(CC1C(N)=O)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C25H28ClN5O4S2/c1-15(2)29-8-7-20-22(14-29)36-24(28-20)25(33)31-10-9-30(13-21(31)23(27)32)37(34,35)19-6-4-16-11-18(26)5-3-17(16)12-19/h3-6,11-12,15,21H,7-10,13-14H2,1-2H3,(H2,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Coagulation factor X |
J Med Chem 47: 5167-82 (2004)
Article DOI: 10.1021/jm049884d
BindingDB Entry DOI: 10.7270/Q2V987JK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50154267

(4-(6-Chloro-naphthalene-2-sulfonyl)-1-(4,5,6,7-tet...)Show SMILES NC(=O)C1CN(CCN1C(=O)c1nc2CCNCc2s1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C22H22ClN5O4S2/c23-15-3-1-14-10-16(4-2-13(14)9-15)34(31,32)27-7-8-28(18(12-27)20(24)29)22(30)21-26-17-5-6-25-11-19(17)33-21/h1-4,9-10,18,25H,5-8,11-12H2,(H2,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Coagulation factor X |
J Med Chem 47: 5167-82 (2004)
Article DOI: 10.1021/jm049884d
BindingDB Entry DOI: 10.7270/Q2V987JK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50154264

(CHEMBL365654 | [4-(6-Chloro-naphthalene-2-sulfonyl...)Show SMILES CN1CCc2cc(sc2C1)C(=O)N1CCN(CC1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C23H24ClN3O3S2/c1-25-7-6-18-14-21(31-22(18)15-25)23(28)26-8-10-27(11-9-26)32(29,30)20-5-3-16-12-19(24)4-2-17(16)13-20/h2-5,12-14H,6-11,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Coagulation factor X |
J Med Chem 47: 5167-82 (2004)
Article DOI: 10.1021/jm049884d
BindingDB Entry DOI: 10.7270/Q2V987JK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50154265

(4-(6-Chloro-naphthalene-2-sulfonyl)-1-(5-ethyl-4,5...)Show SMILES CCN1CCc2nc(sc2C1)C(=O)N1CCN(CC1C(N)=O)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C24H26ClN5O4S2/c1-2-28-8-7-19-21(14-28)35-23(27-19)24(32)30-10-9-29(13-20(30)22(26)31)36(33,34)18-6-4-15-11-17(25)5-3-16(15)12-18/h3-6,11-12,20H,2,7-10,13-14H2,1H3,(H2,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Coagulation factor X |
J Med Chem 47: 5167-82 (2004)
Article DOI: 10.1021/jm049884d
BindingDB Entry DOI: 10.7270/Q2V987JK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50146540
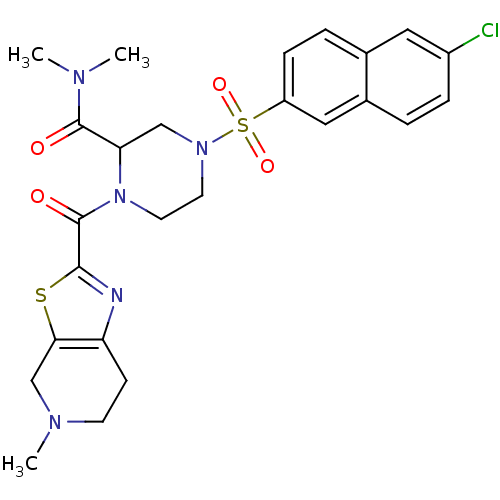
(4-(6-Chloro-naphthalene-2-sulfonyl)-1-(5-methyl-4,...)Show SMILES CN(C)C(=O)C1CN(CCN1C(=O)c1nc2CCN(C)Cc2s1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C25H28ClN5O4S2/c1-28(2)24(32)21-14-30(37(34,35)19-7-5-16-12-18(26)6-4-17(16)13-19)10-11-31(21)25(33)23-27-20-8-9-29(3)15-22(20)36-23/h4-7,12-13,21H,8-11,14-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human Coagulation factor X |
Bioorg Med Chem Lett 14: 2935-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.036
BindingDB Entry DOI: 10.7270/Q2VH5N9B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50154270
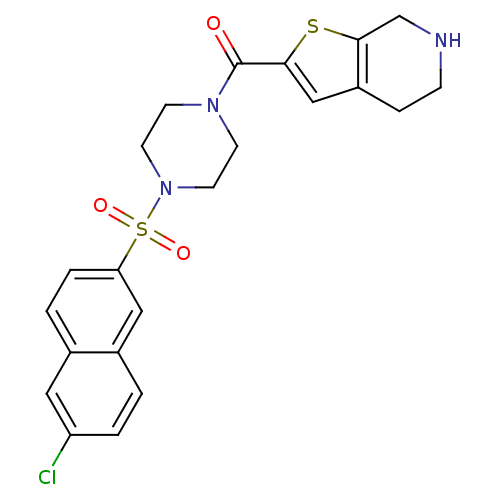
(CHEMBL182957 | [4-(6-Chloro-naphthalene-2-sulfonyl...)Show SMILES Clc1ccc2cc(ccc2c1)S(=O)(=O)N1CCN(CC1)C(=O)c1cc2CCNCc2s1 Show InChI InChI=1S/C22H22ClN3O3S2/c23-18-3-1-16-12-19(4-2-15(16)11-18)31(28,29)26-9-7-25(8-10-26)22(27)20-13-17-5-6-24-14-21(17)30-20/h1-4,11-13,24H,5-10,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Coagulation factor X |
J Med Chem 47: 5167-82 (2004)
Article DOI: 10.1021/jm049884d
BindingDB Entry DOI: 10.7270/Q2V987JK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50154271
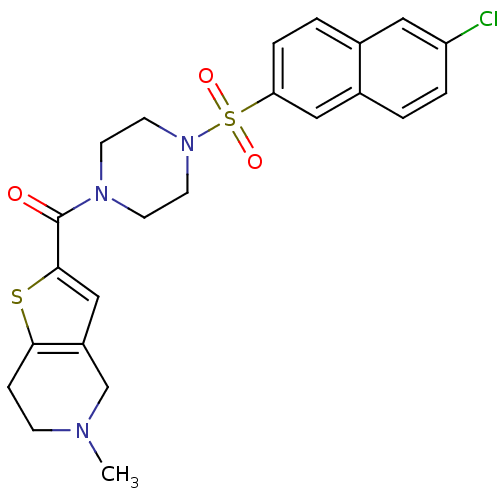
(CHEMBL185539 | [4-(6-Chloro-naphthalene-2-sulfonyl...)Show SMILES CN1CCc2sc(cc2C1)C(=O)N1CCN(CC1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C23H24ClN3O3S2/c1-25-7-6-21-18(15-25)14-22(31-21)23(28)26-8-10-27(11-9-26)32(29,30)20-5-3-16-12-19(24)4-2-17(16)13-20/h2-5,12-14H,6-11,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Coagulation factor X |
J Med Chem 47: 5167-82 (2004)
Article DOI: 10.1021/jm049884d
BindingDB Entry DOI: 10.7270/Q2V987JK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50154266
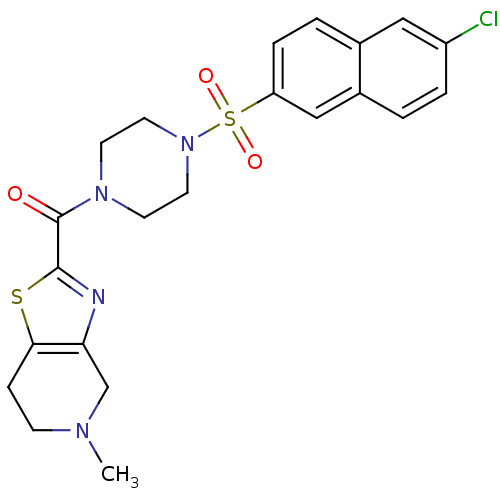
(CHEMBL181749 | [4-(6-Chloro-naphthalene-2-sulfonyl...)Show SMILES CN1CCc2sc(nc2C1)C(=O)N1CCN(CC1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C22H23ClN4O3S2/c1-25-7-6-20-19(14-25)24-21(31-20)22(28)26-8-10-27(11-9-26)32(29,30)18-5-3-15-12-17(23)4-2-16(15)13-18/h2-5,12-13H,6-11,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Coagulation factor X |
J Med Chem 47: 5167-82 (2004)
Article DOI: 10.1021/jm049884d
BindingDB Entry DOI: 10.7270/Q2V987JK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50154275
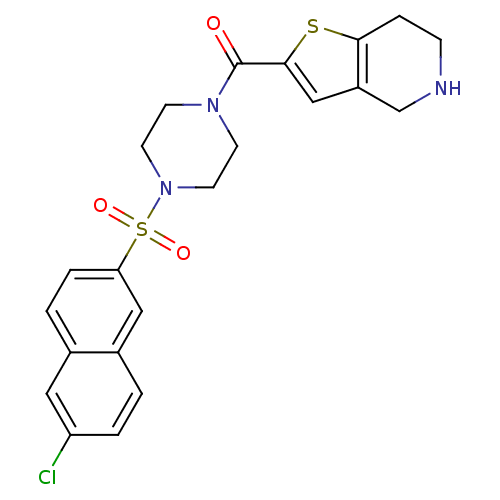
(CHEMBL183960 | [4-(6-Chloro-naphthalene-2-sulfonyl...)Show SMILES Clc1ccc2cc(ccc2c1)S(=O)(=O)N1CCN(CC1)C(=O)c1cc2CNCCc2s1 Show InChI InChI=1S/C22H22ClN3O3S2/c23-18-3-1-16-12-19(4-2-15(16)11-18)31(28,29)26-9-7-25(8-10-26)22(27)21-13-17-14-24-6-5-20(17)30-21/h1-4,11-13,24H,5-10,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Coagulation factor X |
J Med Chem 47: 5167-82 (2004)
Article DOI: 10.1021/jm049884d
BindingDB Entry DOI: 10.7270/Q2V987JK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50154273
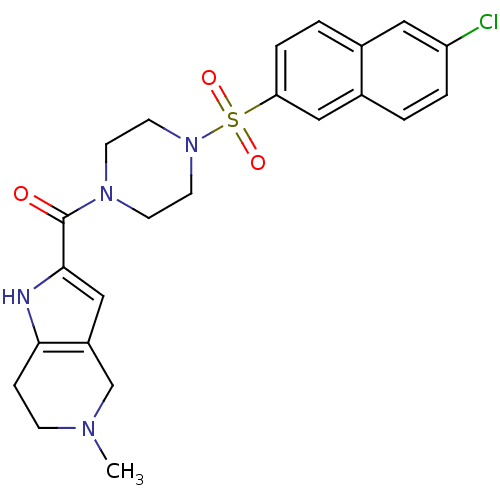
(CHEMBL365826 | [4-(6-Chloro-naphthalene-2-sulfonyl...)Show SMILES CN1CCc2[nH]c(cc2C1)C(=O)N1CCN(CC1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C23H25ClN4O3S/c1-26-7-6-21-18(15-26)14-22(25-21)23(29)27-8-10-28(11-9-27)32(30,31)20-5-3-16-12-19(24)4-2-17(16)13-20/h2-5,12-14,25H,6-11,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Coagulation factor X |
J Med Chem 47: 5167-82 (2004)
Article DOI: 10.1021/jm049884d
BindingDB Entry DOI: 10.7270/Q2V987JK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50154262
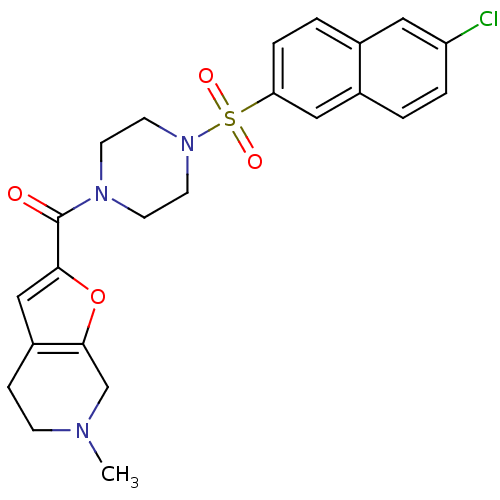
(CHEMBL185141 | [4-(6-Chloro-naphthalene-2-sulfonyl...)Show SMILES CN1CCc2cc(oc2C1)C(=O)N1CCN(CC1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C23H24ClN3O4S/c1-25-7-6-18-14-21(31-22(18)15-25)23(28)26-8-10-27(11-9-26)32(29,30)20-5-3-16-12-19(24)4-2-17(16)13-20/h2-5,12-14H,6-11,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Coagulation factor X |
J Med Chem 47: 5167-82 (2004)
Article DOI: 10.1021/jm049884d
BindingDB Entry DOI: 10.7270/Q2V987JK |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50146534

(CHEMBL100732 | [4-(6-Chloro-naphthalene-2-sulfonyl...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N1CCN(CC1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C22H23ClN4O3S2/c1-25-7-6-19-20(14-25)31-21(24-19)22(28)26-8-10-27(11-9-26)32(29,30)18-5-3-15-12-17(23)4-2-16(15)13-18/h2-5,12-13H,6-11,14H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human thrombin |
Bioorg Med Chem Lett 14: 2935-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.036
BindingDB Entry DOI: 10.7270/Q2VH5N9B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50154268
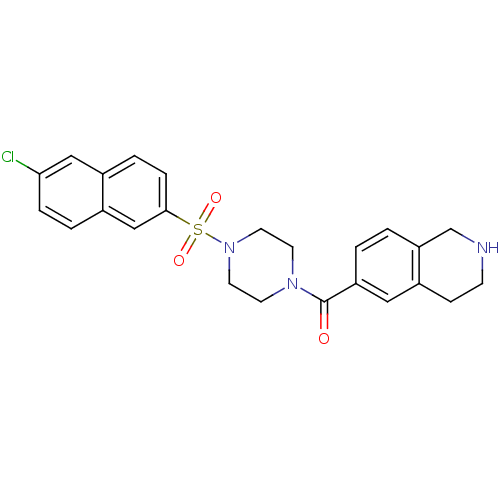
(CHEMBL363048 | [4-(6-Chloro-naphthalene-2-sulfonyl...)Show SMILES Clc1ccc2cc(ccc2c1)S(=O)(=O)N1CCN(CC1)C(=O)c1ccc2CNCCc2c1 Show InChI InChI=1S/C24H24ClN3O3S/c25-22-5-3-18-15-23(6-4-17(18)14-22)32(30,31)28-11-9-27(10-12-28)24(29)20-1-2-21-16-26-8-7-19(21)13-20/h1-6,13-15,26H,7-12,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Coagulation factor X |
J Med Chem 47: 5167-82 (2004)
Article DOI: 10.1021/jm049884d
BindingDB Entry DOI: 10.7270/Q2V987JK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50146533

(4-(6-Chloro-naphthalene-2-sulfonyl)-1-(5-methyl-4,...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N1CCN(CC1C(=O)NCc1ccccc1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C30H30ClN5O4S2/c1-34-12-11-25-27(19-34)41-29(33-25)30(38)36-14-13-35(18-26(36)28(37)32-17-20-5-3-2-4-6-20)42(39,40)24-10-8-21-15-23(31)9-7-22(21)16-24/h2-10,15-16,26H,11-14,17-19H2,1H3,(H,32,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human Coagulation factor X |
Bioorg Med Chem Lett 14: 2935-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.036
BindingDB Entry DOI: 10.7270/Q2VH5N9B |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50146537

(4-(6-Chloro-naphthalene-2-sulfonyl)-1-(5-methyl-4,...)Show SMILES CC(C)NC(=O)C1CN(CCN1C(=O)c1nc2CCN(C)Cc2s1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C26H30ClN5O4S2/c1-16(2)28-24(33)22-14-31(38(35,36)20-7-5-17-12-19(27)6-4-18(17)13-20)10-11-32(22)26(34)25-29-21-8-9-30(3)15-23(21)37-25/h4-7,12-13,16,22H,8-11,14-15H2,1-3H3,(H,28,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human thrombin |
Bioorg Med Chem Lett 14: 2935-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.036
BindingDB Entry DOI: 10.7270/Q2VH5N9B |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50146538

(4-(6-Chloro-naphthalene-2-sulfonyl)-1-(5-methyl-4,...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N1CCN(CC1C(=O)NCC(N)=O)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C25H27ClN6O5S2/c1-30-7-6-19-21(14-30)38-24(29-19)25(35)32-9-8-31(13-20(32)23(34)28-12-22(27)33)39(36,37)18-5-3-15-10-17(26)4-2-16(15)11-18/h2-5,10-11,20H,6-9,12-14H2,1H3,(H2,27,33)(H,28,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human thrombin |
Bioorg Med Chem Lett 14: 2935-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.036
BindingDB Entry DOI: 10.7270/Q2VH5N9B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50154272
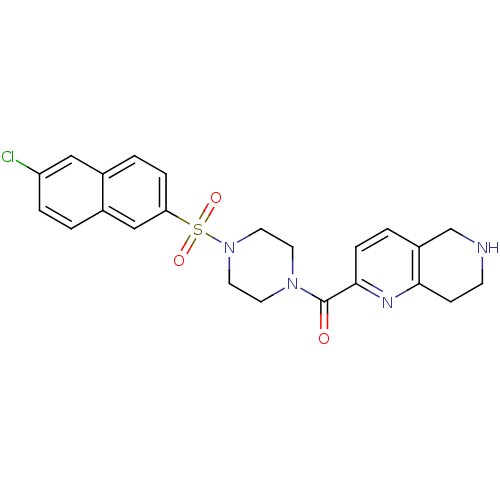
(CHEMBL359966 | [4-(6-Chloro-naphthalene-2-sulfonyl...)Show SMILES Clc1ccc2cc(ccc2c1)S(=O)(=O)N1CCN(CC1)C(=O)c1ccc2CNCCc2n1 Show InChI InChI=1S/C23H23ClN4O3S/c24-19-4-1-17-14-20(5-2-16(17)13-19)32(30,31)28-11-9-27(10-12-28)23(29)22-6-3-18-15-25-8-7-21(18)26-22/h1-6,13-14,25H,7-12,15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Coagulation factor X |
J Med Chem 47: 5167-82 (2004)
Article DOI: 10.1021/jm049884d
BindingDB Entry DOI: 10.7270/Q2V987JK |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50146532

(4-(6-Chloro-naphthalene-2-sulfonyl)-1-(5-methyl-4,...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N1CCN(CC1C(=O)NCC(=O)N1CCOCC1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C29H33ClN6O6S2/c1-33-7-6-23-25(18-33)43-28(32-23)29(39)36-9-8-35(17-24(36)27(38)31-16-26(37)34-10-12-42-13-11-34)44(40,41)22-5-3-19-14-21(30)4-2-20(19)15-22/h2-5,14-15,24H,6-13,16-18H2,1H3,(H,31,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human thrombin |
Bioorg Med Chem Lett 14: 2935-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.036
BindingDB Entry DOI: 10.7270/Q2VH5N9B |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50146536

(4-(6-Chloro-naphthalene-2-sulfonyl)-1-(5-methyl-4,...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N1CCN(CC1C(N)=O)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C23H24ClN5O4S2/c1-27-7-6-18-20(13-27)34-22(26-18)23(31)29-9-8-28(12-19(29)21(25)30)35(32,33)17-5-3-14-10-16(24)4-2-15(14)11-17/h2-5,10-11,19H,6-9,12-13H2,1H3,(H2,25,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human thrombin |
Bioorg Med Chem Lett 14: 2935-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.036
BindingDB Entry DOI: 10.7270/Q2VH5N9B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50154274

(CHEMBL184478 | [4-(6-Chloro-naphthalene-2-sulfonyl...)Show SMILES CN1CCc2cc(ccc2C1)C(=O)N1CCN(CC1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C25H26ClN3O3S/c1-27-9-8-20-14-21(2-3-22(20)17-27)25(30)28-10-12-29(13-11-28)33(31,32)24-7-5-18-15-23(26)6-4-19(18)16-24/h2-7,14-16H,8-13,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Coagulation factor X |
J Med Chem 47: 5167-82 (2004)
Article DOI: 10.1021/jm049884d
BindingDB Entry DOI: 10.7270/Q2V987JK |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50146533

(4-(6-Chloro-naphthalene-2-sulfonyl)-1-(5-methyl-4,...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N1CCN(CC1C(=O)NCc1ccccc1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C30H30ClN5O4S2/c1-34-12-11-25-27(19-34)41-29(33-25)30(38)36-14-13-35(18-26(36)28(37)32-17-20-5-3-2-4-6-20)42(39,40)24-10-8-21-15-23(31)9-7-22(21)16-24/h2-10,15-16,26H,11-14,17-19H2,1H3,(H,32,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human thrombin |
Bioorg Med Chem Lett 14: 2935-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.036
BindingDB Entry DOI: 10.7270/Q2VH5N9B |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50146531

(4-(6-Chloro-naphthalene-2-sulfonyl)-1-(5-methyl-4,...)Show SMILES CNC(=O)C1CN(CCN1C(=O)c1nc2CCN(C)Cc2s1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C24H26ClN5O4S2/c1-26-22(31)20-13-29(36(33,34)18-6-4-15-11-17(25)5-3-16(15)12-18)9-10-30(20)24(32)23-27-19-7-8-28(2)14-21(19)35-23/h3-6,11-12,20H,7-10,13-14H2,1-2H3,(H,26,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human thrombin |
Bioorg Med Chem Lett 14: 2935-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.036
BindingDB Entry DOI: 10.7270/Q2VH5N9B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50154276
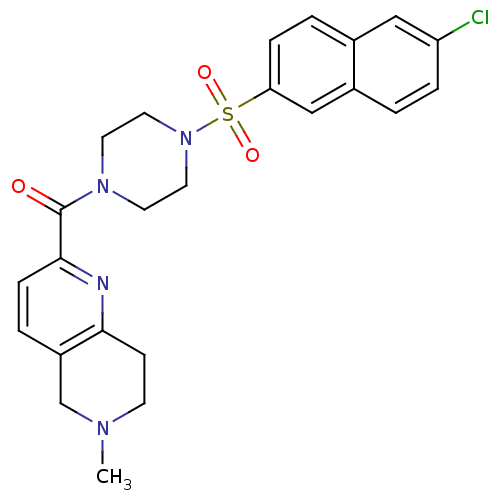
(CHEMBL182374 | [4-(6-Chloro-naphthalene-2-sulfonyl...)Show SMILES CN1CCc2nc(ccc2C1)C(=O)N1CCN(CC1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C24H25ClN4O3S/c1-27-9-8-22-19(16-27)4-7-23(26-22)24(30)28-10-12-29(13-11-28)33(31,32)21-6-3-17-14-20(25)5-2-18(17)15-21/h2-7,14-15H,8-13,16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Coagulation factor X |
J Med Chem 47: 5167-82 (2004)
Article DOI: 10.1021/jm049884d
BindingDB Entry DOI: 10.7270/Q2V987JK |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50146535

(CHEMBL99483 | [4-(6-Chloro-naphthalene-2-sulfonyl)...)Show SMILES Clc1ccc2cc(ccc2c1)S(=O)(=O)N1CCN(CC1)C(=O)c1nc2CCNCc2s1 Show InChI InChI=1S/C21H21ClN4O3S2/c22-16-3-1-15-12-17(4-2-14(15)11-16)31(28,29)26-9-7-25(8-10-26)21(27)20-24-18-5-6-23-13-19(18)30-20/h1-4,11-12,23H,5-10,13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human thrombin |
Bioorg Med Chem Lett 14: 2935-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.036
BindingDB Entry DOI: 10.7270/Q2VH5N9B |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50146539

(2-[4-(6-Chloro-naphthalene-2-sulfonyl)-piperazine-...)Show SMILES C[N+]1(C)CCc2nc(sc2C1)C(=O)N1CCN(CC1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C23H26ClN4O3S2/c1-28(2)12-7-20-21(15-28)32-22(25-20)23(29)26-8-10-27(11-9-26)33(30,31)19-6-4-16-13-18(24)5-3-17(16)14-19/h3-6,13-14H,7-12,15H2,1-2H3/q+1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human thrombin |
Bioorg Med Chem Lett 14: 2935-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.036
BindingDB Entry DOI: 10.7270/Q2VH5N9B |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50146540

(4-(6-Chloro-naphthalene-2-sulfonyl)-1-(5-methyl-4,...)Show SMILES CN(C)C(=O)C1CN(CCN1C(=O)c1nc2CCN(C)Cc2s1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C25H28ClN5O4S2/c1-28(2)24(32)21-14-30(37(34,35)19-7-5-16-12-18(26)6-4-17(16)13-19)10-11-31(21)25(33)23-27-20-8-9-29(3)15-22(20)36-23/h4-7,12-13,21H,8-11,14-15H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human thrombin |
Bioorg Med Chem Lett 14: 2935-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.036
BindingDB Entry DOI: 10.7270/Q2VH5N9B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50154269
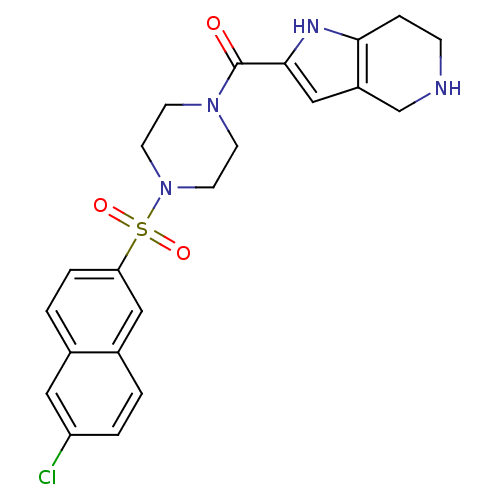
(CHEMBL184873 | [4-(6-Chloro-naphthalene-2-sulfonyl...)Show SMILES Clc1ccc2cc(ccc2c1)S(=O)(=O)N1CCN(CC1)C(=O)c1cc2CNCCc2[nH]1 Show InChI InChI=1S/C22H23ClN4O3S/c23-18-3-1-16-12-19(4-2-15(16)11-18)31(29,30)27-9-7-26(8-10-27)22(28)21-13-17-14-24-6-5-20(17)25-21/h1-4,11-13,24-25H,5-10,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Coagulation factor X |
J Med Chem 47: 5167-82 (2004)
Article DOI: 10.1021/jm049884d
BindingDB Entry DOI: 10.7270/Q2V987JK |
More data for this
Ligand-Target Pair | |
Beta-adrenergic receptor kinase 1
(Homo sapiens (Human)) | BDBM50113255
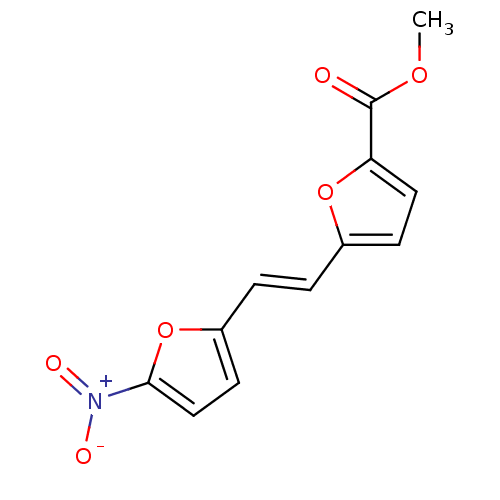
(5-[2-(5-Nitro-furan-2-yl)-vinyl]-furan-2-carboxyli...)Show InChI InChI=1S/C12H9NO6/c1-17-12(14)10-6-4-8(18-10)2-3-9-5-7-11(19-9)13(15)16/h2-7H,1H3/b3-2+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.26E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Beta-adrenergic receptor kinase 1 |
J Med Chem 45: 2150-9 (2002)
BindingDB Entry DOI: 10.7270/Q2Q81CCR |
More data for this
Ligand-Target Pair | |
Beta-adrenergic receptor kinase 1
(Homo sapiens (Human)) | BDBM50113256
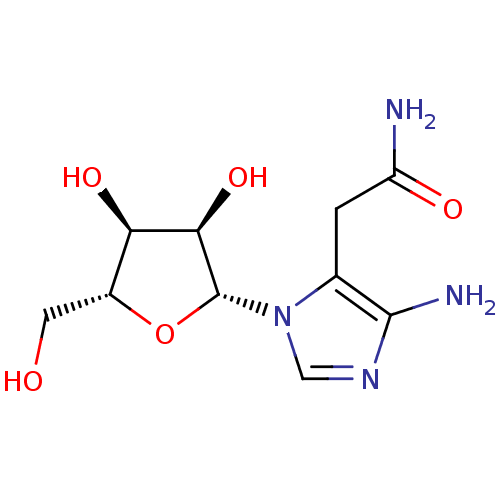
(2-[5-Amino-3-(3,4-dihydroxy-5-hydroxymethyl-tetrah...)Show SMILES NC(=O)Cc1c(N)ncn1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H16N4O5/c11-6(16)1-4-9(12)13-3-14(4)10-8(18)7(17)5(2-15)19-10/h3,5,7-8,10,15,17-18H,1-2,12H2,(H2,11,16)/t5-,7-,8-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 5.57E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Beta-adrenergic receptor kinase 1 |
J Med Chem 45: 2150-9 (2002)
BindingDB Entry DOI: 10.7270/Q2Q81CCR |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50146534

(CHEMBL100732 | [4-(6-Chloro-naphthalene-2-sulfonyl...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N1CCN(CC1)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C22H23ClN4O3S2/c1-25-7-6-19-20(14-25)31-21(24-19)22(28)26-8-10-27(11-9-26)32(29,30)18-5-3-15-12-17(23)4-2-16(15)13-18/h2-5,12-13H,6-11,14H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Concentration required to inhibit thrombin activity by 50% |
J Med Chem 47: 5167-82 (2004)
Article DOI: 10.1021/jm049884d
BindingDB Entry DOI: 10.7270/Q2V987JK |
More data for this
Ligand-Target Pair | |
Beta-adrenergic receptor kinase 1
(Homo sapiens (Human)) | BDBM50113257
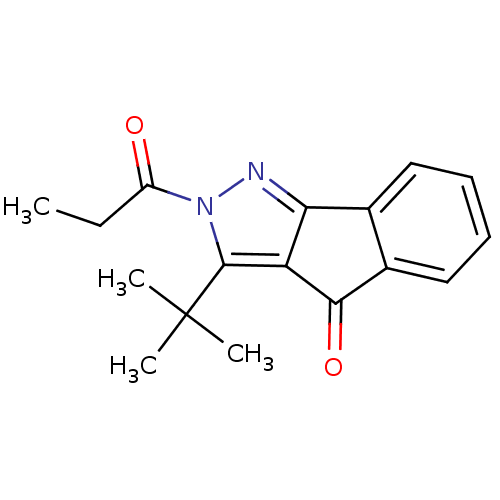
(3-tert-Butyl-2-propionyl-2H-indeno[1,2-c]pyrazol-4...)Show InChI InChI=1S/C17H18N2O2/c1-5-12(20)19-16(17(2,3)4)13-14(18-19)10-8-6-7-9-11(10)15(13)21/h6-9H,5H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 5.63E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Beta-adrenergic receptor kinase 1 |
J Med Chem 45: 2150-9 (2002)
BindingDB Entry DOI: 10.7270/Q2Q81CCR |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50146536

(4-(6-Chloro-naphthalene-2-sulfonyl)-1-(5-methyl-4,...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N1CCN(CC1C(N)=O)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C23H24ClN5O4S2/c1-27-7-6-18-20(13-27)34-22(26-18)23(31)29-9-8-28(12-19(29)21(25)30)35(32,33)17-5-3-14-10-16(24)4-2-15(14)11-17/h2-5,10-11,19H,6-9,12-13H2,1H3,(H2,25,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Concentration required to inhibit thrombin activity by 50% |
J Med Chem 47: 5167-82 (2004)
Article DOI: 10.1021/jm049884d
BindingDB Entry DOI: 10.7270/Q2V987JK |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50146535

(CHEMBL99483 | [4-(6-Chloro-naphthalene-2-sulfonyl)...)Show SMILES Clc1ccc2cc(ccc2c1)S(=O)(=O)N1CCN(CC1)C(=O)c1nc2CCNCc2s1 Show InChI InChI=1S/C21H21ClN4O3S2/c22-16-3-1-15-12-17(4-2-14(15)11-16)31(28,29)26-9-7-25(8-10-26)21(27)20-24-18-5-6-23-13-19(18)30-20/h1-4,11-12,23H,5-10,13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Concentration required to inhibit thrombin activity by 50% |
J Med Chem 47: 5167-82 (2004)
Article DOI: 10.1021/jm049884d
BindingDB Entry DOI: 10.7270/Q2V987JK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data