 Found 174 hits with Last Name = 'gailunas' and Initial = 'a'
Found 174 hits with Last Name = 'gailunas' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-secretase 1
(Homo sapiens (Human)) | BDBM16770
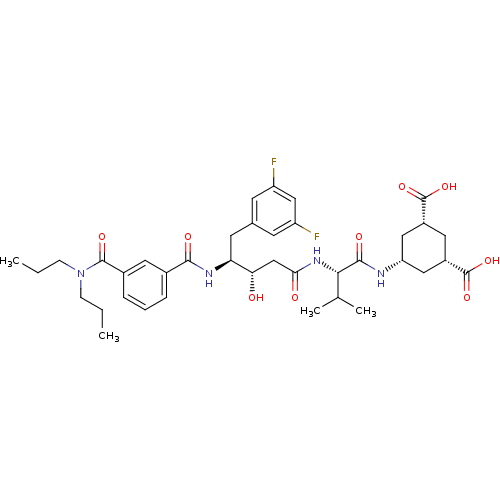
((1R,3S,5S)-5-[(3S,4S)-N-[(1S)-1-carbamoyl-2-methyl...)Show SMILES CCCN(CCC)C(=O)c1cccc(c1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@@H](O)CC(=O)N[C@@H](C(C)C)C(=O)N[C@@H]1C[C@@H](C[C@@H](C1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C38H50F2N4O9/c1-5-10-44(11-6-2)36(49)24-9-7-8-23(15-24)34(47)42-30(14-22-12-27(39)19-28(40)13-22)31(45)20-32(46)43-33(21(3)4)35(48)41-29-17-25(37(50)51)16-26(18-29)38(52)53/h7-9,12-13,15,19,21,25-26,29-31,33,45H,5-6,10-11,14,16-18,20H2,1-4H3,(H,41,48)(H,42,47)(H,43,46)(H,50,51)(H,52,53)/t25-,26+,29-,30-,31-,33-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 4.8 | 37 |
Elan Pharmaceuticals
| Assay Description
Beta-cleavage ELISA assays were carried out in reaction buffer containing enzyme, substrate MBP-C125, and test compounds. Generated beta-cleaved prod... |
J Med Chem 47: 158-64 (2004)
Article DOI: 10.1021/jm0304008
BindingDB Entry DOI: 10.7270/Q2G73BZ6 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50438363
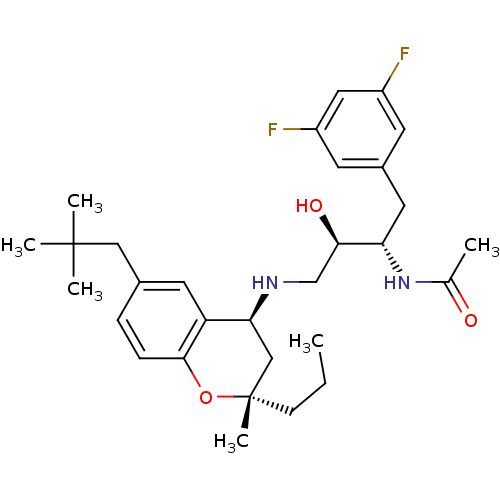
(CHEMBL2408751)Show SMILES CCC[C@@]1(C)C[C@H](NC[C@@H](O)[C@H](Cc2cc(F)cc(F)c2)NC(C)=O)c2cc(CC(C)(C)C)ccc2O1 |r| Show InChI InChI=1S/C30H42F2N2O3/c1-7-10-30(6)17-26(24-13-20(16-29(3,4)5)8-9-28(24)37-30)33-18-27(36)25(34-19(2)35)14-21-11-22(31)15-23(32)12-21/h8-9,11-13,15,25-27,33,36H,7,10,14,16-18H2,1-6H3,(H,34,35)/t25-,26-,27+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin-D (unknown origin) using C-terminal biotinylated peptide substrate treated 30 mins before addition of peptide substrate measu... |
Bioorg Med Chem Lett 23: 4674-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.006
BindingDB Entry DOI: 10.7270/Q29W0GWT |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50438362
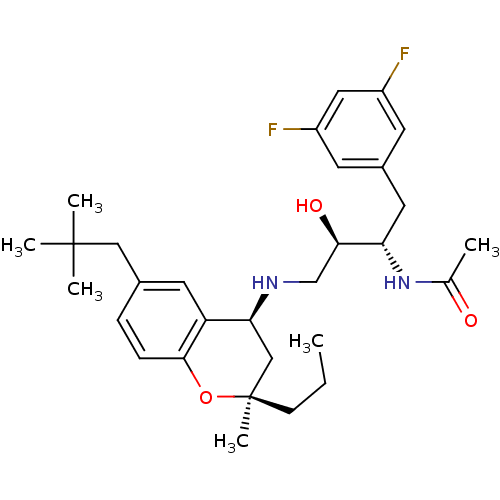
(CHEMBL2408752)Show SMILES CCC[C@]1(C)C[C@H](NC[C@@H](O)[C@H](Cc2cc(F)cc(F)c2)NC(C)=O)c2cc(CC(C)(C)C)ccc2O1 |r| Show InChI InChI=1S/C30H42F2N2O3/c1-7-10-30(6)17-26(24-13-20(16-29(3,4)5)8-9-28(24)37-30)33-18-27(36)25(34-19(2)35)14-21-11-22(31)15-23(32)12-21/h8-9,11-13,15,25-27,33,36H,7,10,14,16-18H2,1-6H3,(H,34,35)/t25-,26-,27+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin-D (unknown origin) using C-terminal biotinylated peptide substrate treated 30 mins before addition of peptide substrate measu... |
Bioorg Med Chem Lett 23: 4674-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.006
BindingDB Entry DOI: 10.7270/Q29W0GWT |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM16250
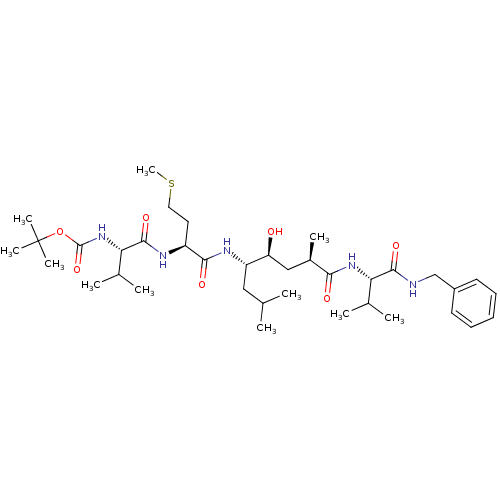
(CHEMBL290001 | N-(tert-butoxycarbonyl)-L-valyl-N-[...)Show SMILES CSCC[C@H](NC(=O)[C@@H](NC(=O)OC(C)(C)C)C(C)C)C(=O)N[C@@H](CC(C)C)[C@@H](O)C[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C37H63N5O7S/c1-22(2)19-28(29(43)20-25(7)32(44)41-30(23(3)4)34(46)38-21-26-15-13-12-14-16-26)40-33(45)27(17-18-50-11)39-35(47)31(24(5)6)42-36(48)49-37(8,9)10/h12-16,22-25,27-31,43H,17-21H2,1-11H3,(H,38,46)(H,39,47)(H,40,45)(H,41,44)(H,42,48)/t25-,27+,28+,29+,30+,31+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 4.8 | 37 |
Elan Pharmaceuticals
| Assay Description
Beta-cleavage ELISA assays were carried out in reaction buffer containing enzyme, substrate MBP-C125, and test compounds. Generated beta-cleaved prod... |
J Med Chem 47: 158-64 (2004)
Article DOI: 10.1021/jm0304008
BindingDB Entry DOI: 10.7270/Q22Z13RC |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50438361
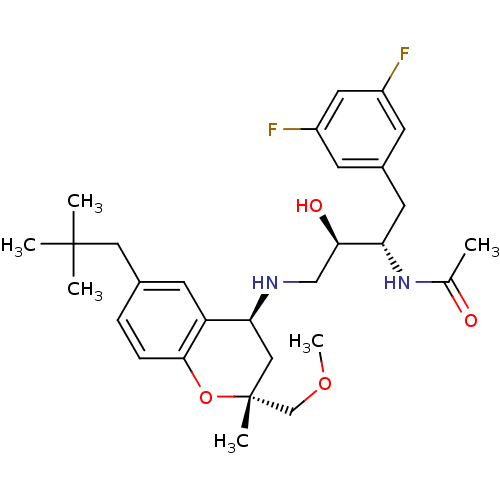
(CHEMBL2408753)Show SMILES COC[C@@]1(C)C[C@H](NC[C@@H](O)[C@H](Cc2cc(F)cc(F)c2)NC(C)=O)c2cc(CC(C)(C)C)ccc2O1 |r| Show InChI InChI=1S/C29H40F2N2O4/c1-18(34)33-24(12-20-9-21(30)13-22(31)10-20)26(35)16-32-25-15-29(5,17-36-6)37-27-8-7-19(11-23(25)27)14-28(2,3)4/h7-11,13,24-26,32,35H,12,14-17H2,1-6H3,(H,33,34)/t24-,25-,26+,29+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin-D (unknown origin) using C-terminal biotinylated peptide substrate treated 30 mins before addition of peptide substrate measu... |
Bioorg Med Chem Lett 23: 4674-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.006
BindingDB Entry DOI: 10.7270/Q29W0GWT |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50438360
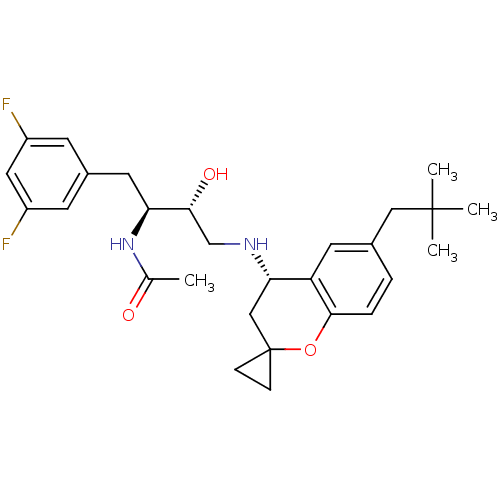
(CHEMBL2408755)Show SMILES CC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CN[C@H]1CC2(CC2)Oc2ccc(CC(C)(C)C)cc12 |r| Show InChI InChI=1S/C28H36F2N2O3/c1-17(33)32-23(12-19-9-20(29)13-21(30)10-19)25(34)16-31-24-15-28(7-8-28)35-26-6-5-18(11-22(24)26)14-27(2,3)4/h5-6,9-11,13,23-25,31,34H,7-8,12,14-16H2,1-4H3,(H,32,33)/t23-,24-,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin-D (unknown origin) using C-terminal biotinylated peptide substrate treated 30 mins before addition of peptide substrate measu... |
Bioorg Med Chem Lett 23: 4674-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.006
BindingDB Entry DOI: 10.7270/Q29W0GWT |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50438359
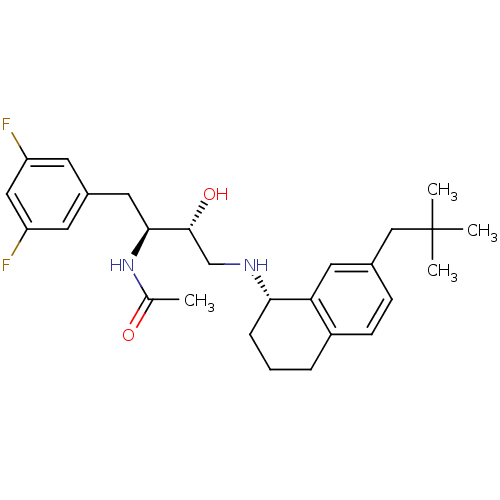
(CHEMBL2408760)Show SMILES CC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CN[C@H]1CCCc2ccc(CC(C)(C)C)cc12 |r| Show InChI InChI=1S/C27H36F2N2O2/c1-17(32)31-25(13-19-10-21(28)14-22(29)11-19)26(33)16-30-24-7-5-6-20-9-8-18(12-23(20)24)15-27(2,3)4/h8-12,14,24-26,30,33H,5-7,13,15-16H2,1-4H3,(H,31,32)/t24-,25-,26+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin-D (unknown origin) using C-terminal biotinylated peptide substrate treated 30 mins before addition of peptide substrate measu... |
Bioorg Med Chem Lett 23: 4674-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.006
BindingDB Entry DOI: 10.7270/Q29W0GWT |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50328048
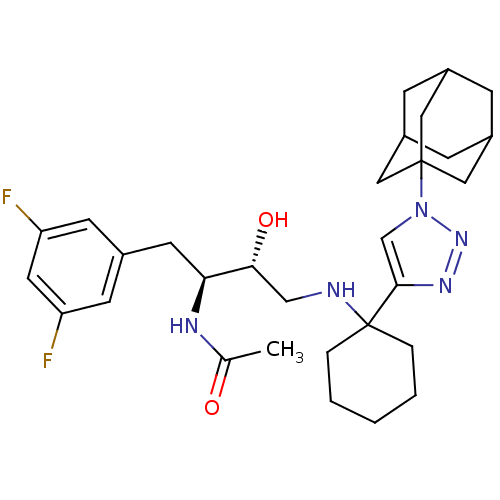
(CHEMBL1257185)Show SMILES CC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNC1(CCCCC1)c1cn(nn1)C12CC3CC(CC(C3)C1)C2 |r,TLB:26:29:32.31.36:34,THB:30:31:34:38.29.37,30:29:32.31.36:34,26:29:32:36.35.34,37:29:32:36.35.34,37:35:32:38.30.29| Show InChI InChI=1S/C30H41F2N5O2/c1-19(38)34-26(12-20-10-24(31)13-25(32)11-20)27(39)17-33-30(5-3-2-4-6-30)28-18-37(36-35-28)29-14-21-7-22(15-29)9-23(8-21)16-29/h10-11,13,18,21-23,26-27,33,39H,2-9,12,14-17H2,1H3,(H,34,38)/t21?,22?,23?,26-,27+,29?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 20: 6034-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.070
BindingDB Entry DOI: 10.7270/Q2XK8FSK |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50438358
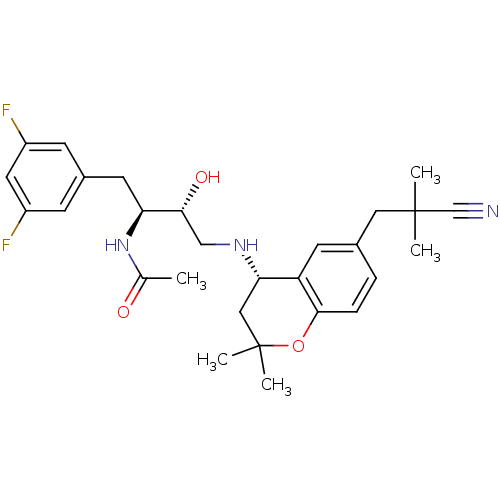
(CHEMBL2408757)Show SMILES CC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CN[C@H]1CC(C)(C)Oc2ccc(CC(C)(C)C#N)cc12 |r| Show InChI InChI=1S/C28H35F2N3O3/c1-17(34)33-23(11-19-8-20(29)12-21(30)9-19)25(35)15-32-24-14-28(4,5)36-26-7-6-18(10-22(24)26)13-27(2,3)16-31/h6-10,12,23-25,32,35H,11,13-15H2,1-5H3,(H,33,34)/t23-,24-,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin-D (unknown origin) using C-terminal biotinylated peptide substrate treated 30 mins before addition of peptide substrate measu... |
Bioorg Med Chem Lett 23: 4674-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.006
BindingDB Entry DOI: 10.7270/Q29W0GWT |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50328038

(CHEMBL1258467 | N-((2S,3R)-1-(3,5-difluorophenyl)-...)Show SMILES CC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNC1(CCCCC1)c1cc(CC(C)(C)C)cs1 |r| Show InChI InChI=1S/C27H38F2N2O2S/c1-18(32)31-23(12-19-10-21(28)14-22(29)11-19)24(33)16-30-27(8-6-5-7-9-27)25-13-20(17-34-25)15-26(2,3)4/h10-11,13-14,17,23-24,30,33H,5-9,12,15-16H2,1-4H3,(H,31,32)/t23-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 20: 6034-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.070
BindingDB Entry DOI: 10.7270/Q2XK8FSK |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50328030
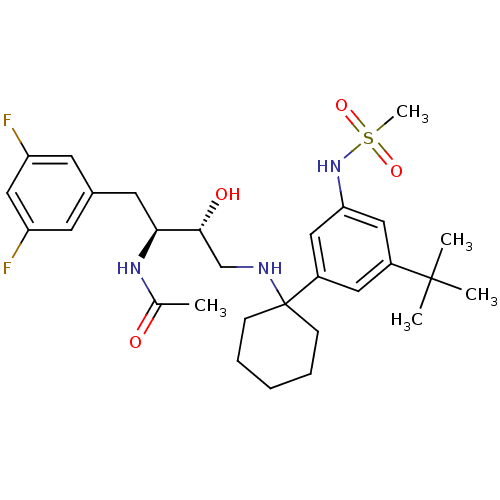
(CHEMBL1258001 | N-((2S,3R)-4-(1-(3-tert-butyl-5-(m...)Show SMILES CC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNC1(CCCCC1)c1cc(NS(C)(=O)=O)cc(c1)C(C)(C)C |r| Show InChI InChI=1S/C29H41F2N3O4S/c1-19(35)33-26(13-20-11-23(30)17-24(31)12-20)27(36)18-32-29(9-7-6-8-10-29)22-14-21(28(2,3)4)15-25(16-22)34-39(5,37)38/h11-12,14-17,26-27,32,34,36H,6-10,13,18H2,1-5H3,(H,33,35)/t26-,27+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 20: 6034-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.070
BindingDB Entry DOI: 10.7270/Q2XK8FSK |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50438357
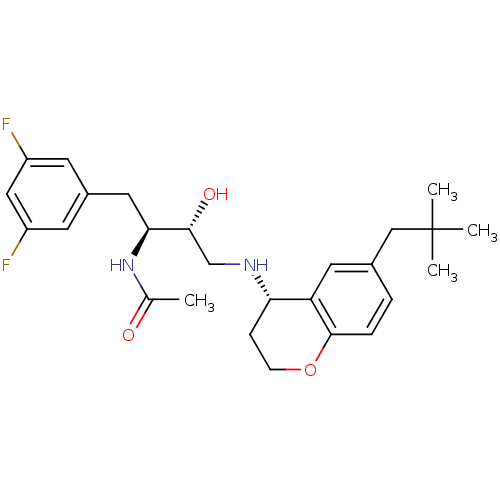
(CHEMBL2408737)Show SMILES CC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CN[C@H]1CCOc2ccc(CC(C)(C)C)cc12 |r| Show InChI InChI=1S/C26H34F2N2O3/c1-16(31)30-23(12-18-9-19(27)13-20(28)10-18)24(32)15-29-22-7-8-33-25-6-5-17(11-21(22)25)14-26(2,3)4/h5-6,9-11,13,22-24,29,32H,7-8,12,14-15H2,1-4H3,(H,30,31)/t22-,23-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin-D (unknown origin) using C-terminal biotinylated peptide substrate treated 30 mins before addition of peptide substrate measu... |
Bioorg Med Chem Lett 23: 4674-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.006
BindingDB Entry DOI: 10.7270/Q29W0GWT |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50328047
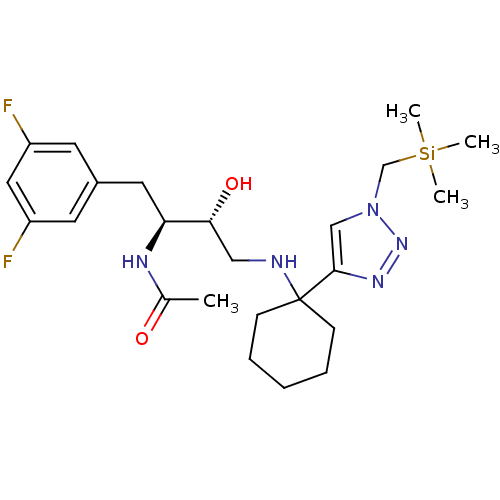
(CHEMBL1257184 | N-((2S,3R)-1-(3,5-difluorophenyl)-...)Show SMILES CC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNC1(CCCCC1)c1cn(C[Si](C)(C)C)nn1 |r| Show InChI InChI=1S/C24H37F2N5O2Si/c1-17(32)28-21(12-18-10-19(25)13-20(26)11-18)22(33)14-27-24(8-6-5-7-9-24)23-15-31(30-29-23)16-34(2,3)4/h10-11,13,15,21-22,27,33H,5-9,12,14,16H2,1-4H3,(H,28,32)/t21-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 20: 6034-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.070
BindingDB Entry DOI: 10.7270/Q2XK8FSK |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50328042
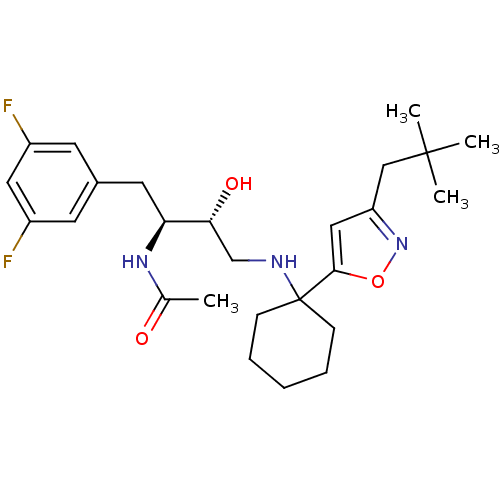
(CHEMBL1258687 | N-((2S,3R)-1-(3,5-difluorophenyl)-...)Show SMILES CC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNC1(CCCCC1)c1cc(CC(C)(C)C)no1 |r| Show InChI InChI=1S/C26H37F2N3O3/c1-17(32)30-22(12-18-10-19(27)13-20(28)11-18)23(33)16-29-26(8-6-5-7-9-26)24-14-21(31-34-24)15-25(2,3)4/h10-11,13-14,22-23,29,33H,5-9,12,15-16H2,1-4H3,(H,30,32)/t22-,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 20: 6034-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.070
BindingDB Entry DOI: 10.7270/Q2XK8FSK |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50438356
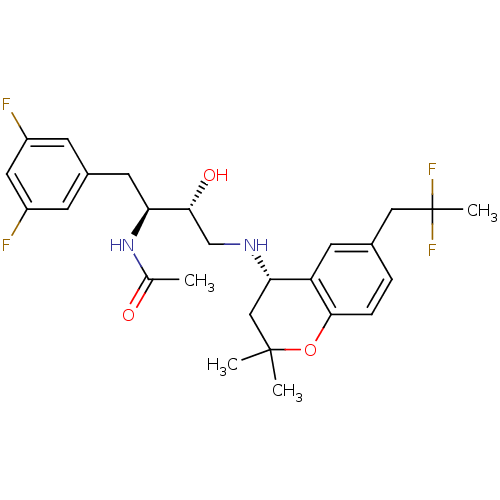
(CHEMBL2408756)Show SMILES CC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CN[C@H]1CC(C)(C)Oc2ccc(CC(C)(F)F)cc12 |r| Show InChI InChI=1S/C26H32F4N2O3/c1-15(33)32-21(10-17-7-18(27)11-19(28)8-17)23(34)14-31-22-13-25(2,3)35-24-6-5-16(9-20(22)24)12-26(4,29)30/h5-9,11,21-23,31,34H,10,12-14H2,1-4H3,(H,32,33)/t21-,22-,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin-D (unknown origin) using C-terminal biotinylated peptide substrate treated 30 mins before addition of peptide substrate measu... |
Bioorg Med Chem Lett 23: 4674-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.006
BindingDB Entry DOI: 10.7270/Q29W0GWT |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50438355
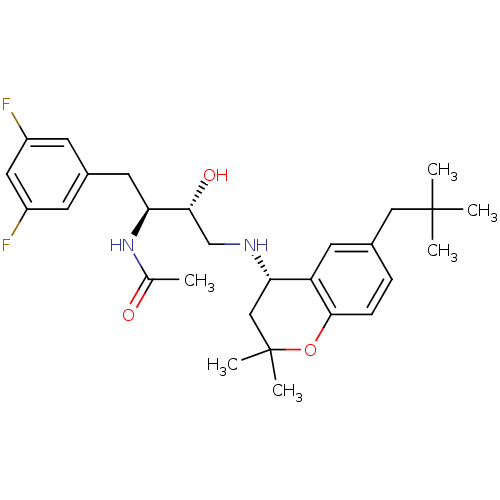
(CHEMBL2408750)Show SMILES CC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CN[C@H]1CC(C)(C)Oc2ccc(CC(C)(C)C)cc12 |r| Show InChI InChI=1S/C28H38F2N2O3/c1-17(33)32-23(12-19-9-20(29)13-21(30)10-19)25(34)16-31-24-15-28(5,6)35-26-8-7-18(11-22(24)26)14-27(2,3)4/h7-11,13,23-25,31,34H,12,14-16H2,1-6H3,(H,32,33)/t23-,24-,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin-D (unknown origin) using C-terminal biotinylated peptide substrate treated 30 mins before addition of peptide substrate measu... |
Bioorg Med Chem Lett 23: 4674-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.006
BindingDB Entry DOI: 10.7270/Q29W0GWT |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM16771
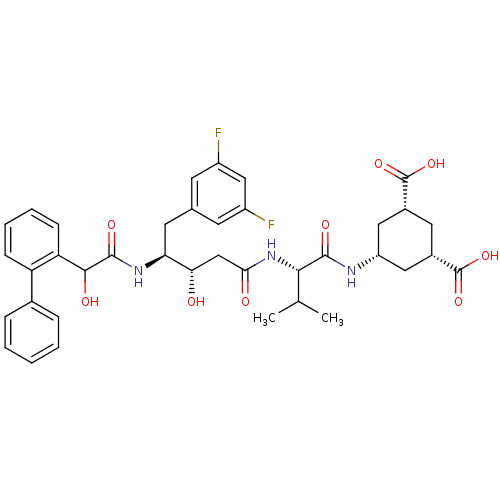
((1R,3S,5S)-5-[(3S,4S)-N-[(1S)-1-carbamoyl-2-methyl...)Show SMILES CC(C)[C@H](NC(=O)C[C@H](O)[C@H](Cc1cc(F)cc(F)c1)NC(=O)C(O)c1ccccc1-c1ccccc1)C(=O)N[C@@H]1C[C@@H](C[C@@H](C1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C38H43F2N3O9/c1-20(2)33(35(47)41-27-16-23(37(49)50)15-24(17-27)38(51)52)43-32(45)19-31(44)30(14-21-12-25(39)18-26(40)13-21)42-36(48)34(46)29-11-7-6-10-28(29)22-8-4-3-5-9-22/h3-13,18,20,23-24,27,30-31,33-34,44,46H,14-17,19H2,1-2H3,(H,41,47)(H,42,48)(H,43,45)(H,49,50)(H,51,52)/t23-,24+,27-,30-,31-,33-,34?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | 4.8 | 37 |
Elan Pharmaceuticals
| Assay Description
Beta-cleavage ELISA assays were carried out in reaction buffer containing enzyme, substrate MBP-C125, and test compounds. Generated beta-cleaved prod... |
J Med Chem 47: 158-64 (2004)
Article DOI: 10.1021/jm0304008
BindingDB Entry DOI: 10.7270/Q2G73BZ6 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50302841

(CHEMBL571860 | N-((2S,3R)-4-(1-(3-tert-butylphenyl...)Show SMILES CC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNC1(CCCCC1)c1cccc(c1)C(C)(C)C |r| Show InChI InChI=1S/C28H38F2N2O2/c1-19(33)32-25(15-20-13-23(29)17-24(30)14-20)26(34)18-31-28(11-6-5-7-12-28)22-10-8-9-21(16-22)27(2,3)4/h8-10,13-14,16-17,25-26,31,34H,5-7,11-12,15,18H2,1-4H3,(H,32,33)/t25-,26+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 20: 6034-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.070
BindingDB Entry DOI: 10.7270/Q2XK8FSK |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50328023
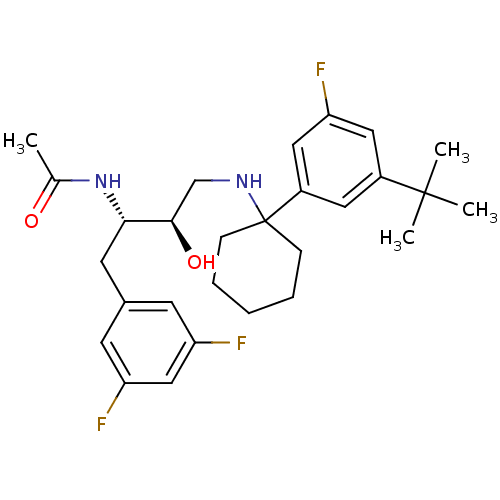
(CHEMBL1257531 | N-((2S,3R)-4-(1-(3-tert-butyl-5-fl...)Show SMILES CC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNC1(CCCCC1)c1cc(F)cc(c1)C(C)(C)C |r| Show InChI InChI=1S/C28H37F3N2O2/c1-18(34)33-25(12-19-10-22(29)16-23(30)11-19)26(35)17-32-28(8-6-5-7-9-28)21-13-20(27(2,3)4)14-24(31)15-21/h10-11,13-16,25-26,32,35H,5-9,12,17H2,1-4H3,(H,33,34)/t25-,26+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 20: 6034-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.070
BindingDB Entry DOI: 10.7270/Q2XK8FSK |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM16765
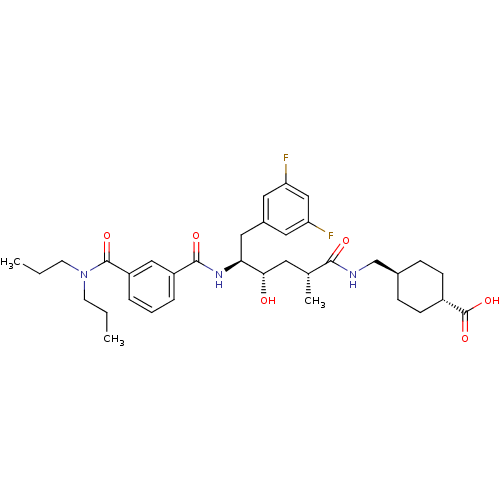
(4-{[(2R,4S,5S)-6-(3,5-difluorophenyl)-5-{[3-(dipro...)Show SMILES CCCN(CCC)C(=O)c1cccc(c1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@@H](O)C[C@@H](C)C(=O)NC[C@H]1CC[C@@H](CC1)C(O)=O |r,wU:18.18,37.38,wD:28.30,31.33,40.45,(-9.78,5.32,;-8.44,4.55,;-7.11,5.32,;-5.78,4.55,;-5.78,3.01,;-4.44,2.24,;-4.44,.7,;-4.44,5.32,;-4.44,6.86,;-3.11,4.55,;-3.11,3.01,;-1.77,2.24,;-.44,3.01,;-.44,4.55,;-1.77,5.32,;.89,5.32,;.89,6.86,;2.23,4.55,;3.56,5.32,;3.56,6.86,;4.65,7.95,;4.06,9.37,;5,10.59,;4.41,12.02,;6.52,10.39,;7.11,8.97,;8.64,8.77,;6.18,7.75,;4.89,4.55,;4.89,3.01,;6.23,5.32,;7.56,4.55,;7.56,3.01,;8.9,5.32,;8.9,6.86,;10.23,4.55,;11.56,5.32,;12.9,4.55,;14.23,5.32,;15.56,4.55,;15.56,3.01,;14.23,2.24,;12.9,3.01,;16.9,2.24,;18.23,3.01,;16.9,.7,)| Show InChI InChI=1S/C35H47F2N3O6/c1-4-13-40(14-5-2)34(44)27-8-6-7-26(19-27)33(43)39-30(18-24-16-28(36)20-29(37)17-24)31(41)15-22(3)32(42)38-21-23-9-11-25(12-10-23)35(45)46/h6-8,16-17,19-20,22-23,25,30-31,41H,4-5,9-15,18,21H2,1-3H3,(H,38,42)(H,39,43)(H,45,46)/t22-,23-,25-,30+,31+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | 4.8 | 37 |
Elan Pharmaceuticals
| Assay Description
Beta-cleavage ELISA assays were carried out in reaction buffer containing enzyme, substrate MBP-C125, and test compounds. Generated beta-cleaved prod... |
J Med Chem 47: 158-64 (2004)
Article DOI: 10.1021/jm0304008
BindingDB Entry DOI: 10.7270/Q2G73BZ6 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50328046
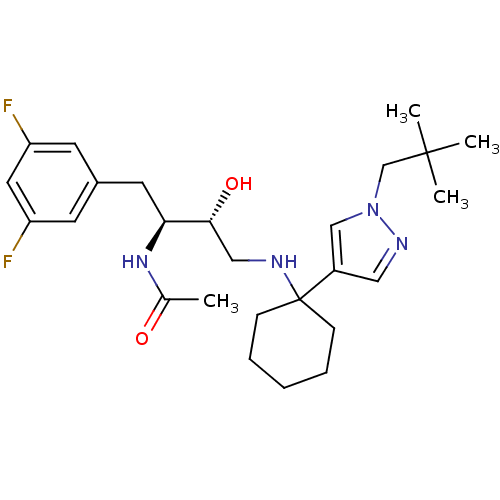
(CHEMBL1258918 | N-((2S,3R)-1-(3,5-difluorophenyl)-...)Show SMILES CC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNC1(CCCCC1)c1cnn(CC(C)(C)C)c1 |r| Show InChI InChI=1S/C26H38F2N4O2/c1-18(33)31-23(12-19-10-21(27)13-22(28)11-19)24(34)15-29-26(8-6-5-7-9-26)20-14-30-32(16-20)17-25(2,3)4/h10-11,13-14,16,23-24,29,34H,5-9,12,15,17H2,1-4H3,(H,31,33)/t23-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 20: 6034-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.070
BindingDB Entry DOI: 10.7270/Q2XK8FSK |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM16798
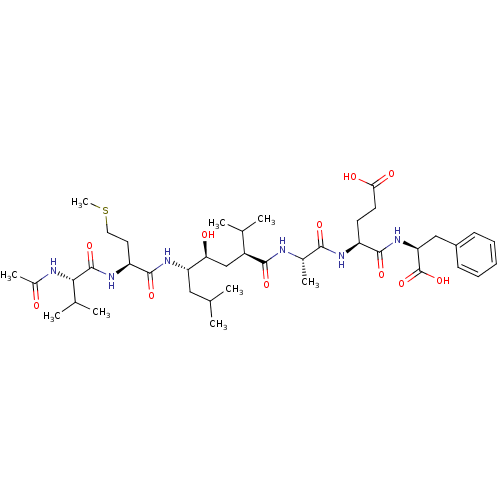
((4S)-4-{[(1S)-1-carboxy-2-phenylethyl]carbamoyl}-4...)Show SMILES CSCC[C@H](NC(=O)[C@@H](NC(C)=O)C(C)C)C(=O)N[C@@H](CC(C)C)[C@@H](O)C[C@@H](C(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C41H66N6O11S/c1-22(2)19-31(46-39(55)30(17-18-59-9)45-40(56)35(24(5)6)43-26(8)48)33(49)21-28(23(3)4)37(53)42-25(7)36(52)44-29(15-16-34(50)51)38(54)47-32(41(57)58)20-27-13-11-10-12-14-27/h10-14,22-25,28-33,35,49H,15-21H2,1-9H3,(H,42,53)(H,43,48)(H,44,52)(H,45,56)(H,46,55)(H,47,54)(H,50,51)(H,57,58)/t25-,28-,29-,30-,31-,32-,33-,35-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | 4.8 | 37 |
Elan Pharmaceuticals
| Assay Description
Beta-cleavage ELISA assays were carried out in reaction buffer containing enzyme, substrate MBP-C125, and test compounds. Generated beta-cleaved prod... |
J Med Chem 47: 158-64 (2004)
Article DOI: 10.1021/jm0304008
BindingDB Entry DOI: 10.7270/Q22Z13RC |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM15790
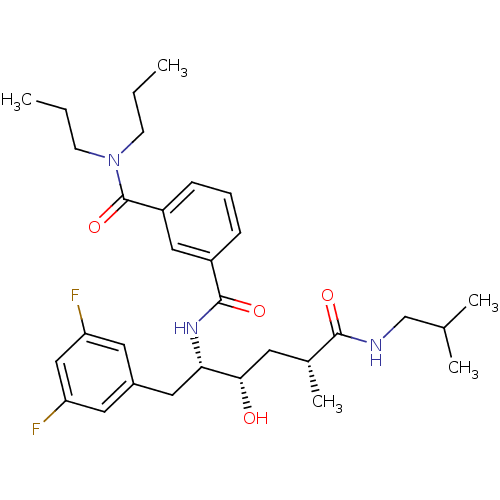
(3-N-[(2S,3S,5R)-1-(3,5-difluorophenyl)-3-hydroxy-5...)Show SMILES CCCN(CCC)C(=O)c1cccc(c1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@@H](O)C[C@@H](C)C(=O)NCC(C)C |r| Show InChI InChI=1S/C31H43F2N3O4/c1-6-11-36(12-7-2)31(40)24-10-8-9-23(17-24)30(39)35-27(16-22-14-25(32)18-26(33)15-22)28(37)13-21(5)29(38)34-19-20(3)4/h8-10,14-15,17-18,20-21,27-28,37H,6-7,11-13,16,19H2,1-5H3,(H,34,38)(H,35,39)/t21-,27+,28+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | 4.8 | 37 |
Elan Pharmaceuticals
| Assay Description
Beta-cleavage ELISA assays were carried out in reaction buffer containing enzyme, substrate MBP-C125, and test compounds. Generated beta-cleaved prod... |
J Med Chem 47: 158-64 (2004)
Article DOI: 10.1021/jm0304008
BindingDB Entry DOI: 10.7270/Q2G73BZ6 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50328037
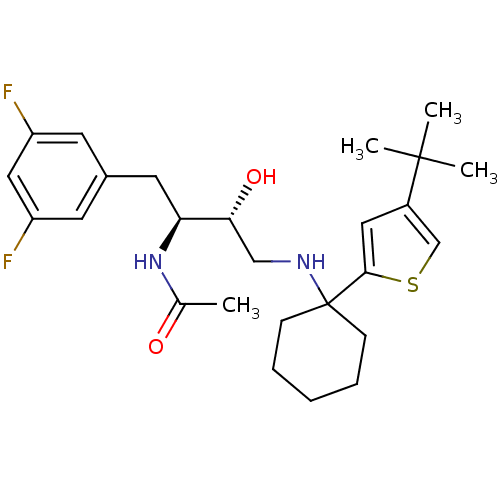
(CHEMBL1258466 | N-((2S,3R)-4-(1-(4-tert-butylthiop...)Show SMILES CC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNC1(CCCCC1)c1cc(cs1)C(C)(C)C |r| Show InChI InChI=1S/C26H36F2N2O2S/c1-17(31)30-22(12-18-10-20(27)14-21(28)11-18)23(32)15-29-26(8-6-5-7-9-26)24-13-19(16-33-24)25(2,3)4/h10-11,13-14,16,22-23,29,32H,5-9,12,15H2,1-4H3,(H,30,31)/t22-,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 20: 6034-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.070
BindingDB Entry DOI: 10.7270/Q2XK8FSK |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50328036
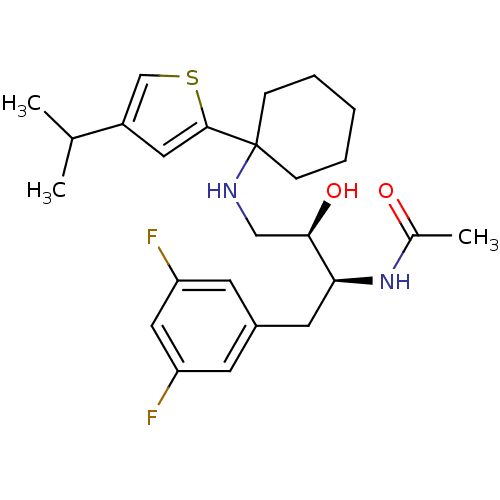
(CHEMBL1258353 | N-((2S,3R)-1-(3,5-difluorophenyl)-...)Show SMILES CC(C)c1csc(c1)C1(CCCCC1)NC[C@@H](O)[C@H](Cc1cc(F)cc(F)c1)NC(C)=O |r| Show InChI InChI=1S/C25H34F2N2O2S/c1-16(2)19-12-24(32-15-19)25(7-5-4-6-8-25)28-14-23(31)22(29-17(3)30)11-18-9-20(26)13-21(27)10-18/h9-10,12-13,15-16,22-23,28,31H,4-8,11,14H2,1-3H3,(H,29,30)/t22-,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 20: 6034-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.070
BindingDB Entry DOI: 10.7270/Q2XK8FSK |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50328035
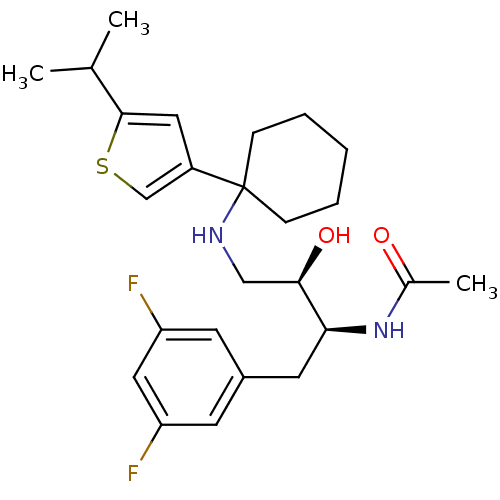
(CHEMBL1258352 | N-((2S,3R)-1-(3,5-difluorophenyl)-...)Show SMILES CC(C)c1cc(cs1)C1(CCCCC1)NC[C@@H](O)[C@H](Cc1cc(F)cc(F)c1)NC(C)=O |r| Show InChI InChI=1S/C25H34F2N2O2S/c1-16(2)24-12-19(15-32-24)25(7-5-4-6-8-25)28-14-23(31)22(29-17(3)30)11-18-9-20(26)13-21(27)10-18/h9-10,12-13,15-16,22-23,28,31H,4-8,11,14H2,1-3H3,(H,29,30)/t22-,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 20: 6034-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.070
BindingDB Entry DOI: 10.7270/Q2XK8FSK |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50328024
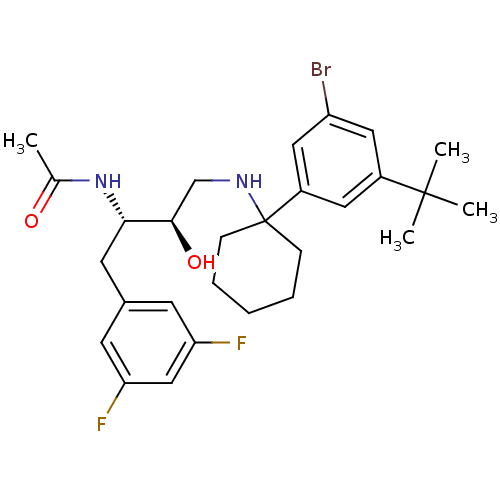
(CHEMBL1257649 | N-((2S,3R)-4-(1-(3-bromo-5-tert-bu...)Show SMILES CC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNC1(CCCCC1)c1cc(Br)cc(c1)C(C)(C)C |r| Show InChI InChI=1S/C28H37BrF2N2O2/c1-18(34)33-25(12-19-10-23(30)16-24(31)11-19)26(35)17-32-28(8-6-5-7-9-28)21-13-20(27(2,3)4)14-22(29)15-21/h10-11,13-16,25-26,32,35H,5-9,12,17H2,1-4H3,(H,33,34)/t25-,26+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 20: 6034-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.070
BindingDB Entry DOI: 10.7270/Q2XK8FSK |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50328031
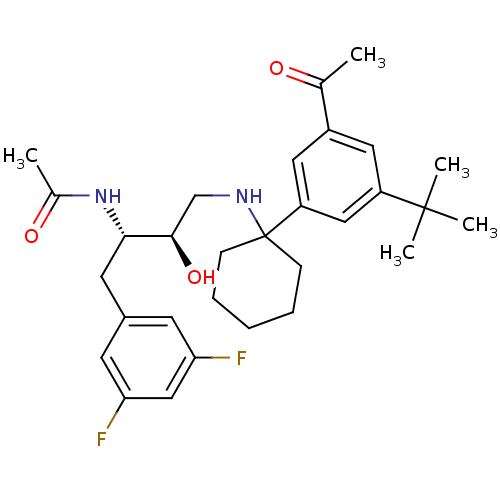
(CHEMBL1258119 | N-((2S,3R)-4-(1-(3-acetyl-5-tert-b...)Show SMILES CC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNC1(CCCCC1)c1cc(cc(c1)C(C)(C)C)C(C)=O |r| Show InChI InChI=1S/C30H40F2N2O3/c1-19(35)22-14-23(29(3,4)5)16-24(15-22)30(9-7-6-8-10-30)33-18-28(37)27(34-20(2)36)13-21-11-25(31)17-26(32)12-21/h11-12,14-17,27-28,33,37H,6-10,13,18H2,1-5H3,(H,34,36)/t27-,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 20: 6034-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.070
BindingDB Entry DOI: 10.7270/Q2XK8FSK |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM16766
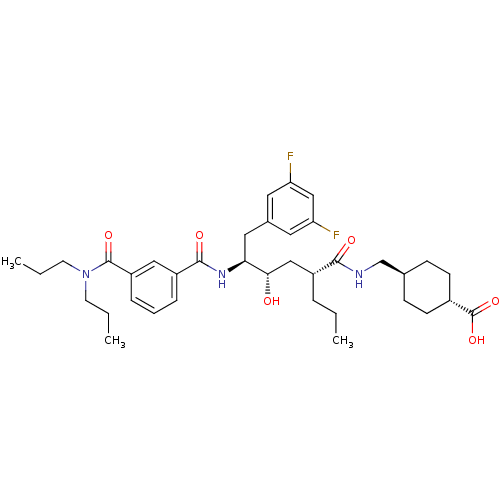
(4-{[(2R,4S,5S)-6-(3,5-difluorophenyl)-5-{[3-(dipro...)Show SMILES CCC[C@H](C[C@H](O)[C@H](Cc1cc(F)cc(F)c1)NC(=O)c1cccc(c1)C(=O)N(CCC)CCC)C(=O)NC[C@H]1CC[C@@H](CC1)C(O)=O |r,wU:7.17,39.40,wD:3.3,5.5,42.47,(8.9,.7,;8.9,2.24,;7.56,3.01,;7.56,4.55,;6.23,5.32,;4.89,4.55,;4.89,3.01,;3.56,5.32,;3.56,6.86,;4.65,7.95,;4.06,9.37,;5,10.59,;4.41,12.02,;6.52,10.39,;7.11,8.97,;8.64,8.77,;6.18,7.75,;2.23,4.55,;.89,5.32,;.89,6.86,;-.44,4.55,;-.44,3.01,;-1.77,2.24,;-3.11,3.01,;-3.11,4.55,;-1.77,5.32,;-4.44,5.32,;-4.44,6.86,;-5.78,4.55,;-7.11,5.32,;-8.44,4.55,;-9.78,5.32,;-5.78,3.01,;-4.44,2.24,;-4.44,.7,;8.9,5.32,;8.9,6.86,;10.23,4.55,;11.56,5.32,;12.9,4.55,;14.23,5.32,;15.56,4.55,;15.56,3.01,;14.23,2.24,;12.9,3.01,;16.9,2.24,;18.23,3.01,;16.9,.7,)| Show InChI InChI=1S/C37H51F2N3O6/c1-4-8-27(34(44)40-23-24-11-13-26(14-12-24)37(47)48)21-33(43)32(19-25-17-30(38)22-31(39)18-25)41-35(45)28-9-7-10-29(20-28)36(46)42(15-5-2)16-6-3/h7,9-10,17-18,20,22,24,26-27,32-33,43H,4-6,8,11-16,19,21,23H2,1-3H3,(H,40,44)(H,41,45)(H,47,48)/t24-,26-,27-,32+,33+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | 4.8 | 37 |
Elan Pharmaceuticals
| Assay Description
Beta-cleavage ELISA assays were carried out in reaction buffer containing enzyme, substrate MBP-C125, and test compounds. Generated beta-cleaved prod... |
J Med Chem 47: 158-64 (2004)
Article DOI: 10.1021/jm0304008
BindingDB Entry DOI: 10.7270/Q2G73BZ6 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50328023

(CHEMBL1257531 | N-((2S,3R)-4-(1-(3-tert-butyl-5-fl...)Show SMILES CC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNC1(CCCCC1)c1cc(F)cc(c1)C(C)(C)C |r| Show InChI InChI=1S/C28H37F3N2O2/c1-18(34)33-25(12-19-10-22(29)16-23(30)11-19)26(35)17-32-28(8-6-5-7-9-28)21-13-20(27(2,3)4)14-24(31)15-21/h10-11,13-16,25-26,32,35H,5-9,12,17H2,1-4H3,(H,33,34)/t25-,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 20: 6034-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.070
BindingDB Entry DOI: 10.7270/Q2XK8FSK |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50328027
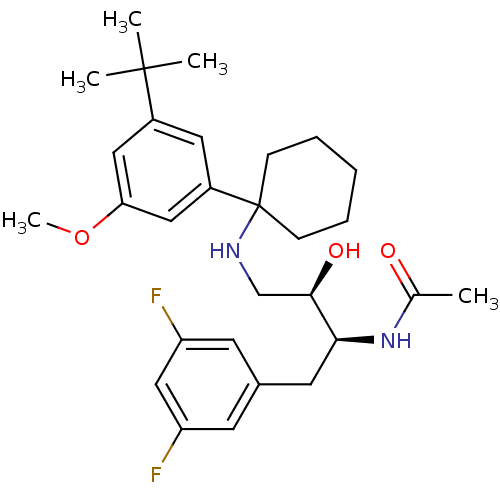
(CHEMBL1257773 | N-((2S,3R)-4-(1-(3-tert-butyl-5-me...)Show SMILES COc1cc(cc(c1)C1(CCCCC1)NC[C@@H](O)[C@H](Cc1cc(F)cc(F)c1)NC(C)=O)C(C)(C)C |r| Show InChI InChI=1S/C29H40F2N2O3/c1-19(34)33-26(13-20-11-23(30)17-24(31)12-20)27(35)18-32-29(9-7-6-8-10-29)22-14-21(28(2,3)4)15-25(16-22)36-5/h11-12,14-17,26-27,32,35H,6-10,13,18H2,1-5H3,(H,33,34)/t26-,27+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 20: 6034-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.070
BindingDB Entry DOI: 10.7270/Q2XK8FSK |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50302841

(CHEMBL571860 | N-((2S,3R)-4-(1-(3-tert-butylphenyl...)Show SMILES CC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNC1(CCCCC1)c1cccc(c1)C(C)(C)C |r| Show InChI InChI=1S/C28H38F2N2O2/c1-19(33)32-25(15-20-13-23(29)17-24(30)14-20)26(34)18-31-28(11-6-5-7-12-28)22-10-8-9-21(16-22)27(2,3)4/h8-10,13-14,16-17,25-26,31,34H,5-7,11-12,15,18H2,1-4H3,(H,32,33)/t25-,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 20: 6034-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.070
BindingDB Entry DOI: 10.7270/Q2XK8FSK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM16761
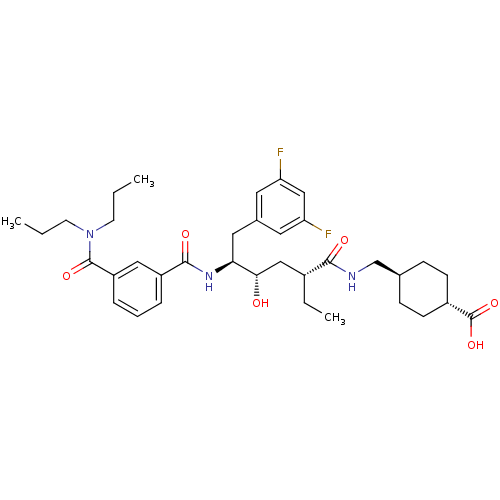
(4-{[(2R,4S,5S)-6-(3,5-difluorophenyl)-5-{[3-(dipro...)Show SMILES CCCN(CCC)C(=O)c1cccc(c1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@@H](O)C[C@@H](CC)C(=O)NC[C@H]1CC[C@@H](CC1)C(O)=O |r,wU:18.18,38.39,wD:31.32,28.30,41.46,(-9.78,5.32,;-8.44,4.55,;-7.11,5.32,;-5.78,4.55,;-5.78,3.01,;-4.44,2.24,;-4.44,.7,;-4.44,5.32,;-4.44,6.86,;-3.11,4.55,;-3.11,3.01,;-1.77,2.24,;-.44,3.01,;-.44,4.55,;-1.77,5.32,;.89,5.32,;.89,6.86,;2.23,4.55,;3.56,5.32,;3.56,6.86,;4.65,7.95,;4.06,9.37,;5,10.59,;4.41,12.02,;6.52,10.39,;7.11,8.97,;8.64,8.77,;6.18,7.75,;4.89,4.55,;4.89,3.01,;6.23,5.32,;7.56,4.55,;7.56,3.01,;6.23,2.24,;8.9,5.32,;8.9,6.86,;10.23,4.55,;11.56,5.32,;12.9,4.55,;14.23,5.32,;15.56,4.55,;15.56,3.01,;14.23,2.24,;12.9,3.01,;16.9,2.24,;18.23,3.01,;16.9,.7,)| Show InChI InChI=1S/C36H49F2N3O6/c1-4-14-41(15-5-2)35(45)28-9-7-8-27(19-28)34(44)40-31(18-24-16-29(37)21-30(38)17-24)32(42)20-25(6-3)33(43)39-22-23-10-12-26(13-11-23)36(46)47/h7-9,16-17,19,21,23,25-26,31-32,42H,4-6,10-15,18,20,22H2,1-3H3,(H,39,43)(H,40,44)(H,46,47)/t23-,25-,26-,31+,32+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | 4.8 | 37 |
Elan Pharmaceuticals
| Assay Description
Beta-cleavage ELISA assays were carried out in reaction buffer containing enzyme, substrate MBP-C125, and test compounds. Generated beta-cleaved prod... |
J Med Chem 47: 158-64 (2004)
Article DOI: 10.1021/jm0304008
BindingDB Entry DOI: 10.7270/Q2G73BZ6 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50328025
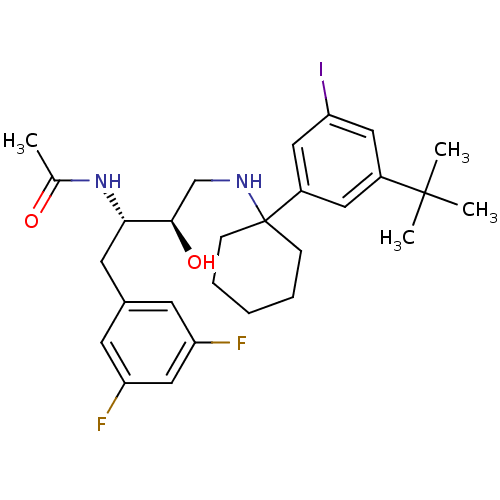
(CHEMBL1257650 | N-((2S,3R)-4-(1-(3-tert-butyl-5-io...)Show SMILES CC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNC1(CCCCC1)c1cc(I)cc(c1)C(C)(C)C |r| Show InChI InChI=1S/C28H37F2IN2O2/c1-18(34)33-25(12-19-10-22(29)16-23(30)11-19)26(35)17-32-28(8-6-5-7-9-28)21-13-20(27(2,3)4)14-24(31)15-21/h10-11,13-16,25-26,32,35H,5-9,12,17H2,1-4H3,(H,33,34)/t25-,26+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 20: 6034-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.070
BindingDB Entry DOI: 10.7270/Q2XK8FSK |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50438354

(CHEMBL2408758)Show SMILES CC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CN[C@H]1CC(C)(C)Oc2ccc(OC(F)(F)F)cc12 |r| Show InChI InChI=1S/C24H27F5N2O4/c1-13(32)31-19(8-14-6-15(25)9-16(26)7-14)21(33)12-30-20-11-23(2,3)35-22-5-4-17(10-18(20)22)34-24(27,28)29/h4-7,9-10,19-21,30,33H,8,11-12H2,1-3H3,(H,31,32)/t19-,20-,21+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin-D (unknown origin) using C-terminal biotinylated peptide substrate treated 30 mins before addition of peptide substrate measu... |
Bioorg Med Chem Lett 23: 4674-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.006
BindingDB Entry DOI: 10.7270/Q29W0GWT |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50328026
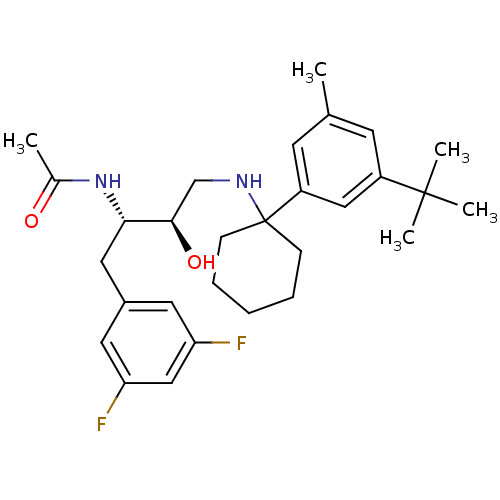
(CHEMBL1257772 | N-((2S,3R)-4-(1-(3-tert-butyl-5-me...)Show SMILES CC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNC1(CCCCC1)c1cc(C)cc(c1)C(C)(C)C |r| Show InChI InChI=1S/C29H40F2N2O2/c1-19-11-22(28(3,4)5)16-23(12-19)29(9-7-6-8-10-29)32-18-27(35)26(33-20(2)34)15-21-13-24(30)17-25(31)14-21/h11-14,16-17,26-27,32,35H,6-10,15,18H2,1-5H3,(H,33,34)/t26-,27+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 20: 6034-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.070
BindingDB Entry DOI: 10.7270/Q2XK8FSK |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50438353
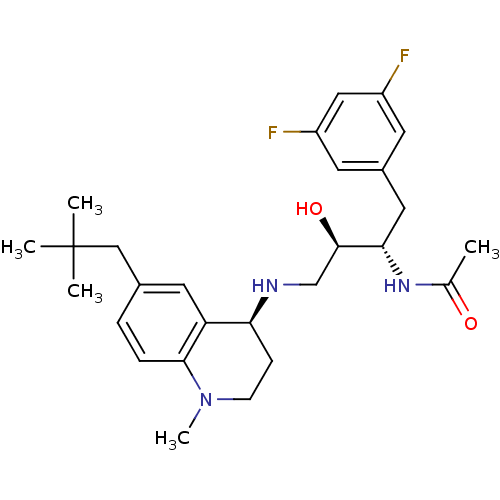
(CHEMBL2408736)Show SMILES CN1CC[C@H](NC[C@@H](O)[C@H](Cc2cc(F)cc(F)c2)NC(C)=O)c2cc(CC(C)(C)C)ccc12 |r| Show InChI InChI=1S/C27H37F2N3O2/c1-17(33)31-24(13-19-10-20(28)14-21(29)11-19)26(34)16-30-23-8-9-32(5)25-7-6-18(12-22(23)25)15-27(2,3)4/h6-7,10-12,14,23-24,26,30,34H,8-9,13,15-16H2,1-5H3,(H,31,33)/t23-,24-,26+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin-D (unknown origin) using C-terminal biotinylated peptide substrate treated 30 mins before addition of peptide substrate measu... |
Bioorg Med Chem Lett 23: 4674-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.006
BindingDB Entry DOI: 10.7270/Q29W0GWT |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50438352
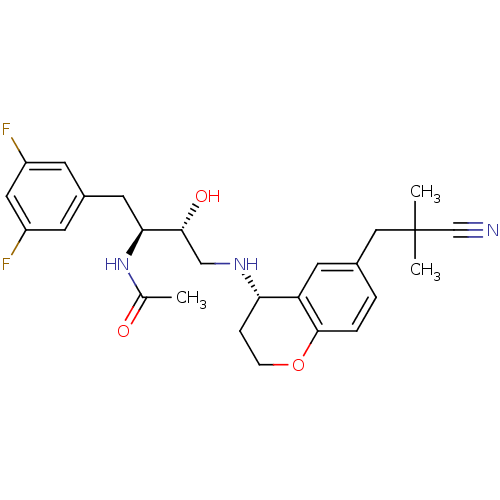
(CHEMBL2408744)Show SMILES CC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CN[C@H]1CCOc2ccc(CC(C)(C)C#N)cc12 |r| Show InChI InChI=1S/C26H31F2N3O3/c1-16(32)31-23(11-18-8-19(27)12-20(28)9-18)24(33)14-30-22-6-7-34-25-5-4-17(10-21(22)25)13-26(2,3)15-29/h4-5,8-10,12,22-24,30,33H,6-7,11,13-14H2,1-3H3,(H,31,32)/t22-,23-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin-D (unknown origin) using C-terminal biotinylated peptide substrate treated 30 mins before addition of peptide substrate measu... |
Bioorg Med Chem Lett 23: 4674-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.006
BindingDB Entry DOI: 10.7270/Q29W0GWT |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50438351
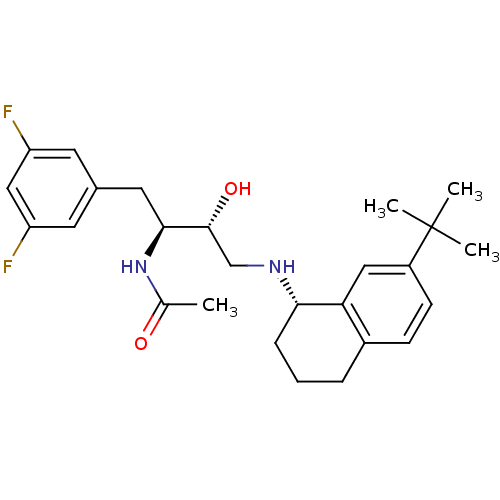
(CHEMBL2408765)Show SMILES CC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CN[C@H]1CCCc2ccc(cc12)C(C)(C)C |r| Show InChI InChI=1S/C26H34F2N2O2/c1-16(31)30-24(12-17-10-20(27)14-21(28)11-17)25(32)15-29-23-7-5-6-18-8-9-19(13-22(18)23)26(2,3)4/h8-11,13-14,23-25,29,32H,5-7,12,15H2,1-4H3,(H,30,31)/t23-,24-,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin-D (unknown origin) using C-terminal biotinylated peptide substrate treated 30 mins before addition of peptide substrate measu... |
Bioorg Med Chem Lett 23: 4674-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.006
BindingDB Entry DOI: 10.7270/Q29W0GWT |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50328027

(CHEMBL1257773 | N-((2S,3R)-4-(1-(3-tert-butyl-5-me...)Show SMILES COc1cc(cc(c1)C1(CCCCC1)NC[C@@H](O)[C@H](Cc1cc(F)cc(F)c1)NC(C)=O)C(C)(C)C |r| Show InChI InChI=1S/C29H40F2N2O3/c1-19(34)33-26(13-20-11-23(30)17-24(31)12-20)27(35)18-32-29(9-7-6-8-10-29)22-14-21(28(2,3)4)15-25(16-22)36-5/h11-12,14-17,26-27,32,35H,6-10,13,18H2,1-5H3,(H,33,34)/t26-,27+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 20: 6034-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.070
BindingDB Entry DOI: 10.7270/Q2XK8FSK |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50438350

(CHEMBL2408763)Show SMILES CCCCc1ccc2CCC[C@H](NC[C@@H](O)[C@H](Cc3cc(F)cc(F)c3)NC(C)=O)c2c1 |r| Show InChI InChI=1S/C26H34F2N2O2/c1-3-4-6-18-9-10-20-7-5-8-24(23(20)13-18)29-16-26(32)25(30-17(2)31)14-19-11-21(27)15-22(28)12-19/h9-13,15,24-26,29,32H,3-8,14,16H2,1-2H3,(H,30,31)/t24-,25-,26+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin-D (unknown origin) using C-terminal biotinylated peptide substrate treated 30 mins before addition of peptide substrate measu... |
Bioorg Med Chem Lett 23: 4674-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.006
BindingDB Entry DOI: 10.7270/Q29W0GWT |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50438349
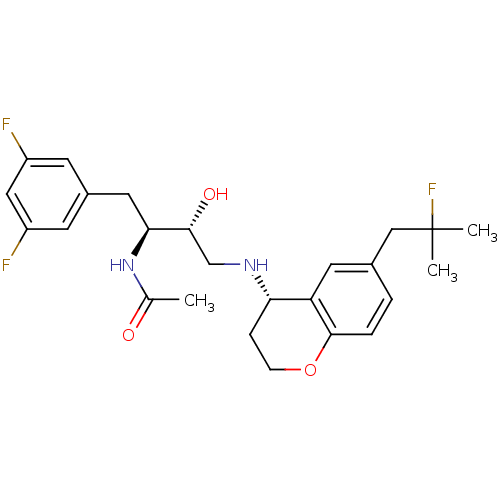
(CHEMBL2408742)Show SMILES CC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CN[C@H]1CCOc2ccc(CC(C)(C)F)cc12 |r| Show InChI InChI=1S/C25H31F3N2O3/c1-15(31)30-22(11-17-8-18(26)12-19(27)9-17)23(32)14-29-21-6-7-33-24-5-4-16(10-20(21)24)13-25(2,3)28/h4-5,8-10,12,21-23,29,32H,6-7,11,13-14H2,1-3H3,(H,30,31)/t21-,22-,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin-D (unknown origin) using C-terminal biotinylated peptide substrate treated 30 mins before addition of peptide substrate measu... |
Bioorg Med Chem Lett 23: 4674-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.006
BindingDB Entry DOI: 10.7270/Q29W0GWT |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50328028
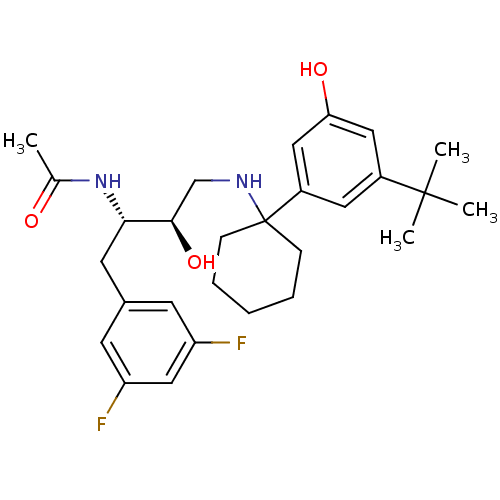
(CHEMBL1257891 | N-((2S,3R)-4-(1-(3-tert-butyl-5-hy...)Show SMILES CC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNC1(CCCCC1)c1cc(O)cc(c1)C(C)(C)C |r| Show InChI InChI=1S/C28H38F2N2O3/c1-18(33)32-25(12-19-10-22(29)16-23(30)11-19)26(35)17-31-28(8-6-5-7-9-28)21-13-20(27(2,3)4)14-24(34)15-21/h10-11,13-16,25-26,31,34-35H,5-9,12,17H2,1-4H3,(H,32,33)/t25-,26+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 20: 6034-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.070
BindingDB Entry DOI: 10.7270/Q2XK8FSK |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50328030

(CHEMBL1258001 | N-((2S,3R)-4-(1-(3-tert-butyl-5-(m...)Show SMILES CC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNC1(CCCCC1)c1cc(NS(C)(=O)=O)cc(c1)C(C)(C)C |r| Show InChI InChI=1S/C29H41F2N3O4S/c1-19(35)33-26(13-20-11-23(30)17-24(31)12-20)27(36)18-32-29(9-7-6-8-10-29)22-14-21(28(2,3)4)15-25(16-22)34-39(5,37)38/h11-12,14-17,26-27,32,34,36H,6-10,13,18H2,1-5H3,(H,33,35)/t26-,27+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 20: 6034-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.070
BindingDB Entry DOI: 10.7270/Q2XK8FSK |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50328037

(CHEMBL1258466 | N-((2S,3R)-4-(1-(4-tert-butylthiop...)Show SMILES CC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNC1(CCCCC1)c1cc(cs1)C(C)(C)C |r| Show InChI InChI=1S/C26H36F2N2O2S/c1-17(31)30-22(12-18-10-20(27)14-21(28)11-18)23(32)15-29-26(8-6-5-7-9-26)24-13-19(16-33-24)25(2,3)4/h10-11,13-14,16,22-23,29,32H,5-9,12,15H2,1-4H3,(H,30,31)/t22-,23+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 20: 6034-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.070
BindingDB Entry DOI: 10.7270/Q2XK8FSK |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50328029
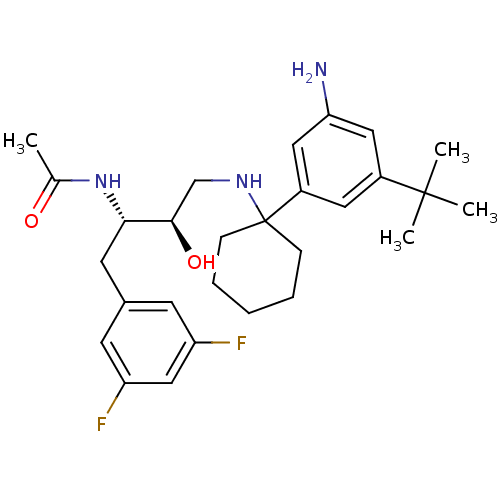
(CHEMBL1257892 | N-((2S,3R)-4-(1-(3-amino-5-tert-bu...)Show SMILES CC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNC1(CCCCC1)c1cc(N)cc(c1)C(C)(C)C |r| Show InChI InChI=1S/C28H39F2N3O2/c1-18(34)33-25(12-19-10-22(29)16-23(30)11-19)26(35)17-32-28(8-6-5-7-9-28)21-13-20(27(2,3)4)14-24(31)15-21/h10-11,13-16,25-26,32,35H,5-9,12,17,31H2,1-4H3,(H,33,34)/t25-,26+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 20: 6034-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.070
BindingDB Entry DOI: 10.7270/Q2XK8FSK |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50127407
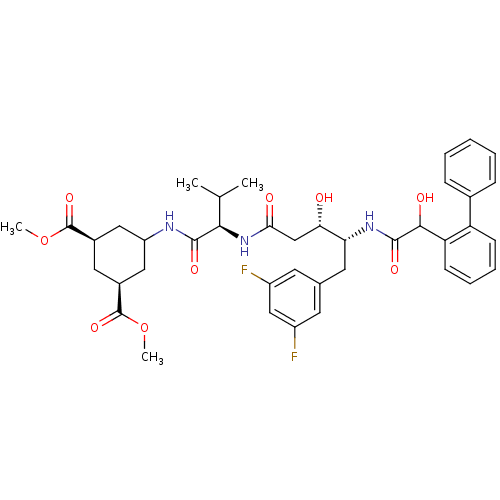
(5-{2-[4-(2-Biphenyl-2-yl-2-hydroxy-acetylamino)-5-...)Show SMILES COC(=O)[C@H]1CC(C[C@H](C1)C(=O)OC)NC(=O)[C@H](NC(=O)C[C@H](O)[C@@H](Cc1cc(F)cc(F)c1)NC(=O)C(O)c1ccccc1-c1ccccc1)C(C)C Show InChI InChI=1S/C40H47F2N3O9/c1-22(2)35(37(49)43-29-18-25(39(51)53-3)17-26(19-29)40(52)54-4)45-34(47)21-33(46)32(16-23-14-27(41)20-28(42)15-23)44-38(50)36(48)31-13-9-8-12-30(31)24-10-6-5-7-11-24/h5-15,20,22,25-26,29,32-33,35-36,46,48H,16-19,21H2,1-4H3,(H,43,49)(H,44,50)(H,45,47)/t25-,26+,29?,32-,33+,35-,36?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human beta-secretase (BACE) in MBP-C125 assay |
J Med Chem 46: 1799-802 (2003)
Article DOI: 10.1021/jm025619l
BindingDB Entry DOI: 10.7270/Q2ZG6RMK |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50328022
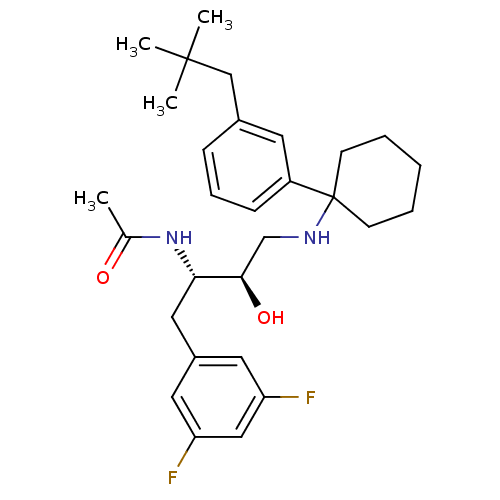
(CHEMBL1257530 | N-((2S,3R)-1-(3,5-difluorophenyl)-...)Show SMILES CC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNC1(CCCCC1)c1cccc(CC(C)(C)C)c1 |r| Show InChI InChI=1S/C29H40F2N2O2/c1-20(34)33-26(16-22-14-24(30)17-25(31)15-22)27(35)19-32-29(11-6-5-7-12-29)23-10-8-9-21(13-23)18-28(2,3)4/h8-10,13-15,17,26-27,32,35H,5-7,11-12,16,18-19H2,1-4H3,(H,33,34)/t26-,27+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 20: 6034-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.070
BindingDB Entry DOI: 10.7270/Q2XK8FSK |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50438348
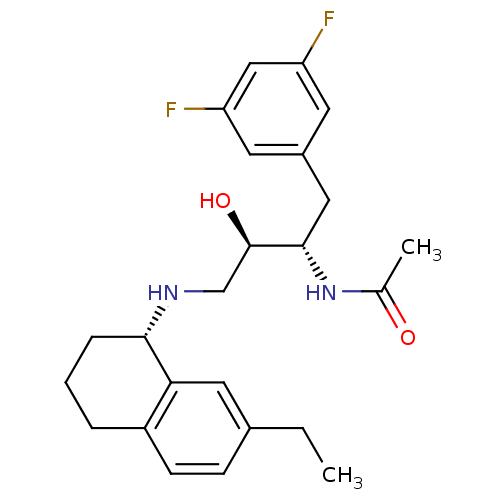
(CHEMBL2408762)Show SMILES CCc1ccc2CCC[C@H](NC[C@@H](O)[C@H](Cc3cc(F)cc(F)c3)NC(C)=O)c2c1 |r| Show InChI InChI=1S/C24H30F2N2O2/c1-3-16-7-8-18-5-4-6-22(21(18)11-16)27-14-24(30)23(28-15(2)29)12-17-9-19(25)13-20(26)10-17/h7-11,13,22-24,27,30H,3-6,12,14H2,1-2H3,(H,28,29)/t22-,23-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin-D (unknown origin) using C-terminal biotinylated peptide substrate treated 30 mins before addition of peptide substrate measu... |
Bioorg Med Chem Lett 23: 4674-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.006
BindingDB Entry DOI: 10.7270/Q29W0GWT |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50328038

(CHEMBL1258467 | N-((2S,3R)-1-(3,5-difluorophenyl)-...)Show SMILES CC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNC1(CCCCC1)c1cc(CC(C)(C)C)cs1 |r| Show InChI InChI=1S/C27H38F2N2O2S/c1-18(32)31-23(12-19-10-21(28)14-22(29)11-19)24(33)16-30-27(8-6-5-7-9-27)25-13-20(17-34-25)15-26(2,3)4/h10-11,13-14,17,23-24,30,33H,5-9,12,15-16H2,1-4H3,(H,31,32)/t23-,24+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 131 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 20: 6034-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.070
BindingDB Entry DOI: 10.7270/Q2XK8FSK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data