 Found 1509 hits with Last Name = 'gao' and Initial = 's'
Found 1509 hits with Last Name = 'gao' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50089636
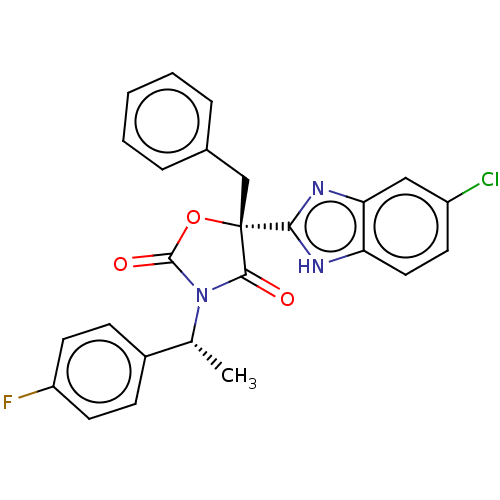
(CHEMBL3578271)Show SMILES C[C@@H](N1C(=O)O[C@@](Cc2ccccc2)(C1=O)c1nc2cc(Cl)ccc2[nH]1)c1ccc(F)cc1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to mineralocorticoid receptor (unknown origin) |
ACS Med Chem Lett 6: 461-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00010
BindingDB Entry DOI: 10.7270/Q21J9CH4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50343759
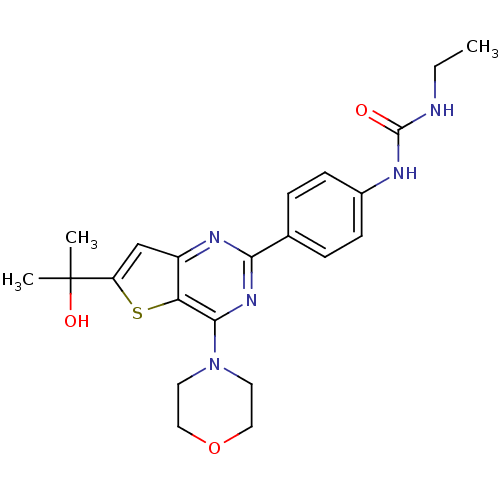
(1-ethyl-3-(4-(6-(2-hydroxypropan-2-yl)-4-morpholin...)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc(N2CCOCC2)c2sc(cc2n1)C(C)(C)O Show InChI InChI=1S/C22H27N5O3S/c1-4-23-21(28)24-15-7-5-14(6-8-15)19-25-16-13-17(22(2,3)29)31-18(16)20(26-19)27-9-11-30-12-10-27/h5-8,13,29H,4,9-12H2,1-3H3,(H2,23,24,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal FLAG-tagged mTOR (1362-end residues) in presence of [gamma33P]ATP after 40 mins |
Eur J Med Chem 129: 135-150 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.015
BindingDB Entry DOI: 10.7270/Q2QN692K |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM496902

(CVD-0018409 | PF-07321332 | US11351149, Example 13...)Show SMILES CC(C)(C)[C@H](NC(=O)C(F)(F)F)C(=O)N1C[C@H]2[C@@H]([C@H]1C(=O)N[C@@H](C[C@@H]1CCNC1=O)C#N)C2(C)C Show InChI InChI=1S/C23H32F3N5O4/c1-21(2,3)16(30-20(35)23(24,25)26)19(34)31-10-13-14(22(13,4)5)15(31)18(33)29-12(9-27)8-11-6-7-28-17(11)32/h11-16H,6-8,10H2,1-5H3,(H,28,32)(H,29,33)(H,30,35)/t11-,12-,13-,14-,15-,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01716
BindingDB Entry DOI: 10.7270/Q2VX0MKR |
More data for this
Ligand-Target Pair | |
Beta-glucuronidase
(Bos taurus (Bovine)) | BDBM50511223

(CHEMBL4589187)Show InChI InChI=1S/C6H11NO4/c8-4-2-7-1-3(5(4)9)6(10)11/h3-5,7-9H,1-2H2,(H,10,11)/t3-,4+,5+/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of bovine GUS pre-incubated for 5 to 30 mins before 4-methylumbelliferone beta-D-glucuronide substrate addition at pH 6.7 |
J Med Chem 63: 4617-4627 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01918
BindingDB Entry DOI: 10.7270/Q2CJ8HTJ |
More data for this
Ligand-Target Pair | |
Beta-glucuronidase
(Escherichia coli (Enterobacteria)) | BDBM50511223

(CHEMBL4589187)Show InChI InChI=1S/C6H11NO4/c8-4-2-7-1-3(5(4)9)6(10)11/h3-5,7-9H,1-2H2,(H,10,11)/t3-,4+,5+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli GUS pre-incubated for 5 to 30 mins before 4-methylumbelliferone beta-D-glucuronide substrate addition at pH 6.7 |
J Med Chem 63: 4617-4627 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01918
BindingDB Entry DOI: 10.7270/Q2CJ8HTJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-glucuronidase
(Escherichia coli (Enterobacteria)) | BDBM50511223

(CHEMBL4589187)Show InChI InChI=1S/C6H11NO4/c8-4-2-7-1-3(5(4)9)6(10)11/h3-5,7-9H,1-2H2,(H,10,11)/t3-,4+,5+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli GUS pre-incubated for 5 to 30 mins before 4-methylumbelliferone beta-D-glucuronide substrate addition at pH 5.5 |
J Med Chem 63: 4617-4627 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01918
BindingDB Entry DOI: 10.7270/Q2CJ8HTJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-glucuronidase
(Bos taurus (Bovine)) | BDBM50511223

(CHEMBL4589187)Show InChI InChI=1S/C6H11NO4/c8-4-2-7-1-3(5(4)9)6(10)11/h3-5,7-9H,1-2H2,(H,10,11)/t3-,4+,5+/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of bovine GUS pre-incubated for 5 to 30 mins before 4-methylumbelliferone beta-D-glucuronide substrate addition at pH 4.5 |
J Med Chem 63: 4617-4627 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01918
BindingDB Entry DOI: 10.7270/Q2CJ8HTJ |
More data for this
Ligand-Target Pair | |
Beta-glucuronidase
(Escherichia coli (Enterobacteria)) | BDBM50511221
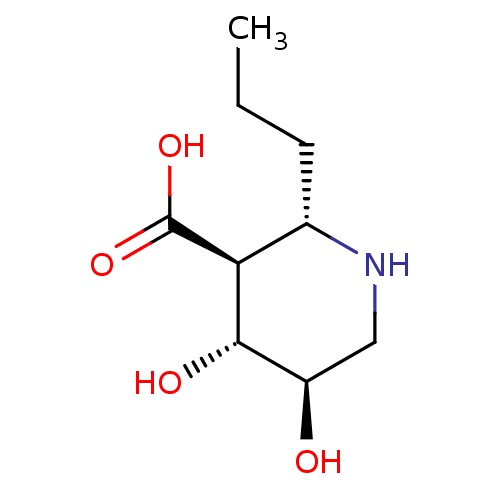
(CHEMBL4451253)Show SMILES CCC[C@@H]1NC[C@@H](O)[C@H](O)[C@H]1C(O)=O |r| Show InChI InChI=1S/C9H17NO4/c1-2-3-5-7(9(13)14)8(12)6(11)4-10-5/h5-8,10-12H,2-4H2,1H3,(H,13,14)/t5-,6+,7-,8-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli GUS pre-incubated for 5 to 30 mins before 4-methylumbelliferone beta-D-glucuronide substrate addition at pH 6.7 |
J Med Chem 63: 4617-4627 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01918
BindingDB Entry DOI: 10.7270/Q2CJ8HTJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-glucuronidase
(Bos taurus (Bovine)) | BDBM50511220
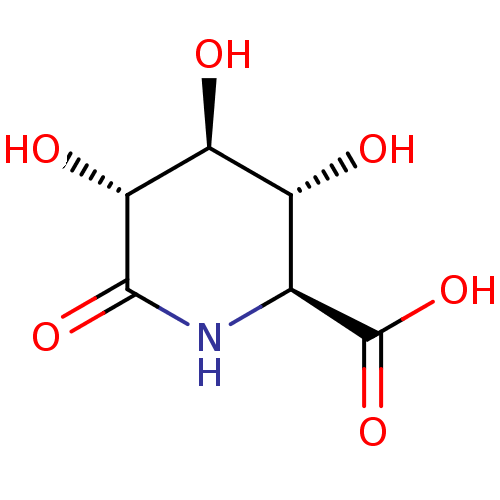
(CHEMBL1232598)Show SMILES O[C@H]1[C@H](O)[C@H](NC(=O)[C@@H]1O)C(O)=O |r| Show InChI InChI=1S/C6H9NO6/c8-2-1(6(12)13)7-5(11)4(10)3(2)9/h1-4,8-10H,(H,7,11)(H,12,13)/t1-,2+,3-,4+/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of bovine GUS pre-incubated for 5 to 30 mins before 4-methylumbelliferone beta-D-glucuronide substrate addition at pH 4.5 |
J Med Chem 63: 4617-4627 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01918
BindingDB Entry DOI: 10.7270/Q2CJ8HTJ |
More data for this
Ligand-Target Pair | |
Beta-glucuronidase
(Escherichia coli (Enterobacteria)) | BDBM50511221

(CHEMBL4451253)Show SMILES CCC[C@@H]1NC[C@@H](O)[C@H](O)[C@H]1C(O)=O |r| Show InChI InChI=1S/C9H17NO4/c1-2-3-5-7(9(13)14)8(12)6(11)4-10-5/h5-8,10-12H,2-4H2,1H3,(H,13,14)/t5-,6+,7-,8-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli GUS pre-incubated for 5 to 30 mins before 4-methylumbelliferone beta-D-glucuronide substrate addition at pH 5.5 |
J Med Chem 63: 4617-4627 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01918
BindingDB Entry DOI: 10.7270/Q2CJ8HTJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-glucuronidase
(Escherichia coli (Enterobacteria)) | BDBM50511220

(CHEMBL1232598)Show SMILES O[C@H]1[C@H](O)[C@H](NC(=O)[C@@H]1O)C(O)=O |r| Show InChI InChI=1S/C6H9NO6/c8-2-1(6(12)13)7-5(11)4(10)3(2)9/h1-4,8-10H,(H,7,11)(H,12,13)/t1-,2+,3-,4+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 232 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli GUS pre-incubated for 5 to 30 mins before 4-methylumbelliferone beta-D-glucuronide substrate addition at pH 5.5 |
J Med Chem 63: 4617-4627 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01918
BindingDB Entry DOI: 10.7270/Q2CJ8HTJ |
More data for this
Ligand-Target Pair | |
Beta-glucuronidase
(Bos taurus (Bovine)) | BDBM50511224
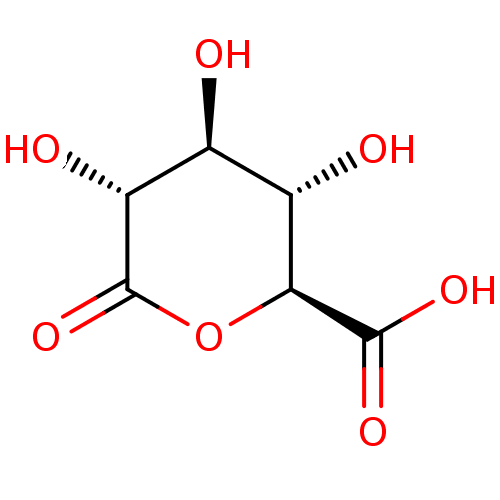
(CHEMBL4589322)Show SMILES O[C@H]1[C@H](O)[C@H](OC(=O)[C@@H]1O)C(O)=O |r| Show InChI InChI=1S/C6H8O7/c7-1-2(8)4(5(10)11)13-6(12)3(1)9/h1-4,7-9H,(H,10,11)/t1-,2-,3+,4-/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 4.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of bovine GUS pre-incubated for 5 to 30 mins before 4-methylumbelliferone beta-D-glucuronide substrate addition at pH 4.5 |
J Med Chem 63: 4617-4627 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01918
BindingDB Entry DOI: 10.7270/Q2CJ8HTJ |
More data for this
Ligand-Target Pair | |
Beta-glucuronidase
(Bos taurus (Bovine)) | BDBM50511221

(CHEMBL4451253)Show SMILES CCC[C@@H]1NC[C@@H](O)[C@H](O)[C@H]1C(O)=O |r| Show InChI InChI=1S/C9H17NO4/c1-2-3-5-7(9(13)14)8(12)6(11)4-10-5/h5-8,10-12H,2-4H2,1H3,(H,13,14)/t5-,6+,7-,8-/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 5.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of bovine GUS pre-incubated for 5 to 30 mins before 4-methylumbelliferone beta-D-glucuronide substrate addition at pH 6.7 |
J Med Chem 63: 4617-4627 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01918
BindingDB Entry DOI: 10.7270/Q2CJ8HTJ |
More data for this
Ligand-Target Pair | |
Beta-glucuronidase
(Escherichia coli (Enterobacteria)) | BDBM50511224

(CHEMBL4589322)Show SMILES O[C@H]1[C@H](O)[C@H](OC(=O)[C@@H]1O)C(O)=O |r| Show InChI InChI=1S/C6H8O7/c7-1-2(8)4(5(10)11)13-6(12)3(1)9/h1-4,7-9H,(H,10,11)/t1-,2-,3+,4-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli GUS pre-incubated for 5 to 30 mins before 4-methylumbelliferone beta-D-glucuronide substrate addition at pH 5.5 |
J Med Chem 63: 4617-4627 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01918
BindingDB Entry DOI: 10.7270/Q2CJ8HTJ |
More data for this
Ligand-Target Pair | |
Beta-glucuronidase
(Escherichia coli (Enterobacteria)) | BDBM50511225

((2S,3R,4R,5S)-3,4,5-Trihydroxypipecolic Acid | CHE...)Show SMILES O[C@H]1CN[C@@H]([C@@H](O)[C@@H]1O)C(O)=O |r| Show InChI InChI=1S/C6H11NO5/c8-2-1-7-3(6(11)12)5(10)4(2)9/h2-5,7-10H,1H2,(H,11,12)/t2-,3-,4+,5+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli GUS pre-incubated for 5 to 30 mins before 4-methylumbelliferone beta-D-glucuronide substrate addition at pH 5.5 |
J Med Chem 63: 4617-4627 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01918
BindingDB Entry DOI: 10.7270/Q2CJ8HTJ |
More data for this
Ligand-Target Pair | |
Beta-glucuronidase
(Bos taurus (Bovine)) | BDBM50511225

((2S,3R,4R,5S)-3,4,5-Trihydroxypipecolic Acid | CHE...)Show SMILES O[C@H]1CN[C@@H]([C@@H](O)[C@@H]1O)C(O)=O |r| Show InChI InChI=1S/C6H11NO5/c8-2-1-7-3(6(11)12)5(10)4(2)9/h2-5,7-10H,1H2,(H,11,12)/t2-,3-,4+,5+/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 2.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of bovine GUS pre-incubated for 5 to 30 mins before 4-methylumbelliferone beta-D-glucuronide substrate addition at pH 4.5 |
J Med Chem 63: 4617-4627 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01918
BindingDB Entry DOI: 10.7270/Q2CJ8HTJ |
More data for this
Ligand-Target Pair | |
Beta-glucuronidase
(Bos taurus (Bovine)) | BDBM50511221

(CHEMBL4451253)Show SMILES CCC[C@@H]1NC[C@@H](O)[C@H](O)[C@H]1C(O)=O |r| Show InChI InChI=1S/C9H17NO4/c1-2-3-5-7(9(13)14)8(12)6(11)4-10-5/h5-8,10-12H,2-4H2,1H3,(H,13,14)/t5-,6+,7-,8-/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 3.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of bovine GUS pre-incubated for 5 to 30 mins before 4-methylumbelliferone beta-D-glucuronide substrate addition at pH 4.5 |
J Med Chem 63: 4617-4627 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01918
BindingDB Entry DOI: 10.7270/Q2CJ8HTJ |
More data for this
Ligand-Target Pair | |
Beta-glucuronidase
(Escherichia coli (Enterobacteria)) | BDBM50511222
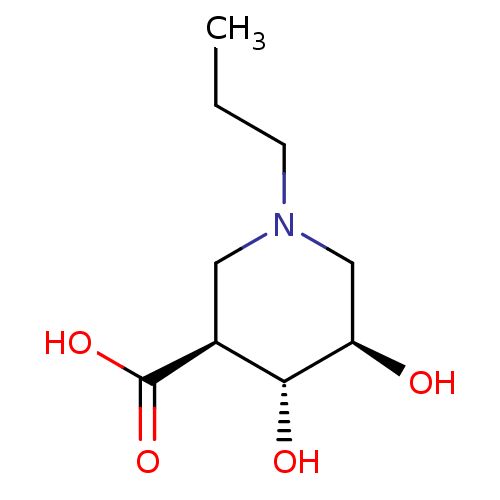
(CHEMBL4441158)Show InChI InChI=1S/C9H17NO4/c1-2-3-10-4-6(9(13)14)8(12)7(11)5-10/h6-8,11-12H,2-5H2,1H3,(H,13,14)/t6-,7+,8+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 4.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli GUS pre-incubated for 5 to 30 mins before 4-methylumbelliferone beta-D-glucuronide substrate addition at pH 6.7 |
J Med Chem 63: 4617-4627 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01918
BindingDB Entry DOI: 10.7270/Q2CJ8HTJ |
More data for this
Ligand-Target Pair | |
Beta-glucuronidase
(Escherichia coli (Enterobacteria)) | BDBM50511222

(CHEMBL4441158)Show InChI InChI=1S/C9H17NO4/c1-2-3-10-4-6(9(13)14)8(12)7(11)5-10/h6-8,11-12H,2-5H2,1H3,(H,13,14)/t6-,7+,8+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 5.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli GUS pre-incubated for 5 to 30 mins before 4-methylumbelliferone beta-D-glucuronide substrate addition at pH 5.5 |
J Med Chem 63: 4617-4627 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01918
BindingDB Entry DOI: 10.7270/Q2CJ8HTJ |
More data for this
Ligand-Target Pair | |
Beta-glucuronidase
(Bos taurus (Bovine)) | BDBM50511222

(CHEMBL4441158)Show InChI InChI=1S/C9H17NO4/c1-2-3-10-4-6(9(13)14)8(12)7(11)5-10/h6-8,11-12H,2-5H2,1H3,(H,13,14)/t6-,7+,8+/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 6.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of bovine GUS pre-incubated for 5 to 30 mins before 4-methylumbelliferone beta-D-glucuronide substrate addition at pH 6.7 |
J Med Chem 63: 4617-4627 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01918
BindingDB Entry DOI: 10.7270/Q2CJ8HTJ |
More data for this
Ligand-Target Pair | |
Beta-glucuronidase
(Bos taurus (Bovine)) | BDBM50511222

(CHEMBL4441158)Show InChI InChI=1S/C9H17NO4/c1-2-3-10-4-6(9(13)14)8(12)7(11)5-10/h6-8,11-12H,2-5H2,1H3,(H,13,14)/t6-,7+,8+/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.87E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of bovine GUS pre-incubated for 5 to 30 mins before 4-methylumbelliferone beta-D-glucuronide substrate addition at pH 4.5 |
J Med Chem 63: 4617-4627 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01918
BindingDB Entry DOI: 10.7270/Q2CJ8HTJ |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM513874

(bioRxiv20220126.477782, S-217622 | bioRxiv20220126...)Show SMILES Cn1cnc(Cn2c(=O)[nH]\c(=N/c3cc4cn(C)nc4cc3Cl)n(Cc3cc(F)c(F)cc3F)c2=O)n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01146
BindingDB Entry DOI: 10.7270/Q2P55SFS |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM420298

(CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C(=O)CO Show InChI InChI=1S/C24H32N4O6/c1-13(2)9-18(23(32)27-17(20(30)12-29)10-14-7-8-25-22(14)31)28-24(33)19-11-15-16(26-19)5-4-6-21(15)34-3/h4-6,11,13-14,17-18,26,29H,7-10,12H2,1-3H3,(H,25,31)(H,27,32)(H,28,33)/t14-,17-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01716
BindingDB Entry DOI: 10.7270/Q2VX0MKR |
More data for this
Ligand-Target Pair | |
Toll-like receptor 7
(Mus musculus) | BDBM21958
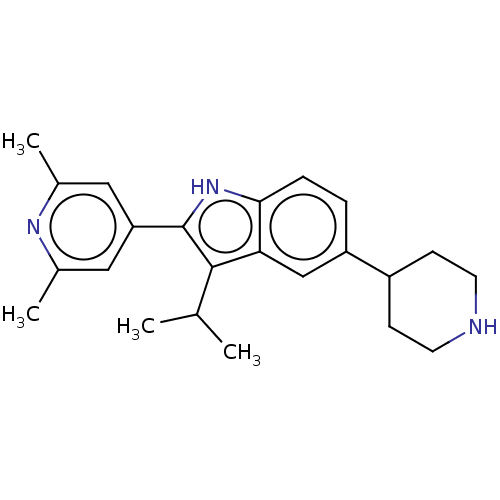
(2-(2,6-dimethylpyridin-4-yl)-3-isopropyl-5-(piperi...)Show SMILES CC(C)c1c([nH]c2ccc(cc12)C1CCNCC1)-c1cc(C)nc(C)c1 Show InChI InChI=1S/C6H13N5O4/c7-4(5(12)13)2-1-3-9-6(8)10-11(14)15/h4H,1-3,7H2,(H,12,13)(H3,8,9,10)/t4-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00049
BindingDB Entry DOI: 10.7270/Q2JH3R6P |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50589777

(CHEMBL5192350)Show SMILES Clc1ccc(cc1Cl)N1CCN(C[C@H]1C(=O)NCc1ccco1)C(=O)c1cc(=O)[nH]c(=O)[nH]1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01146
BindingDB Entry DOI: 10.7270/Q2P55SFS |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50589778

(CHEMBL5204987)Show SMILES Clc1ccc(cc1Cl)N1CCN(C[C@H]1C(=O)NCc1cccs1)C(=O)c1cc(=O)[nH]c(=O)[nH]1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01146
BindingDB Entry DOI: 10.7270/Q2P55SFS |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50589769

(CHEMBL5174111)Show SMILES Clc1ccc(cc1Cl)N1CCN(C[C@H]1C(=O)NCc1cccs1)C(=O)c1cccnc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01146
BindingDB Entry DOI: 10.7270/Q2P55SFS |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50589773

(CHEMBL5188908)Show SMILES Clc1ccc(CN2CCN(C[C@H]2C(=O)NCc2cccs2)C(=O)c2cc(=O)[nH]c(=O)[nH]2)c(Cl)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01146
BindingDB Entry DOI: 10.7270/Q2P55SFS |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50589770

(CHEMBL5188502)Show SMILES Clc1ccc(cc1Cl)N1CCN(C[C@H]1C(=O)NCc1cccs1)C(=O)c1cncc2ccccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01146
BindingDB Entry DOI: 10.7270/Q2P55SFS |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50058752

(CHEMBL3335630)Show SMILES COCCOCc1ccc(CN2CCN(Cc3ccc(cc3)C(=O)Nc3ccc(cc3)C#CC34CC5CC(CC(C5)C3)C4)[C@@H](C)C2)cn1 |r,THB:32:33:36:40.38.39,38:37:34:40.39.41,38:39:36.37.42:34,41:39:36:42.33.34,41:33:36:40.38.39| Show InChI InChI=1S/C41H50N4O3/c1-30-26-44(27-33-7-12-39(42-25-33)29-48-18-17-47-2)15-16-45(30)28-32-3-8-37(9-4-32)40(46)43-38-10-5-31(6-11-38)13-14-41-22-34-19-35(23-41)21-36(20-34)24-41/h3-12,25,30,34-36H,15-24,26-29H2,1-2H3,(H,43,46)/t30-,34?,35?,36?,41?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HIF1 signaling in human U251 cells expressing VEGF by VEGF promoter-driven PLAP reporter gene assay |
Bioorg Med Chem 22: 5513-29 (2014)
Article DOI: 10.1016/j.bmc.2014.07.020
BindingDB Entry DOI: 10.7270/Q24J0GSG |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50589780
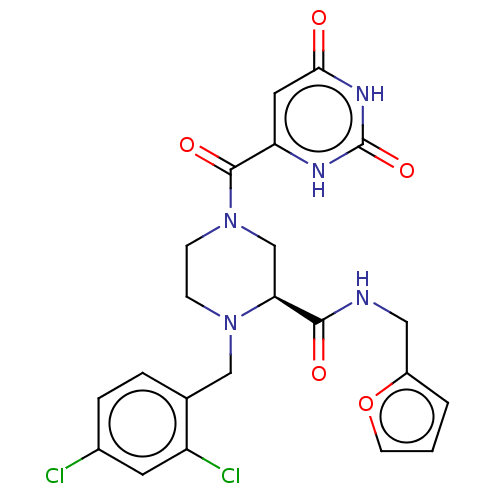
(CHEMBL5188982)Show SMILES Clc1ccc(CN2CCN(C[C@H]2C(=O)NCc2ccco2)C(=O)c2cc(=O)[nH]c(=O)[nH]2)c(Cl)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01146
BindingDB Entry DOI: 10.7270/Q2P55SFS |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
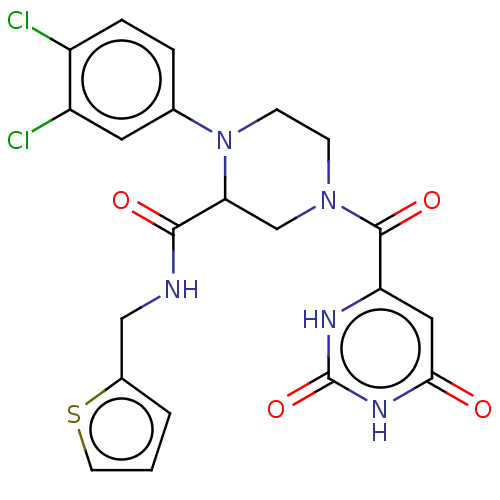
(Mycobacterium smegmatis) | BDBM50010376
(CHEMBL3263618)Show SMILES CCNC(=O)Nc1nc2cc(-c3ccn(CCOC)c(=O)c3)c(OCC3CCOC3)nc2s1 Show InChI InChI=1S/C22H27N5O5S/c1-3-23-21(29)26-22-24-17-11-16(15-4-6-27(7-9-30-2)18(28)10-15)19(25-20(17)33-22)32-13-14-5-8-31-12-14/h4,6,10-11,14H,3,5,7-9,12-13H2,1-2H3,(H2,23,24,26,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca India Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium smegmatis DNA gyrase B ATPase activity assessed as inorganic phosphate release using ATP as substrate by colorimetric ana... |
Cell Chem Biol 56: 8834-48 (2013)
Article DOI: 10.1021/jm401268f
BindingDB Entry DOI: 10.7270/Q2RV0Q7W |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM419133
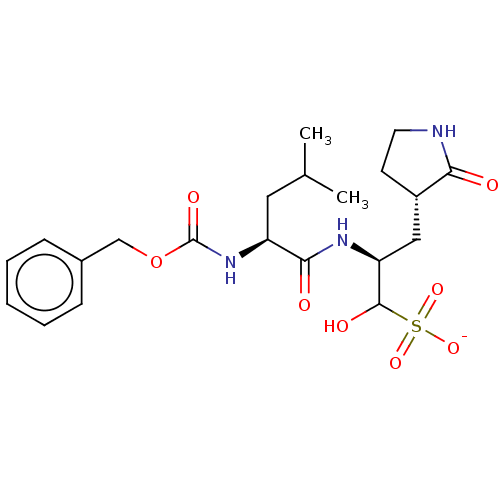
(BDBM429386 | GC376)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C(O)S([O-])(=O)=O Show InChI InChI=1S/C21H31N3O8S/c1-13(2)10-16(24-21(28)32-12-14-6-4-3-5-7-14)19(26)23-17(20(27)33(29,30)31)11-15-8-9-22-18(15)25/h3-7,13,15-17,20,27H,8-12H2,1-2H3,(H,22,25)(H,23,26)(H,24,28)(H,29,30,31)/p-1/t15?,16-,17-,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01146
BindingDB Entry DOI: 10.7270/Q2P55SFS |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50589779

(CHEMBL5181886)Show SMILES Clc1ccc(CN2CCN(CC2C(=O)NCc2ccco2)C(=O)c2cc(=O)[nH]c(=O)[nH]2)c(Cl)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01146
BindingDB Entry DOI: 10.7270/Q2P55SFS |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Plasmodium falciparum (isolate 3D7)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Harvard Medical School
| Assay Description
Recombinant pfHDAC-1 was assayed with substrate in the presence of test compound. The substrate concentration was kept constant at 125 uM while the c... |
J Med Chem 52: 2185-7 (2009)
Article DOI: 10.1021/jm801654y
BindingDB Entry DOI: 10.7270/Q26H4FRD |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50567579
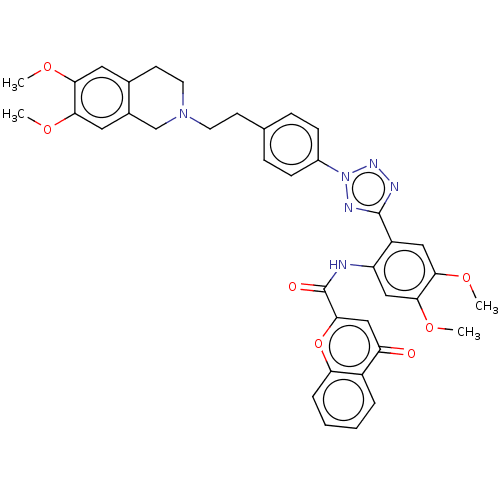
(Encequidar | HM-30181 | HM30181 | HM30181AK | Pgp ...)Show SMILES COc1cc2CCN(CCc3ccc(cc3)-n3nnc(n3)-c3cc(OC)c(OC)cc3NC(=O)c3cc(=O)c4ccccc4o3)Cc2cc1OC | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of paclitaxel stimulated- P-gp ATPase activity (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01826
BindingDB Entry DOI: 10.7270/Q2KP85ZF |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50589775
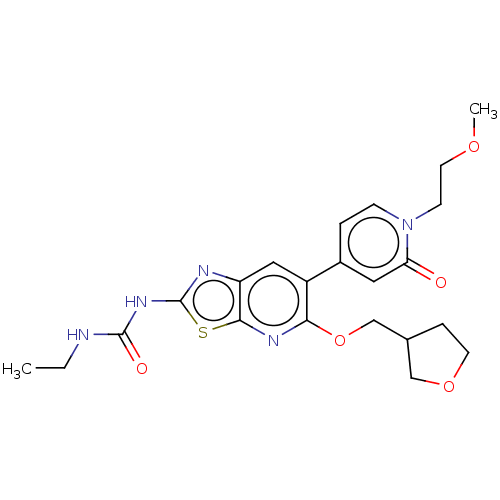
(CHEMBL5172248)Show SMILES Clc1ccc(cc1Cl)N1CCN(CC1C(=O)NCc1cccs1)C(=O)c1cc(=O)[nH]c(=O)[nH]1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01146
BindingDB Entry DOI: 10.7270/Q2P55SFS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50571009

(CHEMBL4865592)Show SMILES CC(C)n1cc(C(=O)Nc2ccc(Oc3ccnc(NC(=O)CC4CCN(C)CC4)c3)cc2)c(=O)n(-c2ccc(F)cc2)c1=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human GST-tagged Axl incubated for 1 hr by ADP-glo based luminometry analysis |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116137
BindingDB Entry DOI: 10.7270/Q2PV6Q46 |
More data for this
Ligand-Target Pair | |
Toll-like receptor 7
(Homo sapiens (Human)) | BDBM21958

(2-(2,6-dimethylpyridin-4-yl)-3-isopropyl-5-(piperi...)Show SMILES CC(C)c1c([nH]c2ccc(cc12)C1CCNCC1)-c1cc(C)nc(C)c1 Show InChI InChI=1S/C6H13N5O4/c7-4(5(12)13)2-1-3-9-6(8)10-11(14)15/h4H,1-3,7H2,(H,12,13)(H3,8,9,10)/t4-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00049
BindingDB Entry DOI: 10.7270/Q2JH3R6P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50571017

(CHEMBL4852684)Show SMILES CC(C)n1cc(C(=O)Nc2cc(F)c(Oc3ccnc(NC(=O)N4CCC(CC4)N4CCN(C)CC4)c3)cc2F)c(=O)n(-c2ccc(F)cc2)c1=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human GST-tagged Axl incubated for 1 hr by ADP-glo based luminometry analysis |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116137
BindingDB Entry DOI: 10.7270/Q2PV6Q46 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50589774

(CHEMBL5201620)Show SMILES Clc1ccc(cc1Cl)N1CCN(CC1C(=O)NCc1ccco1)C(=O)c1cc(=O)[nH]c(=O)[nH]1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01146
BindingDB Entry DOI: 10.7270/Q2P55SFS |
More data for this
Ligand-Target Pair | |
Toll-like receptor 7
(Homo sapiens (Human)) | BDBM443496

(US10660877, Example 73)Show SMILES CC(C)c1c([nH]c2cc(F)c(cc12)C1CCNCC1)-c1ccnc(C)c1 Show InChI InChI=1S/C22H26FN3/c1-13(2)21-18-11-17(15-4-7-24-8-5-15)19(23)12-20(18)26-22(21)16-6-9-25-14(3)10-16/h6,9-13,15,24,26H,4-5,7-8H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00049
BindingDB Entry DOI: 10.7270/Q2JH3R6P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50571010

(CHEMBL4848604)Show SMILES CC(C)n1cc(C(=O)Nc2ccc(Oc3ccnc(NC(=O)CC4CCN(C)CC4)c3)cc2F)c(=O)n(-c2ccc(F)cc2)c1=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human GST-tagged Axl incubated for 1 hr by ADP-glo based luminometry analysis |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116137
BindingDB Entry DOI: 10.7270/Q2PV6Q46 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50571019

(CHEMBL4845957)Show SMILES CC(C)n1cc(C(=O)Nc2ccc(Oc3cc(NC(=O)C4CC4)ncn3)cc2)c(=O)n(-c2ccc(F)cc2)c1=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human GST-tagged Axl incubated for 1 hr by ADP-glo based luminometry analysis |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116137
BindingDB Entry DOI: 10.7270/Q2PV6Q46 |
More data for this
Ligand-Target Pair | |
Toll-like receptor 7
(Homo sapiens (Human)) | BDBM443500

(US10660877, Example 77)Show SMILES CC(C)c1c([nH]c2ccc(C3CCNCC3)c(F)c12)-c1cc(C)nc(C)c1 Show InChI InChI=1S/C23H28FN3/c1-13(2)20-21-19(27-23(20)17-11-14(3)26-15(4)12-17)6-5-18(22(21)24)16-7-9-25-10-8-16/h5-6,11-13,16,25,27H,7-10H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00049
BindingDB Entry DOI: 10.7270/Q2JH3R6P |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50058757
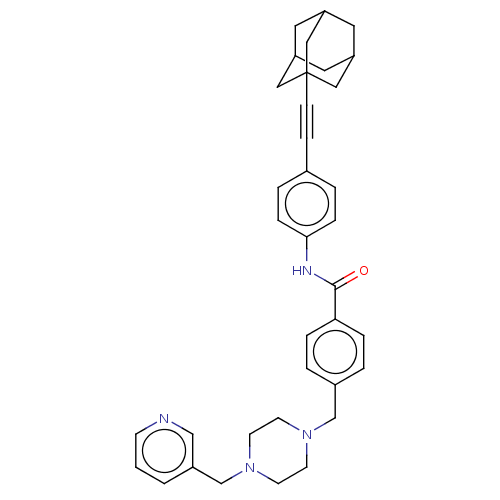
(CHEMBL3335423)Show SMILES O=C(Nc1ccc(cc1)C#CC12CC3CC(CC(C3)C1)C2)c1ccc(CN2CCN(Cc3cccnc3)CC2)cc1 |THB:10:11:14:18.16.17,16:15:12:18.17.19,16:17:14.15.20:12,19:17:14:20.11.12,19:11:14:18.16.17| Show InChI InChI=1S/C36H40N4O/c41-35(33-7-3-28(4-8-33)25-39-14-16-40(17-15-39)26-29-2-1-13-37-24-29)38-34-9-5-27(6-10-34)11-12-36-21-30-18-31(22-36)20-32(19-30)23-36/h1-10,13,24,30-32H,14-23,25-26H2,(H,38,41) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HIF1 signaling in human U251 cells expressing VEGF by VEGF promoter-driven PLAP reporter gene assay |
Bioorg Med Chem 22: 5513-29 (2014)
Article DOI: 10.1016/j.bmc.2014.07.020
BindingDB Entry DOI: 10.7270/Q24J0GSG |
More data for this
Ligand-Target Pair | |
Toll-like receptor 7
(Homo sapiens (Human)) | BDBM21958

(2-(2,6-dimethylpyridin-4-yl)-3-isopropyl-5-(piperi...)Show SMILES CC(C)c1c([nH]c2ccc(cc12)C1CCNCC1)-c1cc(C)nc(C)c1 Show InChI InChI=1S/C6H13N5O4/c7-4(5(12)13)2-1-3-9-6(8)10-11(14)15/h4H,1-3,7H2,(H,12,13)(H3,8,9,10)/t4-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00049
BindingDB Entry DOI: 10.7270/Q2JH3R6P |
More data for this
Ligand-Target Pair | |
Toll-like receptor 8
(Homo sapiens (Human)) | BDBM442004

(US10660877, Example 31)Show SMILES CC(C)c1c([nH]c2ccc(cc12)C1CCNCC1)-c1cc(C)ncc1C Show InChI InChI=1S/C23H29N3/c1-14(2)22-20-12-18(17-7-9-24-10-8-17)5-6-21(20)26-23(22)19-11-16(4)25-13-15(19)3/h5-6,11-14,17,24,26H,7-10H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00049
BindingDB Entry DOI: 10.7270/Q2JH3R6P |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Mycobacterium smegmatis) | BDBM50010373
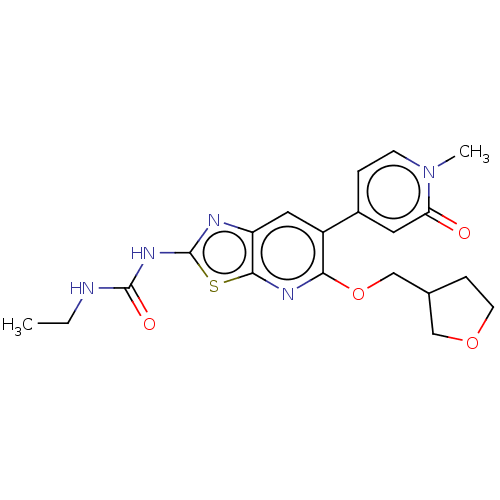
(CHEMBL3263615)Show SMILES CCNC(=O)Nc1nc2cc(-c3ccn(C)c(=O)c3)c(OCC3CCOC3)nc2s1 Show InChI InChI=1S/C20H23N5O4S/c1-3-21-19(27)24-20-22-15-9-14(13-4-6-25(2)16(26)8-13)17(23-18(15)30-20)29-11-12-5-7-28-10-12/h4,6,8-9,12H,3,5,7,10-11H2,1-2H3,(H2,21,22,24,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca India Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium smegmatis DNA gyrase B ATPase activity assessed as inorganic phosphate release using ATP as substrate by colorimetric ana... |
Cell Chem Biol 56: 8834-48 (2013)
Article DOI: 10.1021/jm401268f
BindingDB Entry DOI: 10.7270/Q2RV0Q7W |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Plasmodium falciparum (isolate 3D7)) | BDBM25142
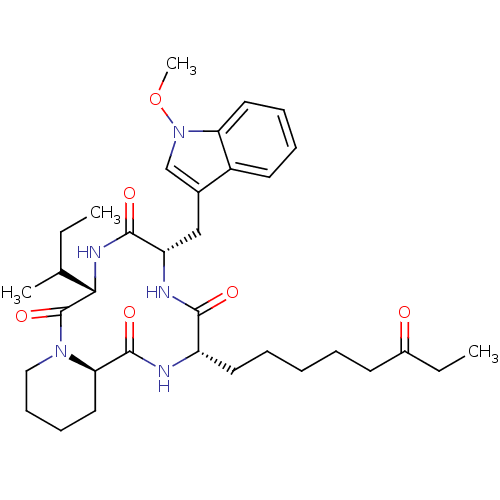
((3S,6S,9S,15aR)-9-[(2R)-butan-2-yl]-6-[(1-methoxy-...)Show SMILES CCC(C)[C@@H]1NC(=O)[C@H](Cc2cn(OC)c3ccccc23)NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@H]2CCCCN2C1=O |r| Show InChI InChI=1S/C34H49N5O6/c1-5-22(3)30-34(44)38-19-13-12-18-29(38)33(43)35-26(16-9-7-8-14-24(40)6-2)31(41)36-27(32(42)37-30)20-23-21-39(45-4)28-17-11-10-15-25(23)28/h10-11,15,17,21-22,26-27,29-30H,5-9,12-14,16,18-20H2,1-4H3,(H,35,43)(H,36,41)(H,37,42)/t22?,26-,27-,29+,30-/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Harvard Medical School
| Assay Description
Recombinant pfHDAC-1 was assayed with substrate in the presence of test compound. The substrate concentration was kept constant at 125 uM while the c... |
J Med Chem 52: 2185-7 (2009)
Article DOI: 10.1021/jm801654y
BindingDB Entry DOI: 10.7270/Q26H4FRD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data