

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (BOVINE) | BDBM50019719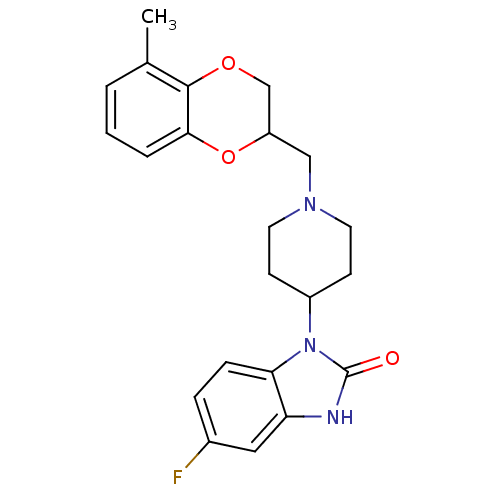 (5-Fluoro-1-[1-(5-methyl-2,3-dihydro-benzo[1,4]diox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-spiroperidol from bovine caudate nucleus membrane Dopamine receptor D2 | J Med Chem 30: 814-9 (1987) BindingDB Entry DOI: 10.7270/Q2PR7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards dopamine receptor D2 was determined by displacement of [3H]-spiroperidol from bovine nucleus caudate membranes. | J Med Chem 30: 814-9 (1987) BindingDB Entry DOI: 10.7270/Q2PR7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50019721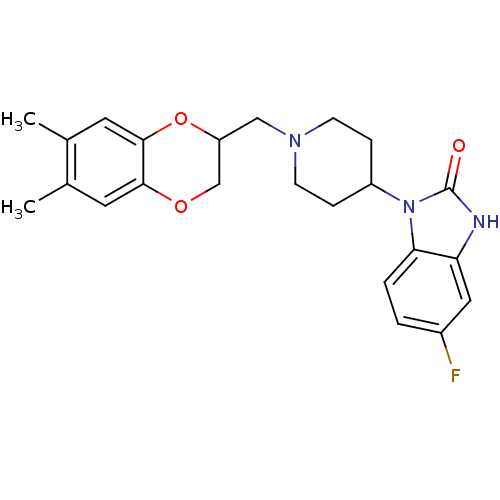 (1-[1-(6,7-Dimethyl-2,3-dihydro-benzo[1,4]dioxin-2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-spiroperidol from bovine caudate nucleus membrane Dopamine receptor D2 | J Med Chem 30: 814-9 (1987) BindingDB Entry DOI: 10.7270/Q2PR7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50019717 (5-Chloro-1-[1-(2,3-dihydro-benzo[1,4]dioxin-2-ylme...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-spiroperidol from bovine caudate nucleus membrane Dopamine receptor D2 | J Med Chem 30: 814-9 (1987) BindingDB Entry DOI: 10.7270/Q2PR7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50019726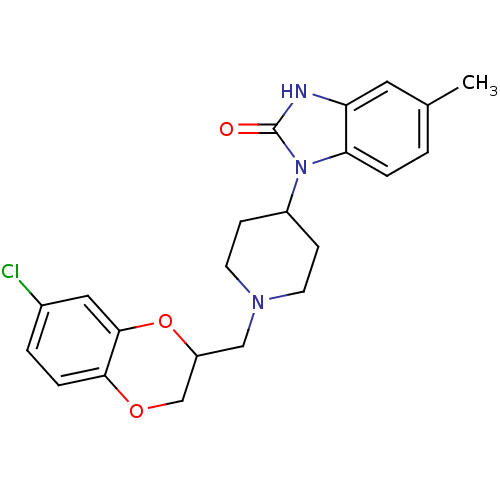 (1-[1-(7-Chloro-2,3-dihydro-benzo[1,4]dioxin-2-ylme...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards dopamine receptor D2 was determined by displacement of [3H]-spiroperidol from bovine nucleus caudate membranes. | J Med Chem 30: 814-9 (1987) BindingDB Entry DOI: 10.7270/Q2PR7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50019725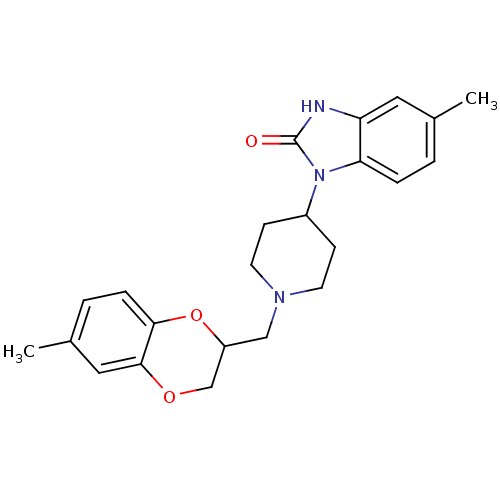 (5-Methyl-1-[1-(6-methyl-2,3-dihydro-benzo[1,4]diox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-spiroperidol from bovine caudate nucleus membrane Dopamine receptor D2 | J Med Chem 30: 814-9 (1987) BindingDB Entry DOI: 10.7270/Q2PR7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50019727 (1-[1-(6,7-Dimethyl-2,3-dihydro-benzo[1,4]dioxin-2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-spiroperidol from bovine caudate nucleus membrane Dopamine receptor D2 | J Med Chem 30: 814-9 (1987) BindingDB Entry DOI: 10.7270/Q2PR7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50019724 (5-Chloro-1-[1-(6-methyl-2,3-dihydro-benzo[1,4]diox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-spiroperidol from bovine caudate nucleus membrane Dopamine receptor D2 | J Med Chem 30: 814-9 (1987) BindingDB Entry DOI: 10.7270/Q2PR7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50019718 (5-Fluoro-1-[1-(6-fluoro-2,3-dihydro-benzo[1,4]diox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-spiroperidol from bovine caudate nucleus membrane Dopamine receptor D2 | J Med Chem 30: 814-9 (1987) BindingDB Entry DOI: 10.7270/Q2PR7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50019728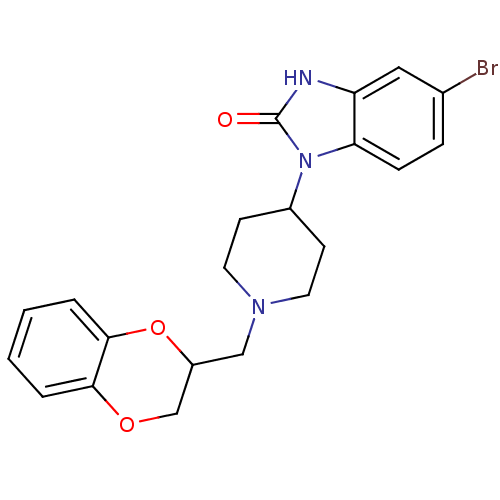 (5-Bromo-1-[1-(2,3-dihydro-benzo[1,4]dioxin-2-ylmet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-spiroperidol from bovine caudate nucleus membrane Dopamine receptor D2 | J Med Chem 30: 814-9 (1987) BindingDB Entry DOI: 10.7270/Q2PR7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50019723 (1-[1-(2,3-Dihydro-benzo[1,4]dioxin-2-ylmethyl)-pip...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-spiroperidol from bovine caudate nucleus membrane Dopamine receptor D2 | J Med Chem 30: 814-9 (1987) BindingDB Entry DOI: 10.7270/Q2PR7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50019720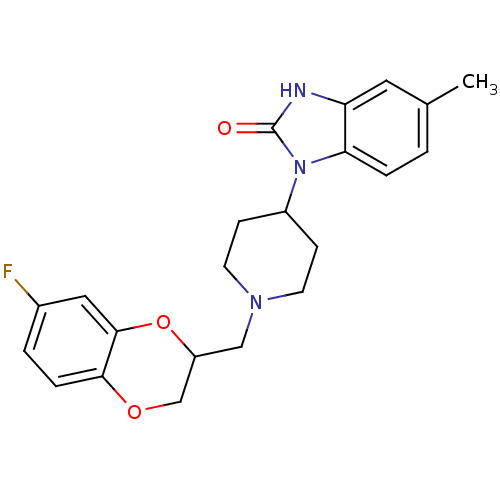 (1-[1-(7-Fluoro-2,3-dihydro-benzo[1,4]dioxin-2-ylme...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-spiroperidol from bovine caudate nucleus membrane Dopamine receptor D2 | J Med Chem 30: 814-9 (1987) BindingDB Entry DOI: 10.7270/Q2PR7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50019722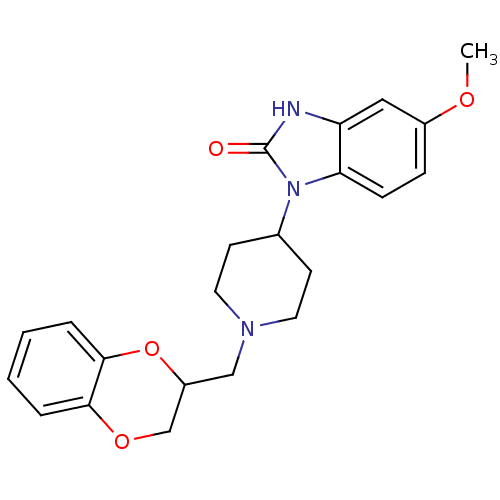 (1-[1-(2,3-Dihydro-benzo[1,4]dioxin-2-ylmethyl)-pip...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-spiroperidol from bovine caudate nucleus membrane Dopamine receptor D2 | J Med Chem 30: 814-9 (1987) BindingDB Entry DOI: 10.7270/Q2PR7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50403371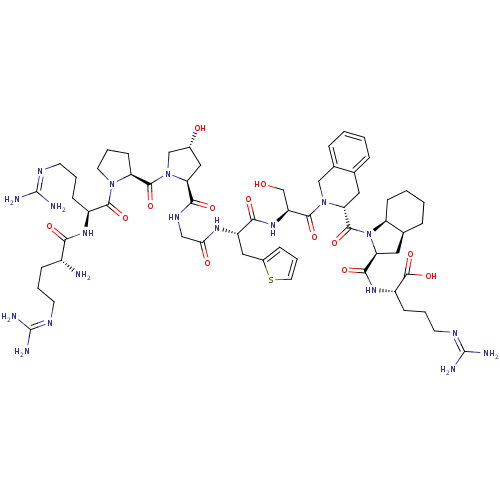 (Firazyr | HOE-140 | ICATIBANT) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Zentrale Pharmaforschung Curated by ChEMBL | Assay Description In vitro antagonistic activity for Bradykinin receptor B2 by guinea pig ileal membrane receptor assay. | J Med Chem 38: 2799-801 (1995) BindingDB Entry DOI: 10.7270/Q2028S70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50403371 (Firazyr | HOE-140 | ICATIBANT) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Zentrale Pharmaforschung Curated by ChEMBL | Assay Description In vitro antagonistic activity for Bradykinin receptor B2 by guinea pig pulmonary artery assay. | J Med Chem 38: 2799-801 (1995) BindingDB Entry DOI: 10.7270/Q2028S70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50032158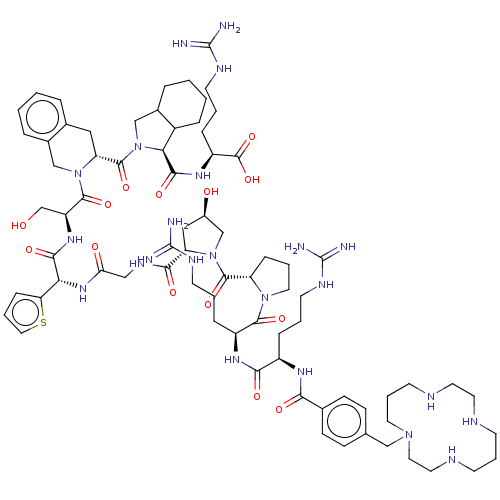 (CHEMBL2370243 | CPTA-D-Arg-Arg-Pro-Hyp-Gly-Thi-Ser...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Zentrale Pharmaforschung Curated by ChEMBL | Assay Description In vitro antagonistic activity for Bradykinin receptor B2 by guinea pig ileal membrane receptor assay. | J Med Chem 38: 2799-801 (1995) BindingDB Entry DOI: 10.7270/Q2028S70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50032158 (CHEMBL2370243 | CPTA-D-Arg-Arg-Pro-Hyp-Gly-Thi-Ser...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Zentrale Pharmaforschung Curated by ChEMBL | Assay Description In vitro antagonistic activity for Bradykinin receptor B2 by guinea pig pulmonary artery assay. | J Med Chem 38: 2799-801 (1995) BindingDB Entry DOI: 10.7270/Q2028S70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||