 Found 16 hits with Last Name = 'gerwick' and Initial = 'l'
Found 16 hits with Last Name = 'gerwick' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Berkeley
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysis |
J Nat Prod 78: 1671-82 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00301
BindingDB Entry DOI: 10.7270/Q22F7Q76 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Berkeley
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysis |
J Nat Prod 78: 1671-82 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00301
BindingDB Entry DOI: 10.7270/Q22F7Q76 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50257325

(CHEMBL4090056)Show SMILES CCCCCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCS(C)(=O)=O)C(=O)N[C@@H](CC(C)C)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C25H45N3O7S/c1-8-9-10-11-20(29)28-21(17(4)5)24(32)26-18(12-13-36(7,33)34)23(31)27-19(14-16(2)3)22(30)25(6)15-35-25/h16-19,21H,8-15H2,1-7H3,(H,26,32)(H,27,31)(H,28,29)/t18-,19-,21-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 375 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pediatrics, School of Medicine, University of California, San Diego , La Jolla, California 92093, United States.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human 20S proteasome beta5 subunit using suc-LLVY-AMC as substrate after 4 hrs at 30 mins time interval by fluorescence as... |
J Med Chem 60: 6721-6732 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00671
BindingDB Entry DOI: 10.7270/Q2CR5WSK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50108987
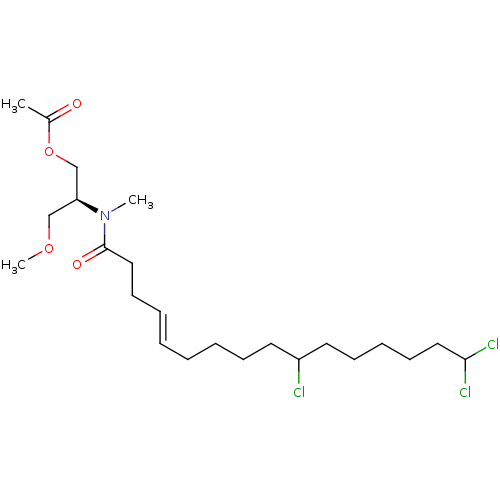
(CHEMBL3597331)Show SMILES COC[C@@H](COC(C)=O)N(C)C(=O)CC\C=C\CCCCC(Cl)CCCCCC(Cl)Cl |r| Show InChI InChI=1S/C23H40Cl3NO4/c1-19(28)31-18-21(17-30-3)27(2)23(29)16-12-7-5-4-6-9-13-20(24)14-10-8-11-15-22(25)26/h5,7,20-22H,4,6,8-18H2,1-3H3/b7-5+/t20?,21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Berkeley
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysis |
J Nat Prod 78: 1671-82 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00301
BindingDB Entry DOI: 10.7270/Q22F7Q76 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50108989
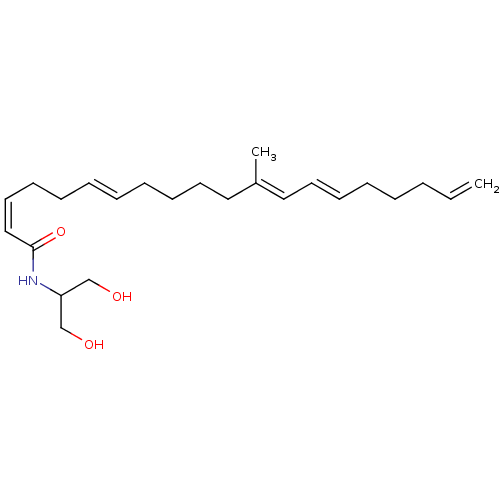
(CHEMBL3597332)Show SMILES C\C(CCCC\C=C\CC\C=C/C(=O)NC(CO)CO)=C/C=C/CCCC=C Show InChI InChI=1S/C24H39NO3/c1-3-4-5-6-11-14-17-22(2)18-15-12-9-7-8-10-13-16-19-24(28)25-23(20-26)21-27/h3,7-8,11,14,16-17,19,23,26-27H,1,4-6,9-10,12-13,15,18,20-21H2,2H3,(H,25,28)/b8-7+,14-11+,19-16-,22-17+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Berkeley
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysis |
J Nat Prod 78: 1671-82 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00301
BindingDB Entry DOI: 10.7270/Q22F7Q76 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50108988
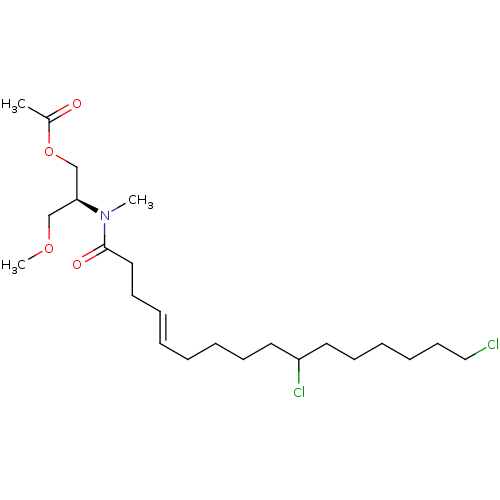
(CHEMBL3597330)Show SMILES COC[C@@H](COC(C)=O)N(C)C(=O)CC\C=C\CCCCC(Cl)CCCCCCCl |r| Show InChI InChI=1S/C23H41Cl2NO4/c1-20(27)30-19-22(18-29-3)26(2)23(28)16-12-7-5-4-6-10-14-21(25)15-11-8-9-13-17-24/h5,7,21-22H,4,6,8-19H2,1-3H3/b7-5+/t21?,22-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Berkeley
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysis |
J Nat Prod 78: 1671-82 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00301
BindingDB Entry DOI: 10.7270/Q22F7Q76 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50108987

(CHEMBL3597331)Show SMILES COC[C@@H](COC(C)=O)N(C)C(=O)CC\C=C\CCCCC(Cl)CCCCCC(Cl)Cl |r| Show InChI InChI=1S/C23H40Cl3NO4/c1-19(28)31-18-21(17-30-3)27(2)23(29)16-12-7-5-4-6-9-13-20(24)14-10-8-11-15-22(25)26/h5,7,20-22H,4,6,8-18H2,1-3H3/b7-5+/t20?,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Berkeley
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysis |
J Nat Prod 78: 1671-82 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00301
BindingDB Entry DOI: 10.7270/Q22F7Q76 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50108988

(CHEMBL3597330)Show SMILES COC[C@@H](COC(C)=O)N(C)C(=O)CC\C=C\CCCCC(Cl)CCCCCCCl |r| Show InChI InChI=1S/C23H41Cl2NO4/c1-20(27)30-19-22(18-29-3)26(2)23(28)16-12-7-5-4-6-10-14-21(25)15-11-8-9-13-17-24/h5,7,21-22H,4,6,8-19H2,1-3H3/b7-5+/t21?,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Berkeley
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysis |
J Nat Prod 78: 1671-82 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00301
BindingDB Entry DOI: 10.7270/Q22F7Q76 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50257327

(CHEMBL4067938)Show SMILES CCCCCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(C)C)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C26H47N3O5/c1-8-10-12-14-21(30)29-22(18(5)6)25(33)27-19(13-11-9-2)24(32)28-20(15-17(3)4)23(31)26(7)16-34-26/h17-20,22H,8-16H2,1-7H3,(H,27,33)(H,28,32)(H,29,30)/t19-,20-,22-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pediatrics, School of Medicine, University of California, San Diego , La Jolla, California 92093, United States.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human 20S proteasome beta5 subunit using suc-LLVY-AMC as substrate after 4 hrs at 30 mins time interval by fluorescence as... |
J Med Chem 60: 6721-6732 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00671
BindingDB Entry DOI: 10.7270/Q2CR5WSK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50257326

(CHEMBL4086345)Show SMILES CCCCCC(=O)N[C@H](C(C)C)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(C)C)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C26H47N3O5/c1-8-10-12-14-21(30)29-22(18(5)6)25(33)27-19(13-11-9-2)24(32)28-20(15-17(3)4)23(31)26(7)16-34-26/h17-20,22H,8-16H2,1-7H3,(H,27,33)(H,28,32)(H,29,30)/t19-,20-,22+,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pediatrics, School of Medicine, University of California, San Diego , La Jolla, California 92093, United States.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human 20S proteasome beta5 subunit using suc-LLVY-AMC as substrate after 4 hrs at 30 mins time interval by fluorescence as... |
J Med Chem 60: 6721-6732 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00671
BindingDB Entry DOI: 10.7270/Q2CR5WSK |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50478445

(Dehydrohirsutanonol | Hirsutanone | Hirsutenone | ...)Show InChI InChI=1S/C19H20O5/c20-15(8-5-14-7-10-17(22)19(24)12-14)4-2-1-3-13-6-9-16(21)18(23)11-13/h2,4,6-7,9-12,21-24H,1,3,5,8H2/b4-2+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c00968
BindingDB Entry DOI: 10.7270/Q27P93C8 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM57938

((1R)-1,6,6-trimethyl-2,7,8,9-tetrahydro-1H-naphtho...)Show SMILES C[C@H]1COC2=C1C(=O)C(=O)c1c3CCCC(C)(C)c3ccc21 |c:4| Show InChI InChI=1S/C19H20O3/c1-10-9-22-18-12-6-7-13-11(5-4-8-19(13,2)3)15(12)17(21)16(20)14(10)18/h6-7,10H,4-5,8-9H2,1-3H3/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c00968
BindingDB Entry DOI: 10.7270/Q27P93C8 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50587668

(Apigenin-7-Neohesperidoside | CHEBI:31227 | RHOIFO...)Show SMILES C[C@@H]1O[C@@H](O[C@H]2[C@H](Oc3cc(O)c4c(c3)oc(cc4=O)-c3ccc(O)cc3)O[C@H](CO)[C@@H](O)[C@@H]2O)[C@H](O)[C@H](O)[C@H]1O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c00968
BindingDB Entry DOI: 10.7270/Q27P93C8 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50304350
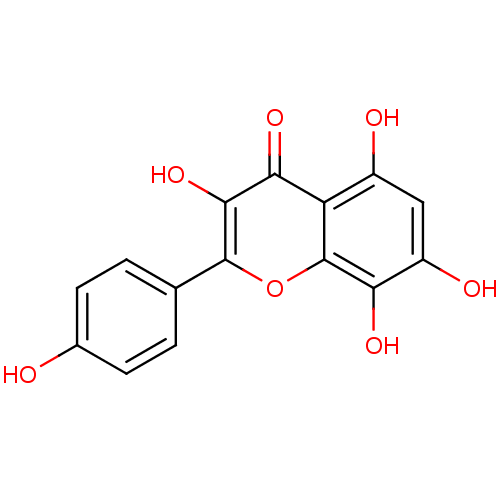
(CHEMBL611029 | Herbacetin)Show InChI InChI=1S/C15H10O7/c16-7-3-1-6(2-4-7)14-13(21)12(20)10-8(17)5-9(18)11(19)15(10)22-14/h1-5,16-19,21H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c00968
BindingDB Entry DOI: 10.7270/Q27P93C8 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50046953
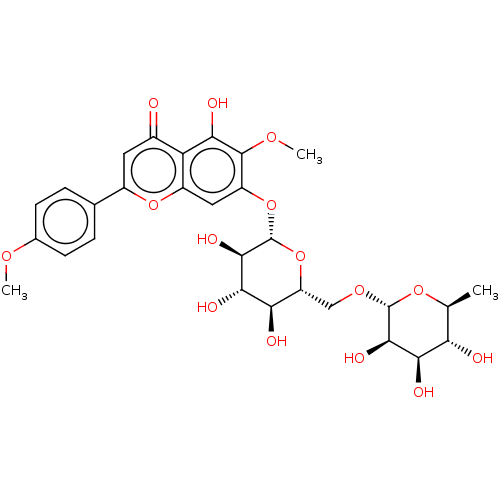
(Pectolinarin)Show SMILES COc1ccc(cc1)-c1cc(=O)c2c(O)c(OC)c(O[C@@H]3O[C@H](CO[C@@H]4O[C@@H](C)[C@H](O)[C@@H](O)[C@H]4O)[C@@H](O)[C@H](O)[C@H]3O)cc2o1 |r| Show InChI InChI=1S/C29H34O15/c1-11-20(31)23(34)25(36)28(41-11)40-10-18-21(32)24(35)26(37)29(44-18)43-17-9-16-19(22(33)27(17)39-3)14(30)8-15(42-16)12-4-6-13(38-2)7-5-12/h4-9,11,18,20-21,23-26,28-29,31-37H,10H2,1-3H3/t11-,18+,20-,21+,23+,24-,25+,26+,28+,29+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c00968
BindingDB Entry DOI: 10.7270/Q27P93C8 |
More data for this
Ligand-Target Pair | |
Spike glycoprotein
(2019-nCoV) | BDBM11318
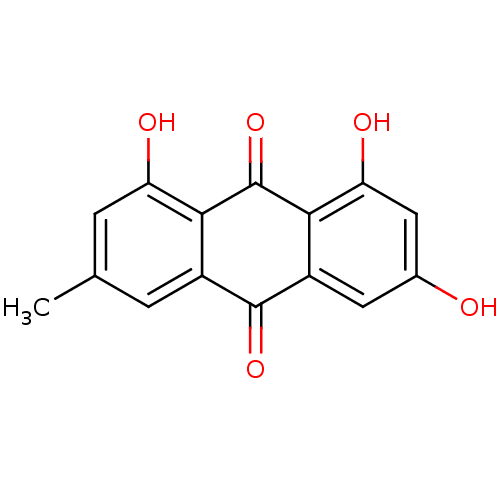
(1,3,8-trihydroxy-6-methyl-9,10-dihydroanthracene-9...)Show InChI InChI=1S/C15H10O5/c1-6-2-8-12(10(17)3-6)15(20)13-9(14(8)19)4-7(16)5-11(13)18/h2-5,16-18H,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c00968
BindingDB Entry DOI: 10.7270/Q27P93C8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data