 Found 382 hits with Last Name = 'gierse' and Initial = 'j'
Found 382 hits with Last Name = 'gierse' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116537
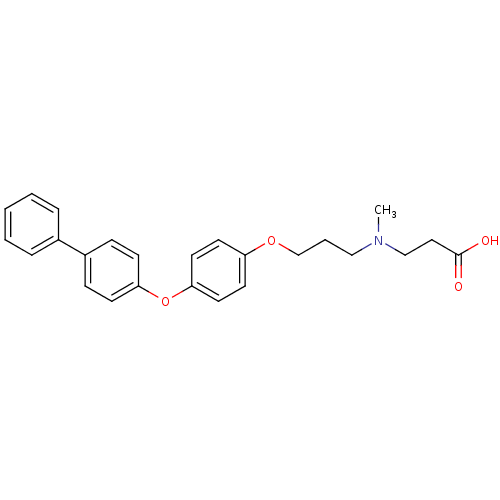
(3-({3-[4-(Biphenyl-4-yloxy)-phenoxy]-propyl}-methy...)Show SMILES CN(CCCOc1ccc(Oc2ccc(cc2)-c2ccccc2)cc1)CCC(O)=O Show InChI InChI=1S/C25H27NO4/c1-26(18-16-25(27)28)17-5-19-29-22-12-14-24(15-13-22)30-23-10-8-21(9-11-23)20-6-3-2-4-7-20/h2-4,6-15H,5,16-19H2,1H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human whole blood LTB-4 production (Leukotriene B-4). |
J Med Chem 45: 3482-90 (2002)
BindingDB Entry DOI: 10.7270/Q2DV1KMF |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50125433
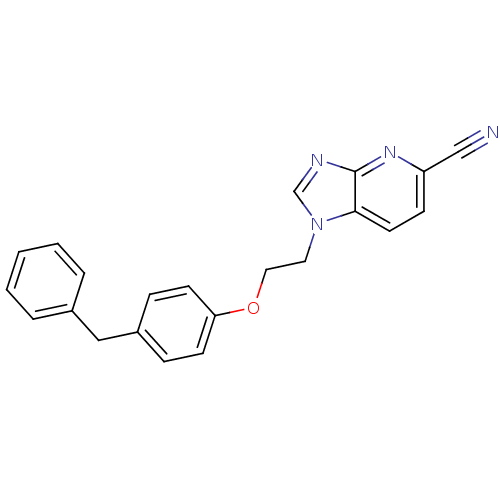
(1-[2-(4-Benzyl-phenoxy)-ethyl]-1H-imidazo[4,5-b]py...)Show InChI InChI=1S/C22H18N4O/c23-15-19-8-11-21-22(25-19)24-16-26(21)12-13-27-20-9-6-18(7-10-20)14-17-4-2-1-3-5-17/h1-11,16H,12-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of recombinant human leukotriene A4 hydrolase. |
Bioorg Med Chem Lett 13: 1137-9 (2003)
BindingDB Entry DOI: 10.7270/Q2891587 |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50271083
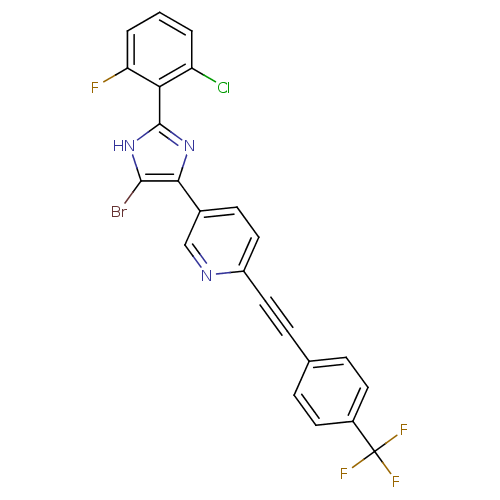
(5-(5-bromo-2-(2-chloro-6-fluorophenyl)-1H-imidazol...)Show SMILES Fc1cccc(Cl)c1-c1nc(c(Br)[nH]1)-c1ccc(nc1)C#Cc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C23H11BrClF4N3/c24-21-20(31-22(32-21)19-17(25)2-1-3-18(19)26)14-7-11-16(30-12-14)10-6-13-4-8-15(9-5-13)23(27,28)29/h1-5,7-9,11-12H,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of mPGES1 |
Bioorg Med Chem Lett 20: 1604-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.060
BindingDB Entry DOI: 10.7270/Q25X292V |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50227631
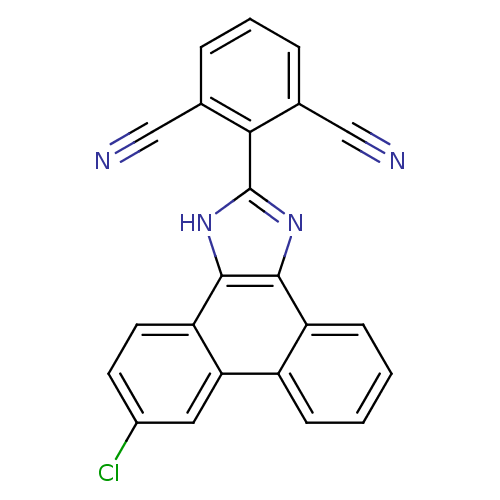
(2-(6-chloro-1H-phenanthro[9,10-d]imidazol-2-yl)iso...)Show SMILES Clc1ccc2c3[nH]c(nc3c3ccccc3c2c1)-c1c(cccc1C#N)C#N Show InChI InChI=1S/C23H11ClN4/c24-15-8-9-18-19(10-15)16-6-1-2-7-17(16)21-22(18)28-23(27-21)20-13(11-25)4-3-5-14(20)12-26/h1-10H,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA |
Bioorg Med Chem Lett 20: 1604-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.060
BindingDB Entry DOI: 10.7270/Q25X292V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116563
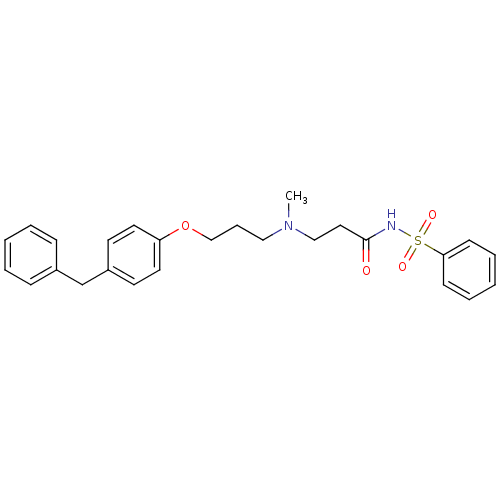
(CHEMBL323686 | N-(3-{[3-(4-Benzyl-phenoxy)-propyl]...)Show SMILES CN(CCCOc1ccc(Cc2ccccc2)cc1)CCC(=O)NS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C26H30N2O4S/c1-28(19-17-26(29)27-33(30,31)25-11-6-3-7-12-25)18-8-20-32-24-15-13-23(14-16-24)21-22-9-4-2-5-10-22/h2-7,9-16H,8,17-21H2,1H3,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase (LTA-4). |
J Med Chem 45: 3482-90 (2002)
BindingDB Entry DOI: 10.7270/Q2DV1KMF |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116562
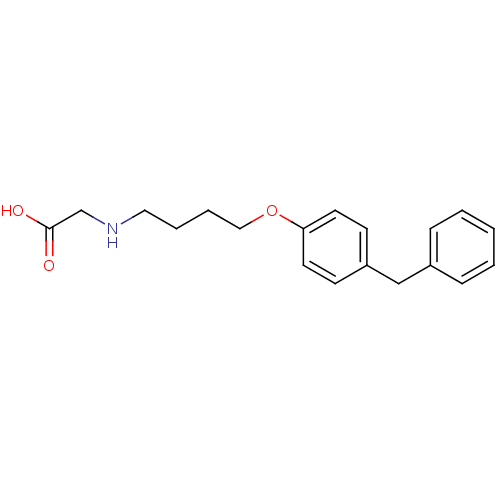
(CHEMBL117549 | [4-(4-Benzyl-phenoxy)-butylamino]-a...)Show InChI InChI=1S/C19H23NO3/c21-19(22)15-20-12-4-5-13-23-18-10-8-17(9-11-18)14-16-6-2-1-3-7-16/h1-3,6-11,20H,4-5,12-15H2,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase (LTA-4). |
J Med Chem 45: 3482-90 (2002)
BindingDB Entry DOI: 10.7270/Q2DV1KMF |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116554
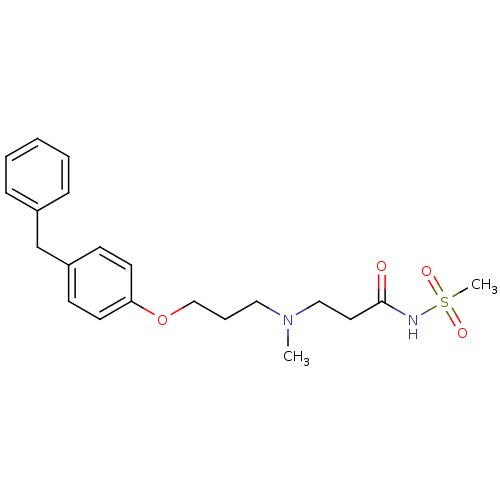
(CHEMBL119683 | N-(3-{[3-(4-Benzyl-phenoxy)-propyl]...)Show SMILES CN(CCCOc1ccc(Cc2ccccc2)cc1)CCC(=O)NS(C)(=O)=O Show InChI InChI=1S/C21H28N2O4S/c1-23(15-13-21(24)22-28(2,25)26)14-6-16-27-20-11-9-19(10-12-20)17-18-7-4-3-5-8-18/h3-5,7-12H,6,13-17H2,1-2H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase (LTA-4). |
J Med Chem 45: 3482-90 (2002)
BindingDB Entry DOI: 10.7270/Q2DV1KMF |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116560

(3-{Methyl-[3-(4-thiophen-3-ylmethyl-phenoxy)-propy...)Show InChI InChI=1S/C18H23NO3S/c1-19(10-7-18(20)21)9-2-11-22-17-5-3-15(4-6-17)13-16-8-12-23-14-16/h3-6,8,12,14H,2,7,9-11,13H2,1H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase (LTA-4). |
J Med Chem 45: 3482-90 (2002)
BindingDB Entry DOI: 10.7270/Q2DV1KMF |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116538
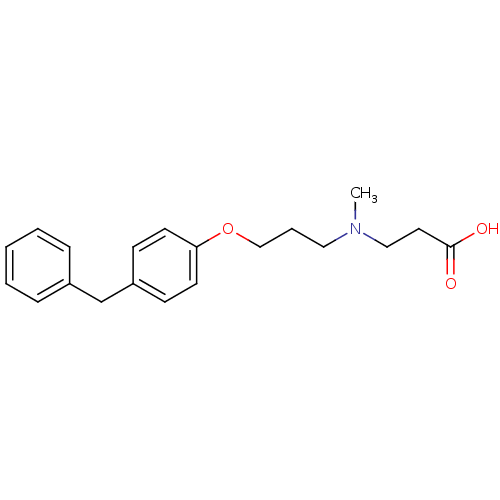
(3-{[3-(4-Benzyl-phenoxy)-propyl]-methyl-amino}-pro...)Show InChI InChI=1S/C20H25NO3/c1-21(14-12-20(22)23)13-5-15-24-19-10-8-18(9-11-19)16-17-6-3-2-4-7-17/h2-4,6-11H,5,12-16H2,1H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation
Curated by ChEMBL
| Assay Description
Inhibition of leukotriene A4 hydrolase in human recombinant assay |
Bioorg Med Chem Lett 12: 3383-6 (2002)
BindingDB Entry DOI: 10.7270/Q2N8795W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116538

(3-{[3-(4-Benzyl-phenoxy)-propyl]-methyl-amino}-pro...)Show InChI InChI=1S/C20H25NO3/c1-21(14-12-20(22)23)13-5-15-24-19-10-8-18(9-11-19)16-17-6-3-2-4-7-17/h2-4,6-11H,5,12-16H2,1H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase (LTA-4). |
J Med Chem 45: 3482-90 (2002)
BindingDB Entry DOI: 10.7270/Q2DV1KMF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116544
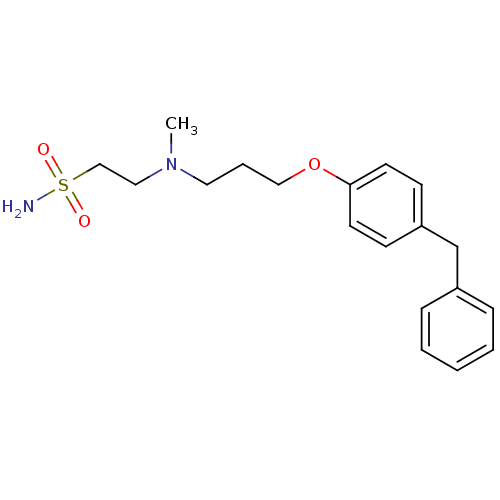
(2-{[3-(4-Benzyl-phenoxy)-propyl]-methyl-amino}-eth...)Show InChI InChI=1S/C19H26N2O3S/c1-21(13-15-25(20,22)23)12-5-14-24-19-10-8-18(9-11-19)16-17-6-3-2-4-7-17/h2-4,6-11H,5,12-16H2,1H3,(H2,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase (LTA-4). |
J Med Chem 45: 3482-90 (2002)
BindingDB Entry DOI: 10.7270/Q2DV1KMF |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116542

(3-{Methyl-[3-(4-thiophen-2-ylmethyl-phenoxy)-propy...)Show InChI InChI=1S/C18H23NO3S/c1-19(11-9-18(20)21)10-3-12-22-16-7-5-15(6-8-16)14-17-4-2-13-23-17/h2,4-8,13H,3,9-12,14H2,1H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase (LTA-4). |
J Med Chem 45: 3482-90 (2002)
BindingDB Entry DOI: 10.7270/Q2DV1KMF |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50105264
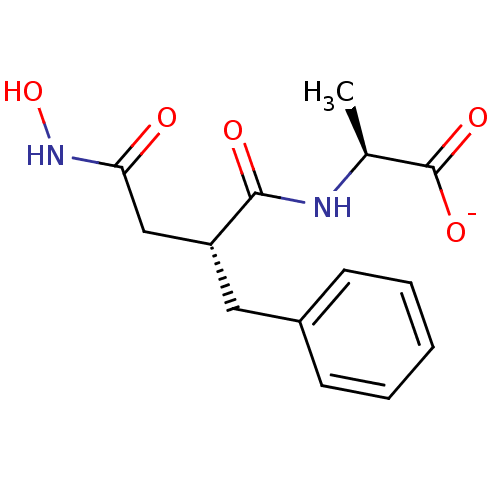
(2-(2-Benzyl-3-hydroxycarbamoyl-propionylamino)-pro...)Show SMILES C[C@H](NC(=O)[C@@H](CC(=O)NO)Cc1ccccc1)C([O-])=O Show InChI InChI=1S/C14H18N2O5/c1-9(14(19)20)15-13(18)11(8-12(17)16-21)7-10-5-3-2-4-6-10/h2-6,9,11,21H,7-8H2,1H3,(H,15,18)(H,16,17)(H,19,20)/p-1/t9-,11+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Leukotriene A4 hydrolase |
J Med Chem 43: 721-35 (2000)
BindingDB Entry DOI: 10.7270/Q2CN74MZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50085261

(CHEMBL163478 | [2-(4-Benzyl-phenoxy)-ethyl]-dimeth...)Show InChI InChI=1S/C17H21NO/c1-18(2)12-13-19-17-10-8-16(9-11-17)14-15-6-4-3-5-7-15/h3-11H,12-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Leukotriene A4 hydrolase |
J Med Chem 43: 721-35 (2000)
BindingDB Entry DOI: 10.7270/Q2CN74MZ |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50085288
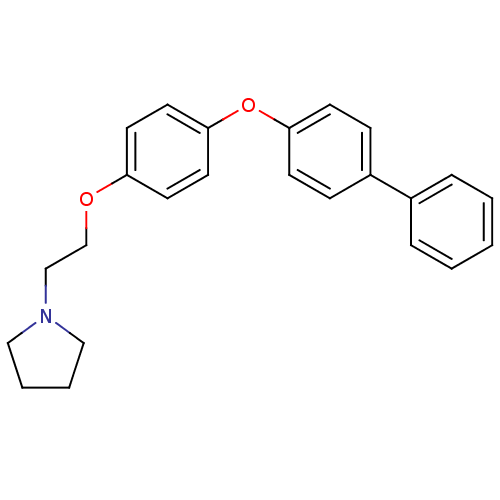
(1-{2-[4-(Biphenyl-4-yloxy)-phenoxy]-ethyl}-pyrroli...)Show InChI InChI=1S/C24H25NO2/c1-2-6-20(7-3-1)21-8-10-23(11-9-21)27-24-14-12-22(13-15-24)26-19-18-25-16-4-5-17-25/h1-3,6-15H,4-5,16-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Leukotriene A4 hydrolase |
J Med Chem 43: 721-35 (2000)
BindingDB Entry DOI: 10.7270/Q2CN74MZ |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116548
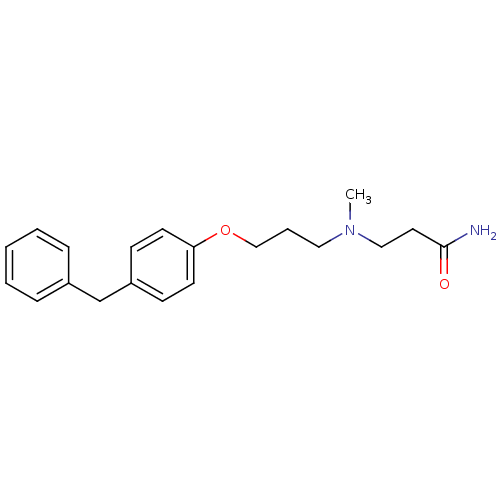
(3-{[3-(4-Benzyl-phenoxy)-propyl]-methyl-amino}-pro...)Show InChI InChI=1S/C20H26N2O2/c1-22(14-12-20(21)23)13-5-15-24-19-10-8-18(9-11-19)16-17-6-3-2-4-7-17/h2-4,6-11H,5,12-16H2,1H3,(H2,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase (LTA-4). |
J Med Chem 45: 3482-90 (2002)
BindingDB Entry DOI: 10.7270/Q2DV1KMF |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50085282

(5-Benzyl-2-(2-pyrrolidin-1-yl-ethoxy)-pyridine | C...)Show InChI InChI=1S/C18H22N2O/c1-2-6-16(7-3-1)14-17-8-9-18(19-15-17)21-13-12-20-10-4-5-11-20/h1-3,6-9,15H,4-5,10-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Leukotriene A4 hydrolase |
J Med Chem 43: 721-35 (2000)
BindingDB Entry DOI: 10.7270/Q2CN74MZ |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116561

(3-[4-(4-Benzyl-phenoxy)-butylamino]-propionic acid...)Show InChI InChI=1S/C22H29NO3/c1-2-25-22(24)14-16-23-15-6-7-17-26-21-12-10-20(11-13-21)18-19-8-4-3-5-9-19/h3-5,8-13,23H,2,6-7,14-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase (LTA-4). |
J Med Chem 45: 3482-90 (2002)
BindingDB Entry DOI: 10.7270/Q2DV1KMF |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50125435

(1-[2-(4-Benzyl-phenoxy)-ethyl]-1H-imidazo[4,5-b]py...)Show InChI InChI=1S/C21H19N3O/c1-2-5-17(6-3-1)15-18-8-10-19(11-9-18)25-14-13-24-16-23-21-20(24)7-4-12-22-21/h1-12,16H,13-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of recombinant human leukotriene A4 hydrolase. |
Bioorg Med Chem Lett 13: 1137-9 (2003)
BindingDB Entry DOI: 10.7270/Q2891587 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50085290
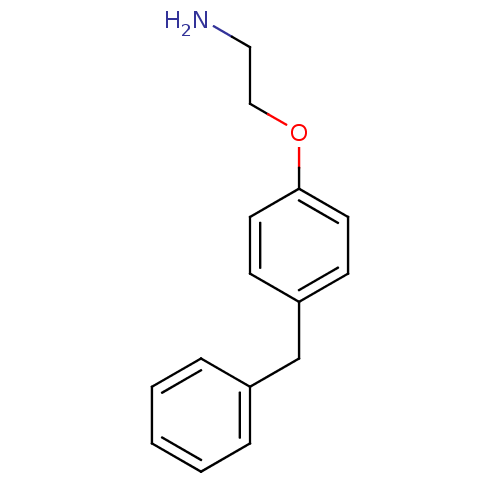
(2-(4-Benzyl-phenoxy)-ethylamine | 2-(4-benzylpheno...)Show InChI InChI=1S/C15H17NO/c16-10-11-17-15-8-6-14(7-9-15)12-13-4-2-1-3-5-13/h1-9H,10-12,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Leukotriene A4 hydrolase |
J Med Chem 43: 721-35 (2000)
BindingDB Entry DOI: 10.7270/Q2CN74MZ |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50085277
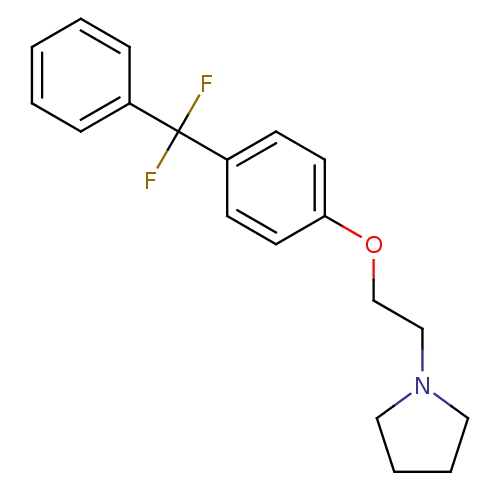
(1-{2-[4-(Difluoro-phenyl-methyl)-phenoxy]-ethyl}-p...)Show InChI InChI=1S/C19H21F2NO/c20-19(21,16-6-2-1-3-7-16)17-8-10-18(11-9-17)23-15-14-22-12-4-5-13-22/h1-3,6-11H,4-5,12-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Leukotriene A4 hydrolase |
J Med Chem 43: 721-35 (2000)
BindingDB Entry DOI: 10.7270/Q2CN74MZ |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50125420

(5-[2-(4-Benzyl-phenoxy)-ethyl]-5H-imidazo[4,5-c]py...)Show InChI InChI=1S/C21H19N3O/c1-2-4-17(5-3-1)14-18-6-8-19(9-7-18)25-13-12-24-11-10-20-21(15-24)23-16-22-20/h1-11,15-16H,12-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of recombinant human leukotriene A4 hydrolase. |
Bioorg Med Chem Lett 13: 1137-9 (2003)
BindingDB Entry DOI: 10.7270/Q2891587 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116532
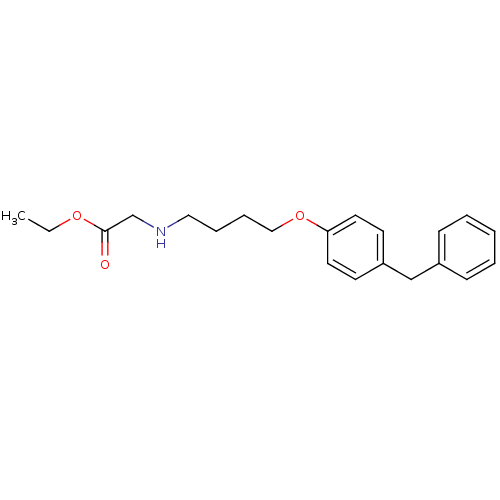
(CHEMBL420502 | [4-(4-Benzyl-phenoxy)-butylamino]-a...)Show InChI InChI=1S/C21H27NO3/c1-2-24-21(23)17-22-14-6-7-15-25-20-12-10-19(11-13-20)16-18-8-4-3-5-9-18/h3-5,8-13,22H,2,6-7,14-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase (LTA-4). |
J Med Chem 45: 3482-90 (2002)
BindingDB Entry DOI: 10.7270/Q2DV1KMF |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50121000
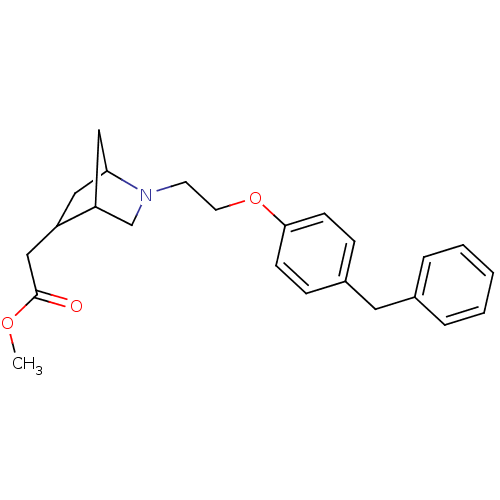
(CHEMBL116174 | {2-[2-(4-Benzyl-phenoxy)-ethyl]-2-a...)Show SMILES COC(=O)CC1CC2CC1CN2CCOc1ccc(Cc2ccccc2)cc1 |THB:4:5:11.10:8| Show InChI InChI=1S/C24H29NO3/c1-27-24(26)16-20-14-22-15-21(20)17-25(22)11-12-28-23-9-7-19(8-10-23)13-18-5-3-2-4-6-18/h2-10,20-22H,11-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation
Curated by ChEMBL
| Assay Description
Inhibition of leukotriene A4 hydrolase in human recombinant assay |
Bioorg Med Chem Lett 12: 3383-6 (2002)
BindingDB Entry DOI: 10.7270/Q2N8795W |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50121000

(CHEMBL116174 | {2-[2-(4-Benzyl-phenoxy)-ethyl]-2-a...)Show SMILES COC(=O)CC1CC2CC1CN2CCOc1ccc(Cc2ccccc2)cc1 |THB:4:5:11.10:8| Show InChI InChI=1S/C24H29NO3/c1-27-24(26)16-20-14-22-15-21(20)17-25(22)11-12-28-23-9-7-19(8-10-23)13-18-5-3-2-4-6-18/h2-10,20-22H,11-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation
Curated by ChEMBL
| Assay Description
Inhibition of leukotriene A4 hydrolase in human recombinant assay |
Bioorg Med Chem Lett 12: 3383-6 (2002)
BindingDB Entry DOI: 10.7270/Q2N8795W |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116529

(3-{Methyl-[3-(4-thiophen-2-ylmethyl-phenoxy)-propy...)Show InChI InChI=1S/C19H25NO3S/c1-20(12-10-19(21)22-2)11-4-13-23-17-8-6-16(7-9-17)15-18-5-3-14-24-18/h3,5-9,14H,4,10-13,15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase (LTA-4). |
J Med Chem 45: 3482-90 (2002)
BindingDB Entry DOI: 10.7270/Q2DV1KMF |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50313205
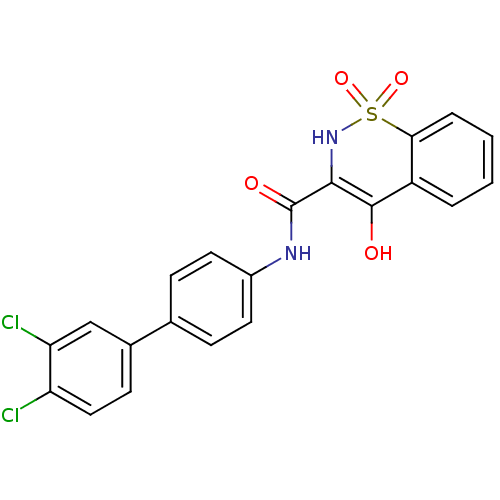
(4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...)Show SMILES OC1=C(NS(=O)(=O)c2ccccc12)C(=O)Nc1ccc(cc1)-c1ccc(Cl)c(Cl)c1 |t:1| Show InChI InChI=1S/C21H14Cl2N2O4S/c22-16-10-7-13(11-17(16)23)12-5-8-14(9-6-12)24-21(27)19-20(26)15-3-1-2-4-18(15)30(28,29)25-19/h1-11,25-26H,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA |
Bioorg Med Chem Lett 20: 1604-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.060
BindingDB Entry DOI: 10.7270/Q25X292V |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50121003

(1-[2-(4-Benzyl-phenoxy)-ethyl]-pyrrolidine-3-carbo...)Show InChI InChI=1S/C20H24N2O2/c21-20(23)18-10-11-22(15-18)12-13-24-19-8-6-17(7-9-19)14-16-4-2-1-3-5-16/h1-9,18H,10-15H2,(H2,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation
Curated by ChEMBL
| Assay Description
Inhibition of leukotriene A4 hydrolase in human recombinant assay |
Bioorg Med Chem Lett 12: 3383-6 (2002)
BindingDB Entry DOI: 10.7270/Q2N8795W |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50085270
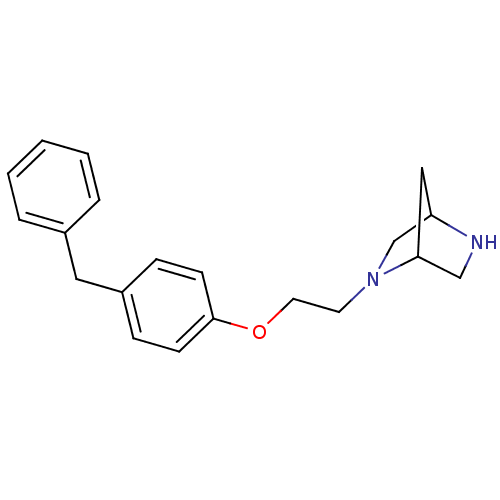
(2-[2-(4-Benzyl-phenoxy)-ethyl]-2,5-diaza-bicyclo[2...)Show SMILES C(CN1CC2CC1CN2)Oc1ccc(Cc2ccccc2)cc1 |THB:1:2:5:8.7| Show InChI InChI=1S/C20H24N2O/c1-2-4-16(5-3-1)12-17-6-8-20(9-7-17)23-11-10-22-15-18-13-19(22)14-21-18/h1-9,18-19,21H,10-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Leukotriene A4 hydrolase |
J Med Chem 43: 721-35 (2000)
BindingDB Entry DOI: 10.7270/Q2CN74MZ |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116531

(3-[4-(4-Benzyl-phenoxy)-butylamino]-propionic acid...)Show InChI InChI=1S/C20H25NO3/c22-20(23)12-14-21-13-4-5-15-24-19-10-8-18(9-11-19)16-17-6-2-1-3-7-17/h1-3,6-11,21H,4-5,12-16H2,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase (LTA-4). |
J Med Chem 45: 3482-90 (2002)
BindingDB Entry DOI: 10.7270/Q2DV1KMF |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116533

(3-{Methyl-[3-(4-phenoxy-phenoxy)-propyl]-amino}-pr...)Show InChI InChI=1S/C19H23NO4/c1-20(14-12-19(21)22)13-5-15-23-16-8-10-18(11-9-16)24-17-6-3-2-4-7-17/h2-4,6-11H,5,12-15H2,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase (LTA-4). |
J Med Chem 45: 3482-90 (2002)
BindingDB Entry DOI: 10.7270/Q2DV1KMF |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50121009
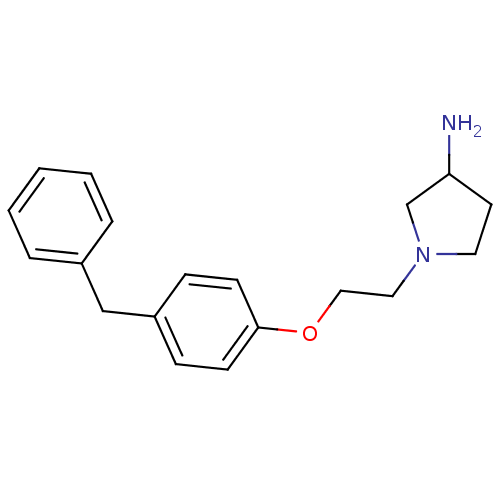
(1-[2-(4-Benzyl-phenoxy)-ethyl]-pyrrolidin-3-ylamin...)Show InChI InChI=1S/C19H24N2O/c20-18-10-11-21(15-18)12-13-22-19-8-6-17(7-9-19)14-16-4-2-1-3-5-16/h1-9,18H,10-15,20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation
Curated by ChEMBL
| Assay Description
Inhibition of leukotriene A4 hydrolase in human recombinant assay |
Bioorg Med Chem Lett 12: 3383-6 (2002)
BindingDB Entry DOI: 10.7270/Q2N8795W |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50085297
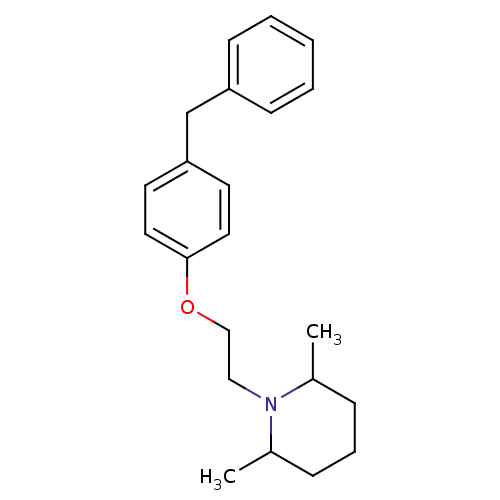
(1-[2-(4-Benzyl-phenoxy)-ethyl]-2,6-dimethyl-piperi...)Show InChI InChI=1S/C22H29NO/c1-18-7-6-8-19(2)23(18)15-16-24-22-13-11-21(12-14-22)17-20-9-4-3-5-10-20/h3-5,9-14,18-19H,6-8,15-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Leukotriene A4 hydrolase |
J Med Chem 43: 721-35 (2000)
BindingDB Entry DOI: 10.7270/Q2CN74MZ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50336012

(6-chloro-8-(4-chlorophenyl)-2-(trifluoromethyl)-2H...)Show SMILES OC(=O)C1=Cc2cc(Cl)cc(-c3ccc(Cl)cc3)c2OC1C(F)(F)F |t:3| Show InChI InChI=1S/C17H9Cl2F3O3/c18-10-3-1-8(2-4-10)12-7-11(19)5-9-6-13(16(23)24)15(17(20,21)22)25-14(9)12/h1-7,15H,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 |
Bioorg Med Chem Lett 21: 993-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.023
BindingDB Entry DOI: 10.7270/Q2QR4XDS |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50006805

(3-(1-(4-chlorobenzyl)-3-(tert-butylthio)-5-isoprop...)Show SMILES CC(C)c1ccc2n(Cc3ccc(Cl)cc3)c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c2c1 Show InChI InChI=1S/C27H34ClNO2S/c1-17(2)19-10-13-22-21(14-19)24(32-26(3,4)5)23(15-27(6,7)25(30)31)29(22)16-18-8-11-20(28)12-9-18/h8-14,17H,15-16H2,1-7H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of FLAP |
Bioorg Med Chem Lett 20: 1604-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.060
BindingDB Entry DOI: 10.7270/Q25X292V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50085279
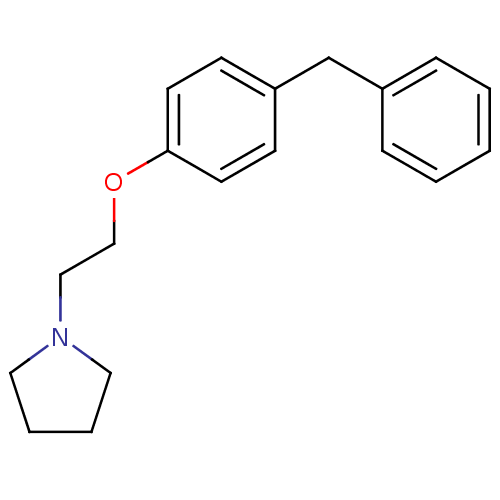
(1-[2-(4-Benzyl-phenoxy)-ethyl]-pyrrolidine | 1-[2-...)Show InChI InChI=1S/C19H23NO/c1-2-6-17(7-3-1)16-18-8-10-19(11-9-18)21-15-14-20-12-4-5-13-20/h1-3,6-11H,4-5,12-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of recombinant human leukotriene A4 hydrolase. |
Bioorg Med Chem Lett 13: 1137-9 (2003)
BindingDB Entry DOI: 10.7270/Q2891587 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50085279

(1-[2-(4-Benzyl-phenoxy)-ethyl]-pyrrolidine | 1-[2-...)Show InChI InChI=1S/C19H23NO/c1-2-6-17(7-3-1)16-18-8-10-19(11-9-18)21-15-14-20-12-4-5-13-20/h1-3,6-11H,4-5,12-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation
Curated by ChEMBL
| Assay Description
Inhibition of leukotriene A4 hydrolase in human recombinant assay |
Bioorg Med Chem Lett 12: 3383-6 (2002)
BindingDB Entry DOI: 10.7270/Q2N8795W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50313223
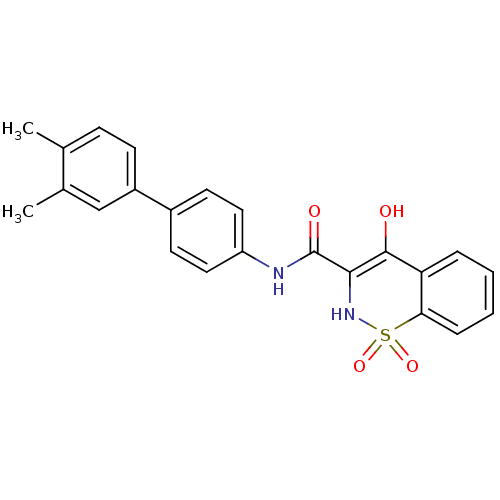
(4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...)Show SMILES Cc1ccc(cc1C)-c1ccc(NC(=O)C2=C(O)c3ccccc3S(=O)(=O)N2)cc1 |c:16| Show InChI InChI=1S/C23H20N2O4S/c1-14-7-8-17(13-15(14)2)16-9-11-18(12-10-16)24-23(27)21-22(26)19-5-3-4-6-20(19)30(28,29)25-21/h3-13,25-26H,1-2H3,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA |
Bioorg Med Chem Lett 20: 1604-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.060
BindingDB Entry DOI: 10.7270/Q25X292V |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50085279

(1-[2-(4-Benzyl-phenoxy)-ethyl]-pyrrolidine | 1-[2-...)Show InChI InChI=1S/C19H23NO/c1-2-6-17(7-3-1)16-18-8-10-19(11-9-18)21-15-14-20-12-4-5-13-20/h1-3,6-11H,4-5,12-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Leukotriene A4 hydrolase |
J Med Chem 43: 721-35 (2000)
BindingDB Entry DOI: 10.7270/Q2CN74MZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116535

(3-(Methyl-{3-[4-(4-oxazol-2-yl-phenoxy)-phenoxy]-p...)Show SMILES CN(CCCOc1ccc(Oc2ccc(cc2)-c2ncco2)cc1)CCC(O)=O Show InChI InChI=1S/C22H24N2O5/c1-24(14-11-21(25)26)13-2-15-27-18-7-9-20(10-8-18)29-19-5-3-17(4-6-19)22-23-12-16-28-22/h3-10,12,16H,2,11,13-15H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase (LTA-4). |
J Med Chem 45: 3482-90 (2002)
BindingDB Entry DOI: 10.7270/Q2DV1KMF |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50085252

(CHEMBL162180 | Phenyl-[4-(2-pyrrolidin-1-yl-ethoxy...)Show InChI InChI=1S/C19H24N2O/c1-2-6-18(7-3-1)20-16-17-8-10-19(11-9-17)22-15-14-21-12-4-5-13-21/h1-3,6-11,20H,4-5,12-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Leukotriene A4 hydrolase |
J Med Chem 43: 721-35 (2000)
BindingDB Entry DOI: 10.7270/Q2CN74MZ |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50121023

(1-[2-(4-Benzyl-phenoxy)-ethyl]-pyrrolidin-3-ol | C...)Show InChI InChI=1S/C19H23NO2/c21-18-10-11-20(15-18)12-13-22-19-8-6-17(7-9-19)14-16-4-2-1-3-5-16/h1-9,18,21H,10-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation
Curated by ChEMBL
| Assay Description
Inhibition of leukotriene A4 hydrolase in human recombinant assay |
Bioorg Med Chem Lett 12: 3383-6 (2002)
BindingDB Entry DOI: 10.7270/Q2N8795W |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50085245

(1-[2-(4-Phenoxy-phenoxy)-ethyl]-pyrrolidine | CHEM...)Show InChI InChI=1S/C18H21NO2/c1-2-6-17(7-3-1)21-18-10-8-16(9-11-18)20-15-14-19-12-4-5-13-19/h1-3,6-11H,4-5,12-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Leukotriene A4 hydrolase |
J Med Chem 43: 721-35 (2000)
BindingDB Entry DOI: 10.7270/Q2CN74MZ |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50085248

(1-[2-(4-Benzyloxy-phenoxy)-ethyl]-pyrrolidine | CH...)Show InChI InChI=1S/C19H23NO2/c1-2-6-17(7-3-1)16-22-19-10-8-18(9-11-19)21-15-14-20-12-4-5-13-20/h1-3,6-11H,4-5,12-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Leukotriene A4 hydrolase |
J Med Chem 43: 721-35 (2000)
BindingDB Entry DOI: 10.7270/Q2CN74MZ |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116536

(3-({3-[4-(4-Fluoro-benzyl)-phenoxy]-propyl}-methyl...)Show InChI InChI=1S/C20H24FNO3/c1-22(13-11-20(23)24)12-2-14-25-19-9-5-17(6-10-19)15-16-3-7-18(21)8-4-16/h3-10H,2,11-15H2,1H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase (LTA-4). |
J Med Chem 45: 3482-90 (2002)
BindingDB Entry DOI: 10.7270/Q2DV1KMF |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50125429
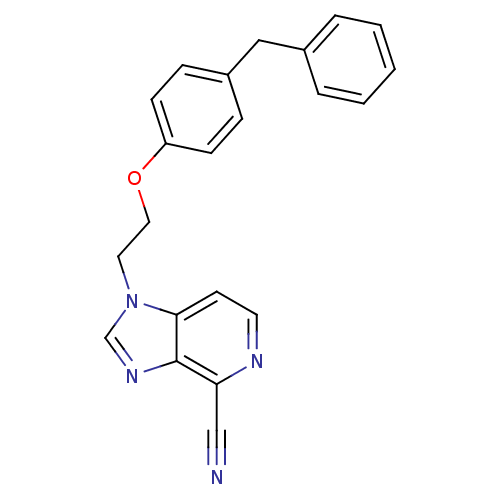
(1-[2-(4-Benzyl-phenoxy)-ethyl]-1H-imidazo[4,5-c]py...)Show InChI InChI=1S/C22H18N4O/c23-15-20-22-21(10-11-24-20)26(16-25-22)12-13-27-19-8-6-18(7-9-19)14-17-4-2-1-3-5-17/h1-11,16H,12-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of recombinant human leukotriene A4 hydrolase. |
Bioorg Med Chem Lett 13: 1137-9 (2003)
BindingDB Entry DOI: 10.7270/Q2891587 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50085299

(1-[2-(4-Thiophen-3-ylmethyl-phenoxy)-ethyl]-pyrrol...)Show InChI InChI=1S/C17H21NOS/c1-2-9-18(8-1)10-11-19-17-5-3-15(4-6-17)13-16-7-12-20-14-16/h3-7,12,14H,1-2,8-11,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Leukotriene A4 hydrolase |
J Med Chem 43: 721-35 (2000)
BindingDB Entry DOI: 10.7270/Q2CN74MZ |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50125421
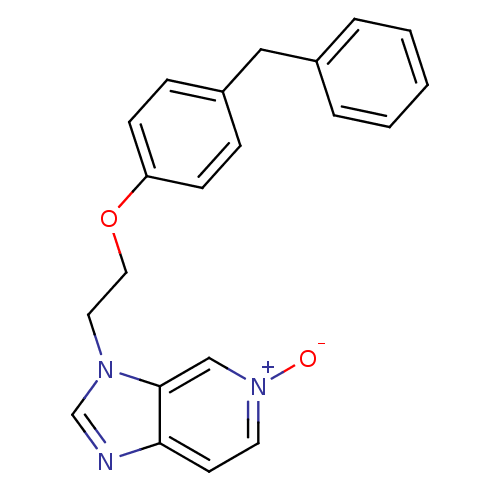
(3-[2-(4-Benzyl-phenoxy)-ethyl]-3H-imidazo[4,5-c]py...)Show SMILES [O-][n+]1ccc2ncn(CCOc3ccc(Cc4ccccc4)cc3)c2c1 Show InChI InChI=1S/C21H19N3O2/c25-24-11-10-20-21(15-24)23(16-22-20)12-13-26-19-8-6-18(7-9-19)14-17-4-2-1-3-5-17/h1-11,15-16H,12-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of recombinant human leukotriene A4 hydrolase. |
Bioorg Med Chem Lett 13: 1137-9 (2003)
BindingDB Entry DOI: 10.7270/Q2891587 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50085262

(1-[2-(5-Benzyl-thiophen-2-yloxy)-ethyl]-pyrrolidin...)Show InChI InChI=1S/C17H21NOS/c1-2-6-15(7-3-1)14-16-8-9-17(20-16)19-13-12-18-10-4-5-11-18/h1-3,6-9H,4-5,10-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Leukotriene A4 hydrolase |
J Med Chem 43: 721-35 (2000)
BindingDB Entry DOI: 10.7270/Q2CN74MZ |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50085283
(1-[2-(4-Benzyl-phenoxy)-ethyl]-azepane | CHEMBL159...)Show InChI InChI=1S/C21H27NO/c1-2-7-15-22(14-6-1)16-17-23-21-12-10-20(11-13-21)18-19-8-4-3-5-9-19/h3-5,8-13H,1-2,6-7,14-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Leukotriene A4 hydrolase |
J Med Chem 43: 721-35 (2000)
BindingDB Entry DOI: 10.7270/Q2CN74MZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data