

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arginase-1 (Bos taurus) | BDBM50553165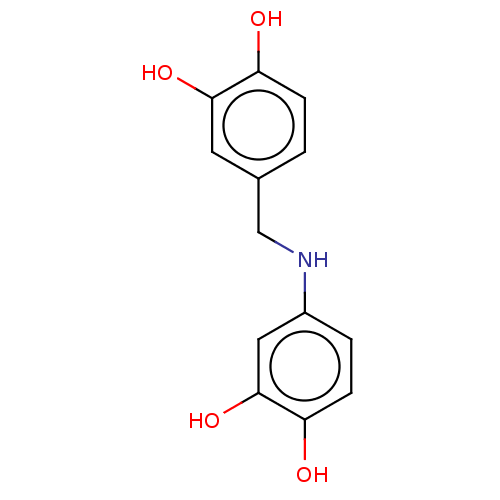 (CHEMBL4754674) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed type inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by Cornish-Bowden plot analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50553165 (CHEMBL4754674) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed type inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50553165 (CHEMBL4754674) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed type inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by Dixon plot analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50045936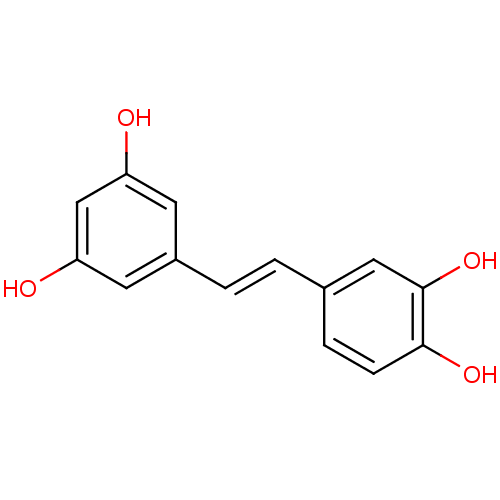 ((E)-4-(3,5-dihydroxystyryl)benzene-1,2-diol | (E)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed type inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by Dixon plot analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50045936 ((E)-4-(3,5-dihydroxystyryl)benzene-1,2-diol | (E)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed type inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50045936 ((E)-4-(3,5-dihydroxystyryl)benzene-1,2-diol | (E)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed type inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by Cornish-Bowden plot analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50008099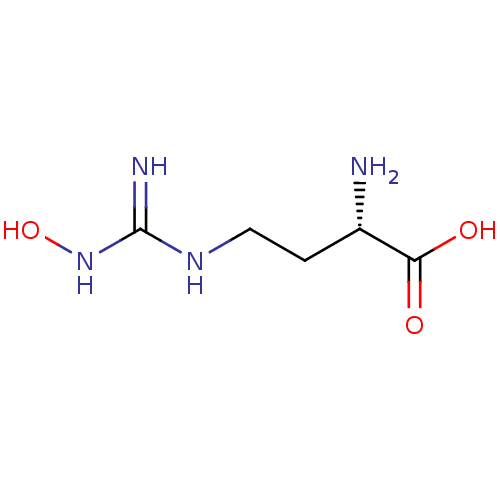 (CHEMBL1234777) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by spectroscopic analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50008099 (CHEMBL1234777) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human liver ARGI using L-arginine as substrate incubated for 60 mins by spectroscopic analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50350311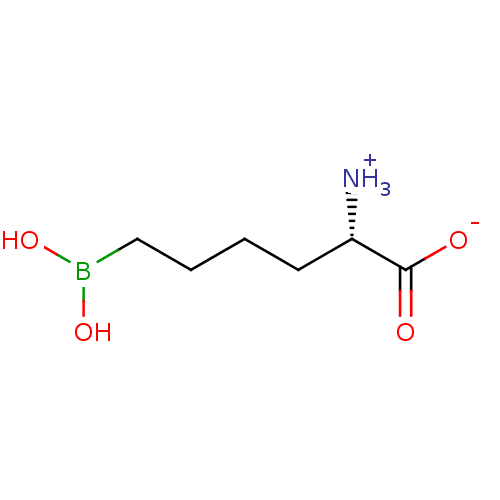 (CHEMBL1812661) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ARG1 expressed in CHO-K1 cells assessed as reduction in urea level incubated for 24 hrs by colorimetric assay | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50045936 ((E)-4-(3,5-dihydroxystyryl)benzene-1,2-diol | (E)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by spectroscopic analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50045936 ((E)-4-(3,5-dihydroxystyryl)benzene-1,2-diol | (E)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human liver ARGI using L-arginine as substrate incubated for 60 mins by spectroscopic analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50553165 (CHEMBL4754674) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by spectroscopic analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50553164 (CHEMBL4741701) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by spectroscopic analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50553165 (CHEMBL4754674) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human liver ARGI using L-arginine as substrate incubated for 60 mins by spectroscopic analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50553157 (CHEMBL440107) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by spectroscopic analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50553177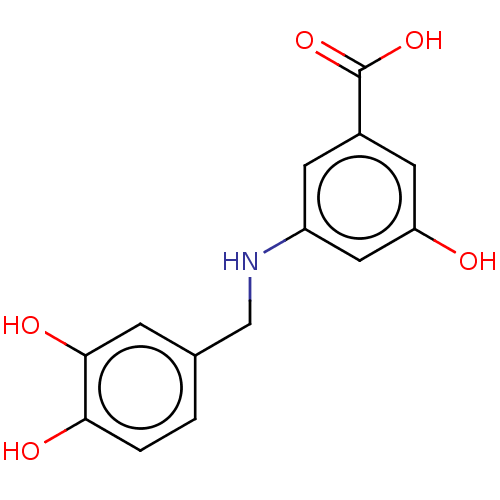 (CHEMBL4742032) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.08E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by spectroscopic analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM26188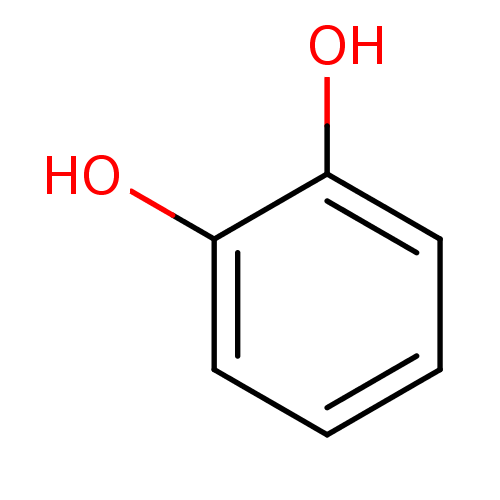 (α-CA inhibitor, 12 | 1,2-Dihydroxybenzene, XI...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.23E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by spectroscopic analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM55121 (3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.28E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by spectroscopic analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM4375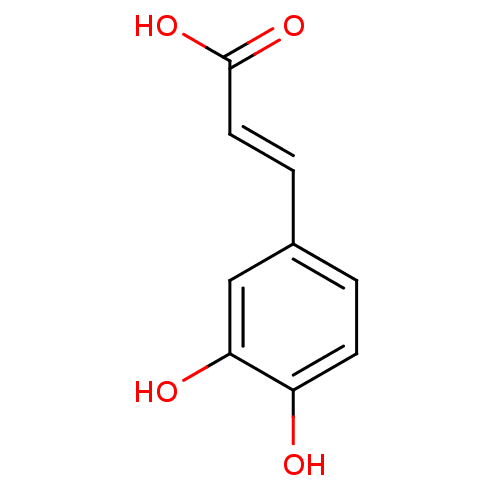 ((2E)-3-(3,4-dihydroxyphenyl)prop-2-enoic acid | (2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.31E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by spectroscopic analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50553162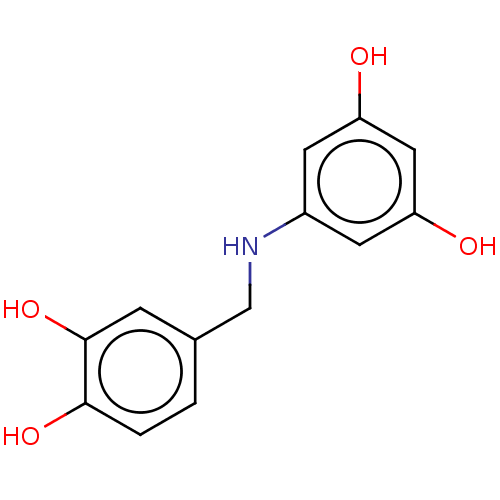 (CHEMBL4798237) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.41E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by spectroscopic analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50553171 (CHEMBL4794440) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by spectroscopic analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50553161 (CHEMBL4757794) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.54E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by spectroscopic analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50553160 (CHEMBL4791480) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.64E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by spectroscopic analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50553158 (CHEMBL2009732) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.64E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by spectroscopic analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50553159 (CHEMBL4764741) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.85E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by spectroscopic analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50553178 (CHEMBL4756480) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.87E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by spectroscopic analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50553176 (CHEMBL4597903) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.26E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by spectroscopic analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50553163 (CHEMBL4741885) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.38E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by spectroscopic analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM92758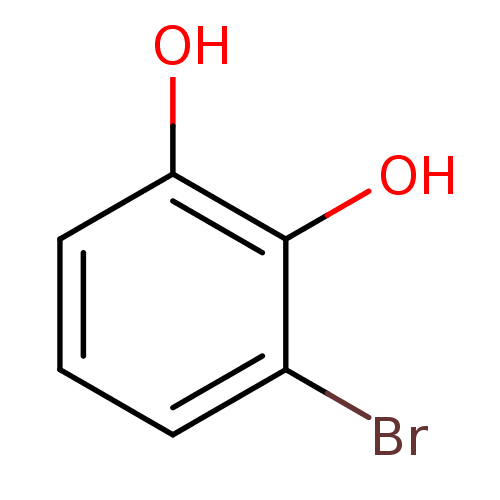 (3-Bromocatechol) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.74E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by spectroscopic analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50553175 (CHEMBL4748612) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.77E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by spectroscopic analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50242283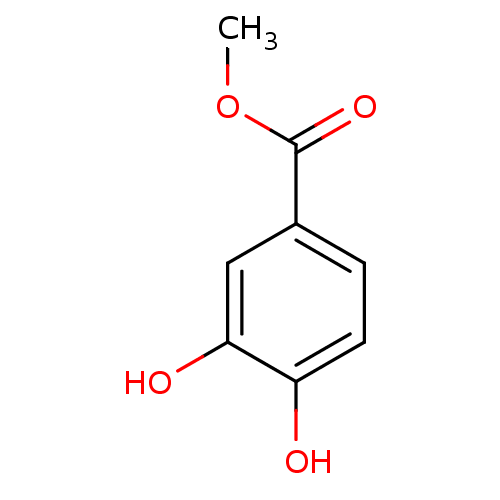 (3,4-dihydroxymethylbenzoate | CHEMBL486027 | Methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.85E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by spectroscopic analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50553173 (CHEMBL4750334) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by spectroscopic analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50553169 (CHEMBL4794775) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.18E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by spectroscopic analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50553174 (CHEMBL4780884) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.29E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by spectroscopic analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50553167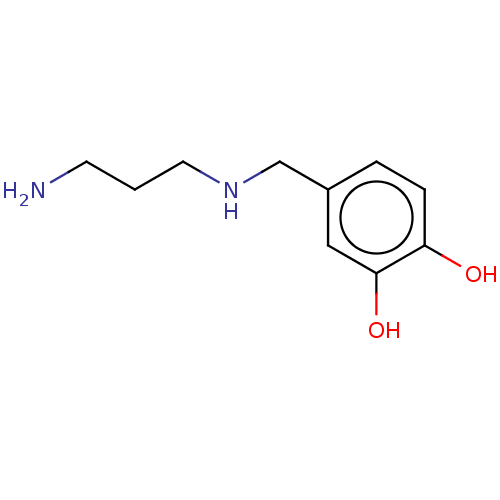 (CHEMBL4793535) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.43E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by spectroscopic analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50240171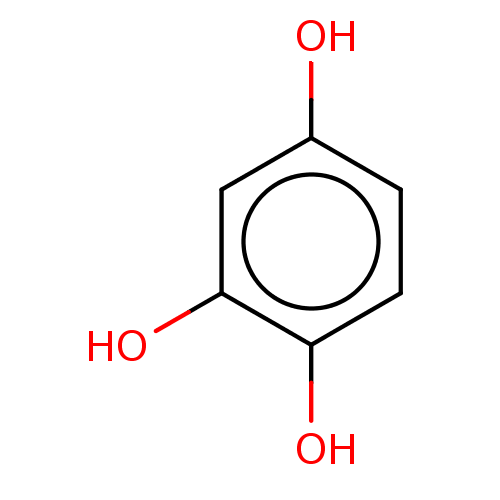 (CHEBI:16971 | CHEMBL3092389) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 3.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by spectroscopic analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50553168 (CHEMBL4788184) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.62E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by spectroscopic analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50553170 (CHEMBL4798533) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.67E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by spectroscopic analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50553172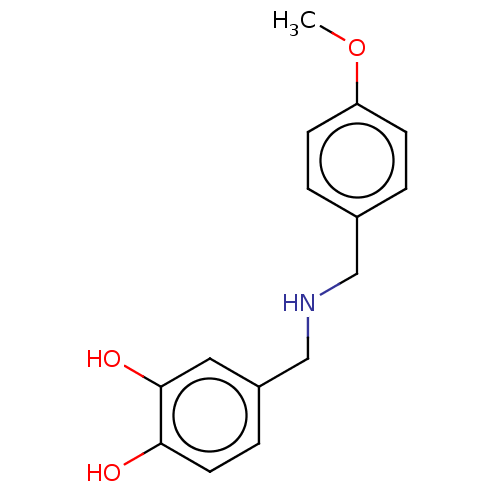 (CHEMBL4781582) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.89E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by spectroscopic analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50553166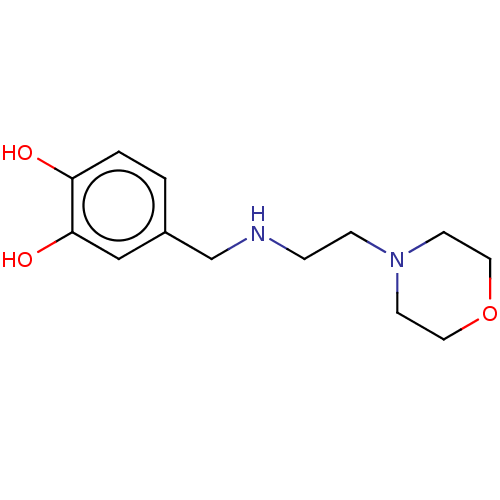 (CHEMBL4761488) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.67E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by spectroscopic analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50553156 (CHEMBL4753152) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.78E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by spectroscopic analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E-selectin (Homo sapiens (Human)) | BDBM50168310 (CHEMBL425373 | [ alpha-Neu5Ac-(2,3)-beta-D-Gal-(1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibition of HL-60 cell adhesion to recombinant human Selectin E | Bioorg Med Chem Lett 15: 3224-8 (2005) Article DOI: 10.1016/j.bmcl.2005.05.004 BindingDB Entry DOI: 10.7270/Q23N22WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50553165 (CHEMBL4754674) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.94E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ARG1 expressed in CHO-K1 cells assessed as reduction in urea level incubated for 24 hrs by colorimetric assay | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50045936 ((E)-4-(3,5-dihydroxystyryl)benzene-1,2-diol | (E)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.18E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ARG1 expressed in CHO-K1 cells assessed as reduction in urea level incubated for 24 hrs by colorimetric assay | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50168310 (CHEMBL425373 | [ alpha-Neu5Ac-(2,3)-beta-D-Gal-(1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibition of HL-60 cell adhesion to recombinant human Selectin P | Bioorg Med Chem Lett 15: 3224-8 (2005) Article DOI: 10.1016/j.bmcl.2005.05.004 BindingDB Entry DOI: 10.7270/Q23N22WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50168307 (3-Methyl-2-[((3R,5R)-1,3,5-trihydroxy-4-(S)-hydrox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibition of HL-60 cell adhesion to recombinant human Selectin P | Bioorg Med Chem Lett 15: 3224-8 (2005) Article DOI: 10.1016/j.bmcl.2005.05.004 BindingDB Entry DOI: 10.7270/Q23N22WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50168303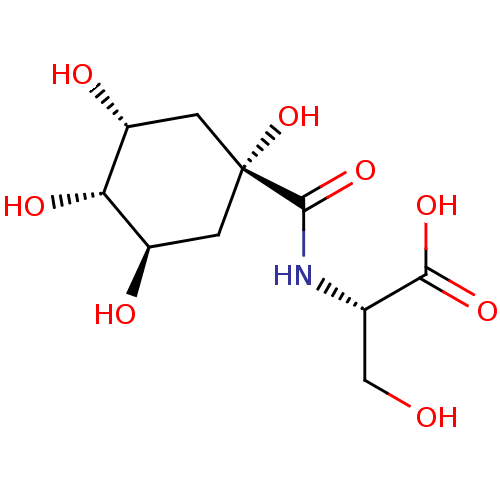 (3-Hydroxy-2-[((3R,5R)-1,3,5-trihydroxy-4-(S)-hydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.95E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibition of HL-60 cell adhesion to recombinant human Selectin P | Bioorg Med Chem Lett 15: 3224-8 (2005) Article DOI: 10.1016/j.bmcl.2005.05.004 BindingDB Entry DOI: 10.7270/Q23N22WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50168306 ((S)-2-[((3R,5R)-1,3,4,5-Tetrahydroxy-cyclohexaneca...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibition of HL-60 cell adhesion to recombinant human Selectin P | Bioorg Med Chem Lett 15: 3224-8 (2005) Article DOI: 10.1016/j.bmcl.2005.05.004 BindingDB Entry DOI: 10.7270/Q23N22WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50168305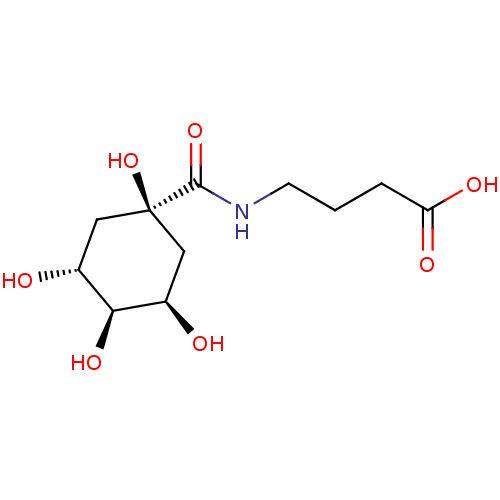 (4-[((3R,5R)-1,3,4,5-Tetrahydroxy-cyclohexanecarbon...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibition of HL-60 cell adhesion to recombinant human Selectin P | Bioorg Med Chem Lett 15: 3224-8 (2005) Article DOI: 10.1016/j.bmcl.2005.05.004 BindingDB Entry DOI: 10.7270/Q23N22WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E-selectin (Homo sapiens (Human)) | BDBM50168308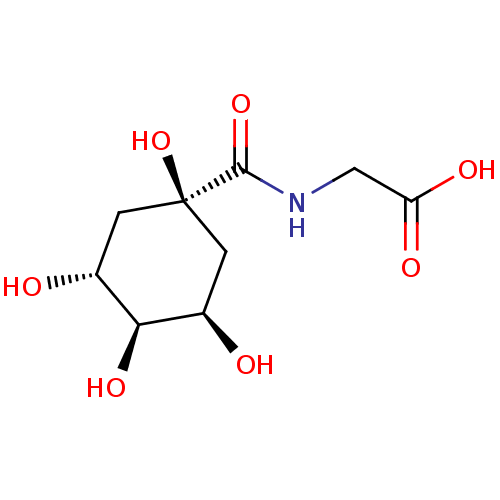 (((3R,5R)-1,3,4,5-Tetrahydroxy-cyclohexanecarbonyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibition of HL-60 cell adhesion to recombinant human Selectin E | Bioorg Med Chem Lett 15: 3224-8 (2005) Article DOI: 10.1016/j.bmcl.2005.05.004 BindingDB Entry DOI: 10.7270/Q23N22WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 86 total ) | Next | Last >> |