 Found 2058 hits with Last Name = 'gobec' and Initial = 's'
Found 2058 hits with Last Name = 'gobec' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholinesterase
(Homo sapiens (Human)) | BDBM50247011

(CHEMBL4080419)Show SMILES Cl.CN(C)CCN(CC1CCCN(C1)C1Cc2ccccc2C1)C(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C30H37N3O.ClH/c1-31(2)16-17-33(30(34)28-14-13-24-9-3-4-10-25(24)18-28)22-23-8-7-15-32(21-23)29-19-26-11-5-6-12-27(26)20-29;/h3-6,9-14,18,23,29H,7-8,15-17,19-22H2,1-2H3;1H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human BChE using butyrylthiocholine iodide as substrate at pH 8 by stopped flow assay |
J Med Chem 61: 119-139 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01086
BindingDB Entry DOI: 10.7270/Q2S75JRJ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50247011

(CHEMBL4080419)Show SMILES Cl.CN(C)CCN(CC1CCCN(C1)C1Cc2ccccc2C1)C(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C30H37N3O.ClH/c1-31(2)16-17-33(30(34)28-14-13-24-9-3-4-10-25(24)18-28)22-23-8-7-15-32(21-23)29-19-26-11-5-6-12-27(26)20-29;/h3-6,9-14,18,23,29H,7-8,15-17,19-22H2,1-2H3;1H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human BChE using butyrylthiocholine iodide as substrate at pH 8 by stopped flow assay |
J Med Chem 61: 119-139 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01086
BindingDB Entry DOI: 10.7270/Q2S75JRJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50579331
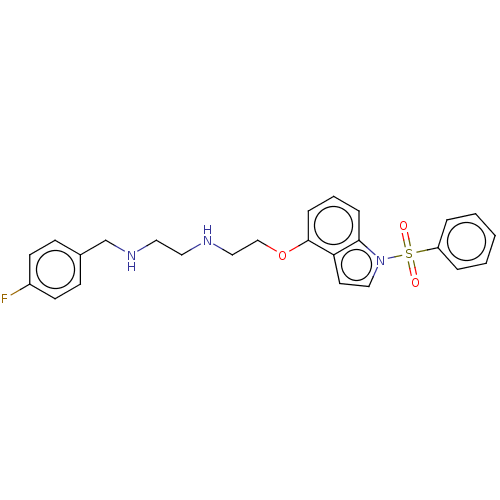
(CHEMBL4852099)Show SMILES Fc1ccc(CNCCNCCOc2cccc3n(ccc23)S(=O)(=O)c2ccccc2)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113783
BindingDB Entry DOI: 10.7270/Q21V5JSC |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8963

(CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...)Show SMILES C(CCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C33H40N4/c1(2-12-22-34-32-24-14-4-8-18-28(24)36-29-19-9-5-15-25(29)32)3-13-23-35-33-26-16-6-10-20-30(26)37-31-21-11-7-17-27(31)33/h4,6,8,10,14,16,18,20H,1-3,5,7,9,11-13,15,17,19,21-23H2,(H,34,36)(H,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human BChE using butyrylthiocholine iodide as substrate at pH 8 by stopped flow assay |
J Med Chem 61: 119-139 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01086
BindingDB Entry DOI: 10.7270/Q2S75JRJ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50604405
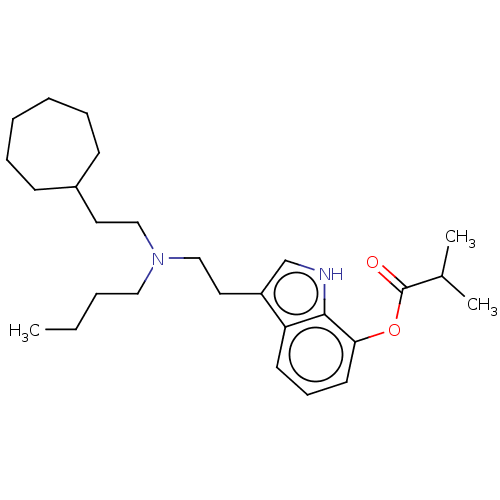
(CHEMBL5186889)Show SMILES CCCCN(CCC1CCCCCC1)CCc1c[nH]c2c(OC(=O)C(C)C)cccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114248
BindingDB Entry DOI: 10.7270/Q2125XRJ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50604405

(CHEMBL5186889)Show SMILES CCCCN(CCC1CCCCCC1)CCc1c[nH]c2c(OC(=O)C(C)C)cccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114248
BindingDB Entry DOI: 10.7270/Q2125XRJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM78940

(METHIOTHEPIN | MLS000859918 | Methiothepin mesylat...)Show InChI InChI=1S/C20H24N2S2/c1-21-9-11-22(12-10-21)18-13-15-5-3-4-6-19(15)24-20-8-7-16(23-2)14-17(18)20/h3-8,14,18H,9-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in CHO-K1 membrane incubated for 60 mins by solid scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113792
BindingDB Entry DOI: 10.7270/Q2514325 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM78940

(METHIOTHEPIN | MLS000859918 | Methiothepin mesylat...)Show InChI InChI=1S/C20H24N2S2/c1-21-9-11-22(12-10-21)18-13-15-5-3-4-6-19(15)24-20-8-7-16(23-2)14-17(18)20/h3-8,14,18H,9-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113783
BindingDB Entry DOI: 10.7270/Q21V5JSC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50574183

(CHEMBL4855991)Show SMILES CC(=O)Nc1ccc(CNCCOc2cccc3n(ccc23)S(=O)(=O)c2ccccc2)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in CHO-K1 membrane incubated for 60 mins by solid scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113792
BindingDB Entry DOI: 10.7270/Q2514325 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50174269

(1-(phenylsulfonyl)-4-(piperazin-1-yl)-1H-indole | ...)Show InChI InChI=1S/C18H19N3O2S/c22-24(23,15-5-2-1-3-6-15)21-12-9-16-17(7-4-8-18(16)21)20-13-10-19-11-14-20/h1-9,12,19H,10-11,13-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human recombinant 5-HT6 receptor expressed in CHOK1 cell membranes measured after 60 mins by scintillation counter |
Eur J Med Chem 124: 63-81 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.016
BindingDB Entry DOI: 10.7270/Q2PZ5BT8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50574192

(CHEMBL4850738)Show SMILES O=S(=O)(c1ccccc1)n1ccc2c(OCCNCc3ccccn3)cccc12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in CHO-K1 membrane incubated for 60 mins by solid scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113792
BindingDB Entry DOI: 10.7270/Q2514325 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50574177

(CHEMBL4871652)Show SMILES O=S(=O)(c1ccccc1)n1ccc2c(OCCNCc3ccc4OCCOc4c3)cccc12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in CHO-K1 membrane incubated for 60 mins by solid scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113792
BindingDB Entry DOI: 10.7270/Q2514325 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50574176

(CHEMBL4866390)Show SMILES COc1ccc(CNCCOc2cccc3n(ccc23)S(=O)(=O)c2ccccc2)cc1OC | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in CHO-K1 membrane incubated for 60 mins by solid scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113792
BindingDB Entry DOI: 10.7270/Q2514325 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50579329

(CHEMBL4863218)Show SMILES Clc1ccc(CNCCNCCOc2cccc3n(ccc23)S(=O)(=O)c2ccccc2)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113783
BindingDB Entry DOI: 10.7270/Q21V5JSC |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50468733
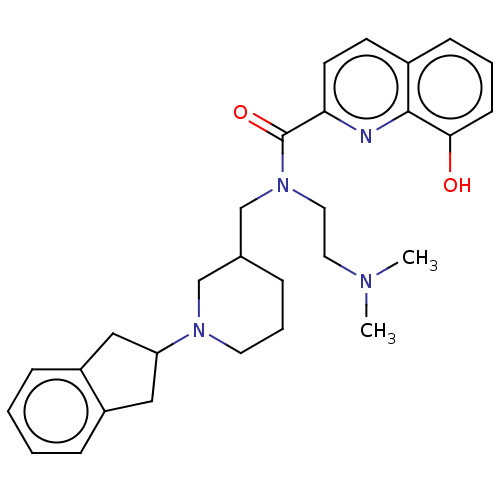
(CHEMBL4294570)Show SMILES CN(C)CCN(CC1CCCN(C1)C1Cc2ccccc2C1)C(=O)c1ccc2cccc(O)c2n1 Show InChI InChI=1S/C29H36N4O2/c1-31(2)15-16-33(29(35)26-13-12-22-10-5-11-27(34)28(22)30-26)20-21-7-6-14-32(19-21)25-17-23-8-3-4-9-24(23)18-25/h3-5,8-13,21,25,34H,6-7,14-20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana
Curated by ChEMBL
| Assay Description
Competitive inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... |
Eur J Med Chem 156: 598-617 (2018)
Article DOI: 10.1016/j.ejmech.2018.07.033
BindingDB Entry DOI: 10.7270/Q27W6FW2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50574175

(CHEMBL4864854)Show SMILES COc1ccc(CNCCOc2cccc3n(ccc23)S(=O)(=O)c2ccccc2)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in CHO-K1 membrane incubated for 60 mins by solid scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113792
BindingDB Entry DOI: 10.7270/Q2514325 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50574191

(CHEMBL178254) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in CHO-K1 membrane incubated for 60 mins by solid scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113792
BindingDB Entry DOI: 10.7270/Q2514325 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50468736
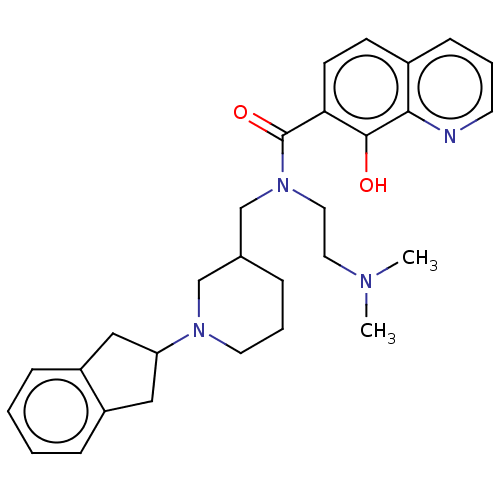
(CHEMBL4283585)Show SMILES CN(C)CCN(CC1CCCN(C1)C1Cc2ccccc2C1)C(=O)c1ccc2cccnc2c1O Show InChI InChI=1S/C29H36N4O2/c1-31(2)15-16-33(29(35)26-12-11-22-10-5-13-30-27(22)28(26)34)20-21-7-6-14-32(19-21)25-17-23-8-3-4-9-24(23)18-25/h3-5,8-13,21,25,34H,6-7,14-20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana
Curated by ChEMBL
| Assay Description
Competitive inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... |
Eur J Med Chem 156: 598-617 (2018)
Article DOI: 10.1016/j.ejmech.2018.07.033
BindingDB Entry DOI: 10.7270/Q27W6FW2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50579335
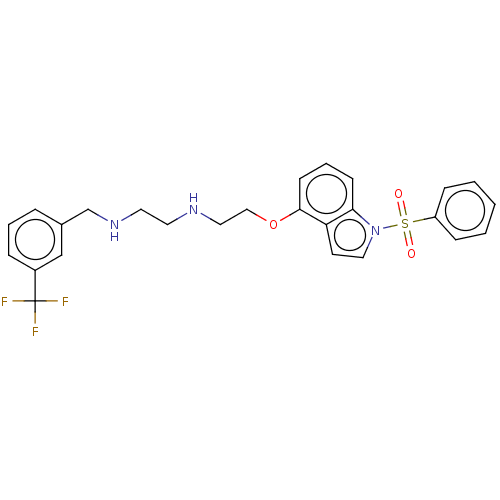
(CHEMBL4854972)Show SMILES FC(F)(F)c1cccc(CNCCNCCOc2cccc3n(ccc23)S(=O)(=O)c2ccccc2)c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113783
BindingDB Entry DOI: 10.7270/Q21V5JSC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50208217
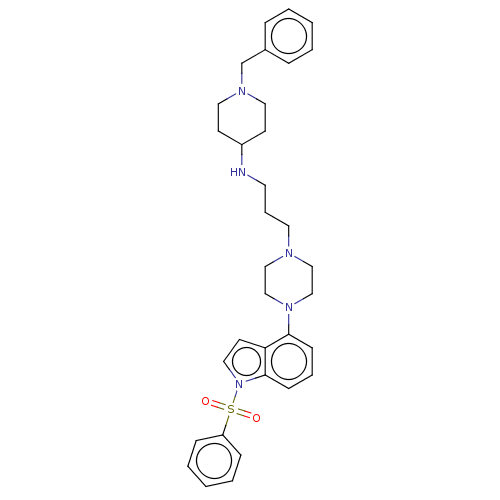
(CHEMBL3884195)Show SMILES O=S(=O)(c1ccccc1)n1ccc2c(cccc12)N1CCN(CCCNC2CCN(Cc3ccccc3)CC2)CC1 Show InChI InChI=1S/C33H41N5O2S/c39-41(40,30-11-5-2-6-12-30)38-22-17-31-32(13-7-14-33(31)38)37-25-23-35(24-26-37)19-8-18-34-29-15-20-36(21-16-29)27-28-9-3-1-4-10-28/h1-7,9-14,17,22,29,34H,8,15-16,18-21,23-27H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human recombinant 5-HT6 receptor expressed in CHOK1 cell membranes measured after 60 mins by scintillation counter |
Eur J Med Chem 124: 63-81 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.016
BindingDB Entry DOI: 10.7270/Q2PZ5BT8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50579332

(CHEMBL4851595)Show SMILES [O-][N+](=O)c1ccc(CNCCNCCOc2cccc3n(ccc23)S(=O)(=O)c2ccccc2)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113783
BindingDB Entry DOI: 10.7270/Q21V5JSC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50579334

(CHEMBL4864206)Show SMILES Cc1cccc(CNCCNCCOc2cccc3n(ccc23)S(=O)(=O)c2ccccc2)c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113783
BindingDB Entry DOI: 10.7270/Q21V5JSC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50579327

(CHEMBL4847532)Show SMILES Clc1cccc(CNCCNCCOc2cccc3n(ccc23)S(=O)(=O)c2ccccc2)c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113783
BindingDB Entry DOI: 10.7270/Q21V5JSC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50208258
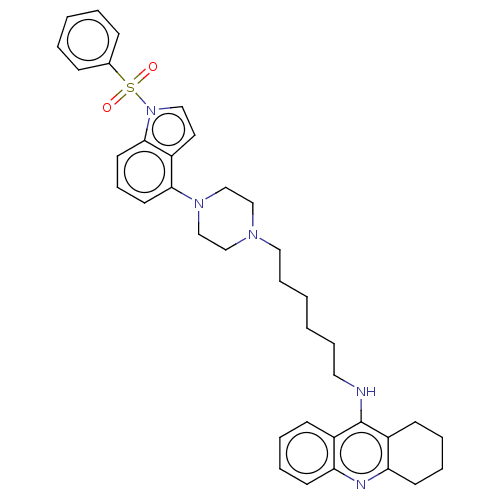
(CHEMBL3885186)Show SMILES O=S(=O)(c1ccccc1)n1ccc2c(cccc12)N1CCN(CCCCCCNc2c3CCCCc3nc3ccccc23)CC1 Show InChI InChI=1S/C37H43N5O2S/c43-45(44,29-13-4-3-5-14-29)42-24-21-32-35(19-12-20-36(32)42)41-27-25-40(26-28-41)23-11-2-1-10-22-38-37-30-15-6-8-17-33(30)39-34-18-9-7-16-31(34)37/h3-6,8,12-15,17,19-21,24H,1-2,7,9-11,16,18,22-23,25-28H2,(H,38,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human recombinant 5-HT6 receptor expressed in CHOK1 cell membranes measured after 60 mins by scintillation counter |
Eur J Med Chem 124: 63-81 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.016
BindingDB Entry DOI: 10.7270/Q2PZ5BT8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50208263
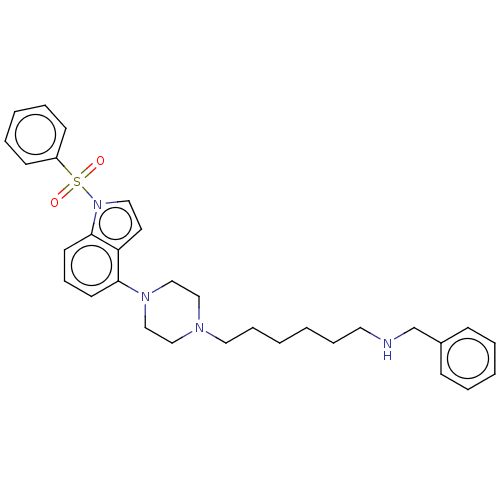
(CHEMBL3884227)Show SMILES O=S(=O)(c1ccccc1)n1ccc2c(cccc12)N1CCN(CCCCCCNCc2ccccc2)CC1 Show InChI InChI=1S/C31H38N4O2S/c36-38(37,28-14-7-4-8-15-28)35-21-18-29-30(16-11-17-31(29)35)34-24-22-33(23-25-34)20-10-2-1-9-19-32-26-27-12-5-3-6-13-27/h3-8,11-18,21,32H,1-2,9-10,19-20,22-26H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human recombinant 5-HT6 receptor expressed in CHOK1 cell membranes measured after 60 mins by scintillation counter |
Eur J Med Chem 124: 63-81 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.016
BindingDB Entry DOI: 10.7270/Q2PZ5BT8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50574162

(CHEMBL368698)Show SMILES O=S(=O)(c1ccccc1)n1ccc2c(OCCNCc3ccccc3)cccc12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in CHO-K1 membrane incubated for 60 mins by solid scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113792
BindingDB Entry DOI: 10.7270/Q2514325 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50574187

(CHEMBL4873700) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in CHO-K1 membrane incubated for 60 mins by solid scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113792
BindingDB Entry DOI: 10.7270/Q2514325 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50027375

(CHEMBL3338394)Show SMILES COCCN(C[C@@H]1CCCN(C1)C1Cc2ccccc2C1)C(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C29H34N2O2/c1-33-16-15-31(29(32)27-13-12-23-8-2-3-9-24(23)17-27)21-22-7-6-14-30(20-22)28-18-25-10-4-5-11-26(25)19-28/h2-5,8-13,17,22,28H,6-7,14-16,18-21H2,1H3/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BChE at 50 nM by stopped flow apparatus method |
J Med Chem 57: 8167-79 (2014)
Article DOI: 10.1021/jm501195e
BindingDB Entry DOI: 10.7270/Q22V2HQ7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50579328

(CHEMBL4878383)Show SMILES Clc1ccccc1CNCCNCCOc1cccc2n(ccc12)S(=O)(=O)c1ccccc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113783
BindingDB Entry DOI: 10.7270/Q21V5JSC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50574193
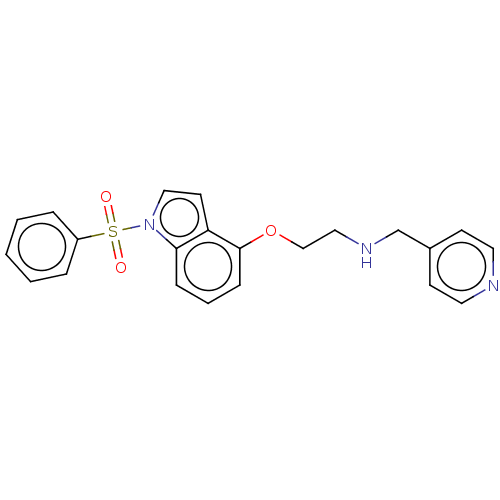
(CHEMBL4863037)Show SMILES O=S(=O)(c1ccccc1)n1ccc2c(OCCNCc3ccncc3)cccc12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in CHO-K1 membrane incubated for 60 mins by solid scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113792
BindingDB Entry DOI: 10.7270/Q2514325 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50579323
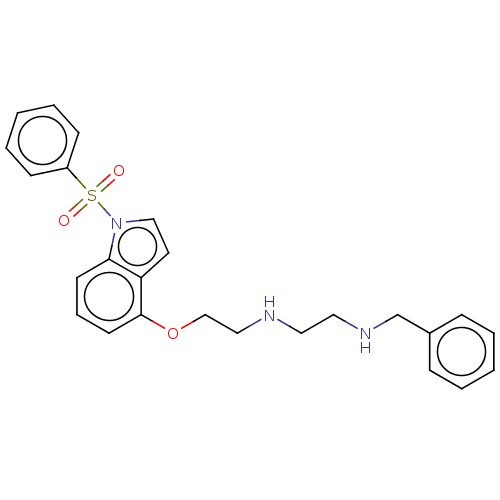
(CHEMBL4878361)Show SMILES O=S(=O)(c1ccccc1)n1ccc2c(OCCNCCNCc3ccccc3)cccc12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113783
BindingDB Entry DOI: 10.7270/Q21V5JSC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50574178

(CHEMBL4862752)Show SMILES CCOc1cccc(CNCCOc2cccc3n(ccc23)S(=O)(=O)c2ccccc2)c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in CHO-K1 membrane incubated for 60 mins by solid scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113792
BindingDB Entry DOI: 10.7270/Q2514325 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50574195

(CHEMBL4860124)Show SMILES O=S(=O)(N1CCc2c1cccc2OCCNCc1ccccc1)c1ccccc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in CHO-K1 membrane incubated for 60 mins by solid scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113792
BindingDB Entry DOI: 10.7270/Q2514325 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50604404
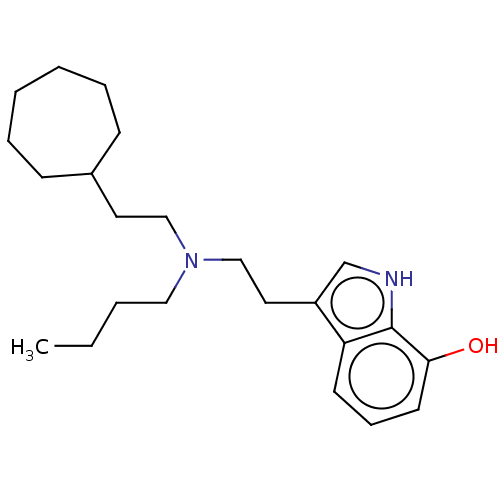
(CHEMBL5186857) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114248
BindingDB Entry DOI: 10.7270/Q2125XRJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50574194

(CHEMBL4862289)Show SMILES Cc1ccc(CNCCOc2cccc3n(ccc23)S(=O)(=O)c2ccccc2)o1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in CHO-K1 membrane incubated for 60 mins by solid scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113792
BindingDB Entry DOI: 10.7270/Q2514325 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50579320
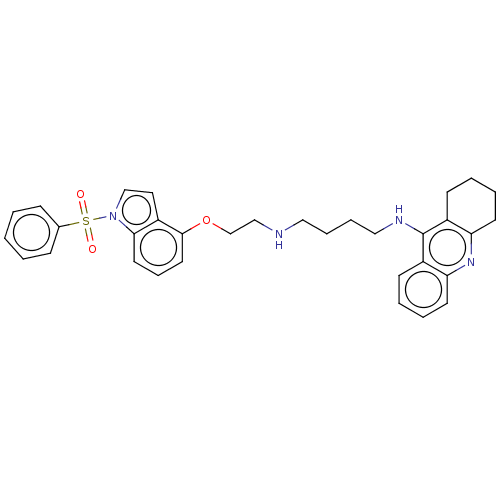
(CHEMBL4874513)Show SMILES O=S(=O)(c1ccccc1)n1ccc2c(OCCNCCCCNc3c4CCCCc4nc4ccccc34)cccc12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113783
BindingDB Entry DOI: 10.7270/Q21V5JSC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50574173
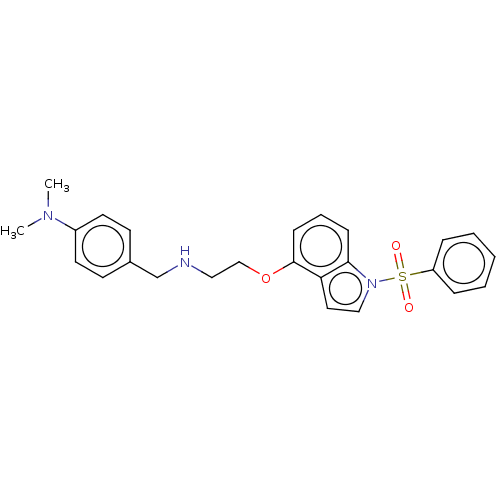
(CHEMBL4871678)Show SMILES CN(C)c1ccc(CNCCOc2cccc3n(ccc23)S(=O)(=O)c2ccccc2)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in CHO-K1 membrane incubated for 60 mins by solid scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113792
BindingDB Entry DOI: 10.7270/Q2514325 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50579333

(CHEMBL4868597)Show SMILES COc1ccc(CNCCNCCOc2cccc3n(ccc23)S(=O)(=O)c2ccccc2)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113783
BindingDB Entry DOI: 10.7270/Q21V5JSC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50574174
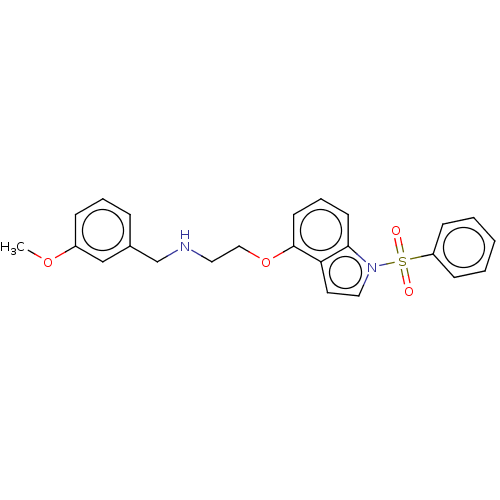
(CHEMBL4871357)Show SMILES COc1cccc(CNCCOc2cccc3n(ccc23)S(=O)(=O)c2ccccc2)c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in CHO-K1 membrane incubated for 60 mins by solid scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113792
BindingDB Entry DOI: 10.7270/Q2514325 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50208254
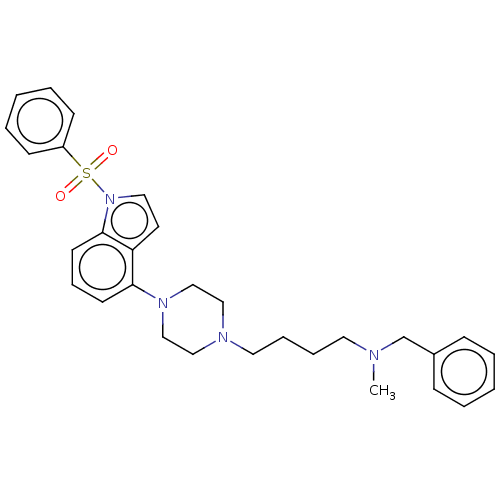
(CHEMBL3883921)Show SMILES CN(CCCCN1CCN(CC1)c1cccc2n(ccc12)S(=O)(=O)c1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C30H36N4O2S/c1-31(25-26-11-4-2-5-12-26)18-8-9-19-32-21-23-33(24-22-32)29-15-10-16-30-28(29)17-20-34(30)37(35,36)27-13-6-3-7-14-27/h2-7,10-17,20H,8-9,18-19,21-25H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human recombinant 5-HT6 receptor expressed in CHOK1 cell membranes measured after 60 mins by scintillation counter |
Eur J Med Chem 124: 63-81 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.016
BindingDB Entry DOI: 10.7270/Q2PZ5BT8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50574169

(CHEMBL4849702)Show SMILES FC(F)(F)c1cccc(CNCCOc2cccc3n(ccc23)S(=O)(=O)c2ccccc2)c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in CHO-K1 membrane incubated for 60 mins by solid scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113792
BindingDB Entry DOI: 10.7270/Q2514325 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50219490
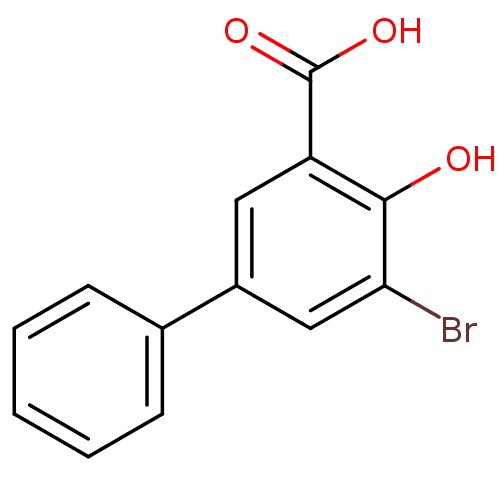
(3-Bromo-5-phenylsalicylc acid | 5-bromo-4-hydroxyb...)Show InChI InChI=1S/C13H9BrO3/c14-11-7-9(8-4-2-1-3-5-8)6-10(12(11)15)13(16)17/h1-7,15H,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AKR1C1 assessed as 1-acenaphthenol oxidation at 400 uM by spectrophotometry |
J Med Chem 55: 7417-24 (2012)
Article DOI: 10.1021/jm300841n
BindingDB Entry DOI: 10.7270/Q2125TR9 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50574185
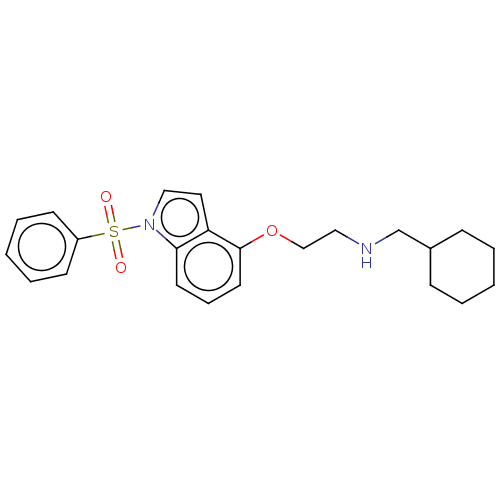
(CHEMBL4854460)Show SMILES O=S(=O)(c1ccccc1)n1ccc2c(OCCNCC3CCCCC3)cccc12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in CHO-K1 membrane incubated for 60 mins by solid scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113792
BindingDB Entry DOI: 10.7270/Q2514325 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50574166

(CHEMBL4849917)Show SMILES Clc1cccc(CNCCOc2cccc3n(ccc23)S(=O)(=O)c2ccccc2)c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in CHO-K1 membrane incubated for 60 mins by solid scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113792
BindingDB Entry DOI: 10.7270/Q2514325 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50574201

(CHEMBL4861756)Show SMILES CC(C)(C)OC(=O)N1CCCC(CNCCOc2cccc3n(ccc23)S(=O)(=O)c2cccc3ccccc23)C1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in CHO-K1 membrane incubated for 60 mins by solid scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113792
BindingDB Entry DOI: 10.7270/Q2514325 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50574172
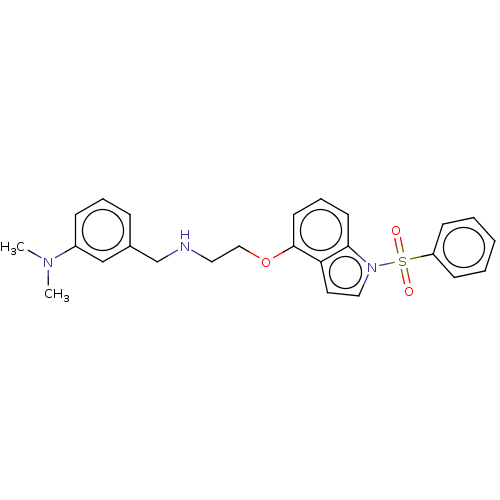
(CHEMBL4850764)Show SMILES CN(C)c1cccc(CNCCOc2cccc3n(ccc23)S(=O)(=O)c2ccccc2)c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in CHO-K1 membrane incubated for 60 mins by solid scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113792
BindingDB Entry DOI: 10.7270/Q2514325 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50208256
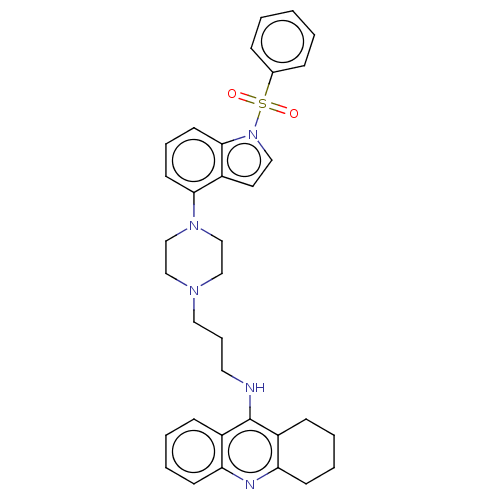
(CHEMBL3884618)Show SMILES O=S(=O)(c1ccccc1)n1ccc2c(cccc12)N1CCN(CCCNc2c3CCCCc3nc3ccccc23)CC1 Show InChI InChI=1S/C34H37N5O2S/c40-42(41,26-10-2-1-3-11-26)39-21-18-29-32(16-8-17-33(29)39)38-24-22-37(23-25-38)20-9-19-35-34-27-12-4-6-14-30(27)36-31-15-7-5-13-28(31)34/h1-4,6,8,10-12,14,16-18,21H,5,7,9,13,15,19-20,22-25H2,(H,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human recombinant 5-HT6 receptor expressed in CHOK1 cell membranes measured after 60 mins by scintillation counter |
Eur J Med Chem 124: 63-81 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.016
BindingDB Entry DOI: 10.7270/Q2PZ5BT8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50574170

(CHEMBL4863101)Show SMILES Fc1ccc(CNCCOc2cccc3n(ccc23)S(=O)(=O)c2ccccc2)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in CHO-K1 membrane incubated for 60 mins by solid scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113792
BindingDB Entry DOI: 10.7270/Q2514325 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50574180

(CHEMBL4867606)Show SMILES CC(=O)Oc1cccc(CNCCOc2cccc3n(ccc23)S(=O)(=O)c2ccccc2)c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in CHO-K1 membrane incubated for 60 mins by solid scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113792
BindingDB Entry DOI: 10.7270/Q2514325 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50604404

(CHEMBL5186857) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114248
BindingDB Entry DOI: 10.7270/Q2125XRJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data