

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50185473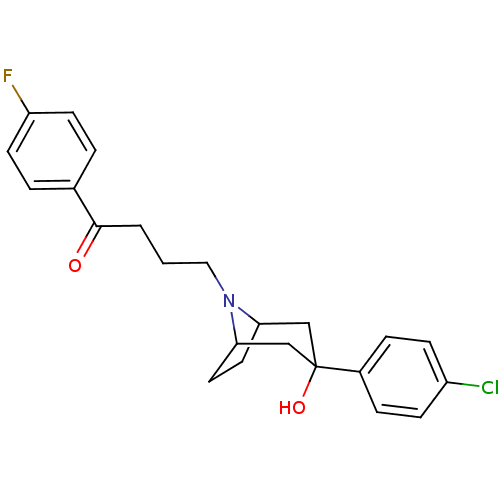 (4-(3-(4-chlorophenyl)-3-hydroxy-8-aza-bicyclo[3.2....) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University Curated by ChEMBL | Assay Description Binding affinity to human cloned dopamine D2 receptor by radioligand binding assay | Bioorg Med Chem Lett 16: 3219-23 (2006) Article DOI: 10.1016/j.bmcl.2006.03.057 BindingDB Entry DOI: 10.7270/Q2571BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50185474 ((+)-4-(3-(4-chlorophenyl)-3-hydroxypyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University Curated by ChEMBL | Assay Description Binding affinity to human cloned dopamine D4.4 receptor by radioligand binding assay | Bioorg Med Chem Lett 16: 3219-23 (2006) Article DOI: 10.1016/j.bmcl.2006.03.057 BindingDB Entry DOI: 10.7270/Q2571BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50185474 ((+)-4-(3-(4-chlorophenyl)-3-hydroxypyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University Curated by ChEMBL | Assay Description Binding affinity to human cloned dopamine D4.4 receptor by radioligand binding assay | Bioorg Med Chem Lett 16: 3219-23 (2006) Article DOI: 10.1016/j.bmcl.2006.03.057 BindingDB Entry DOI: 10.7270/Q2571BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50185473 (4-(3-(4-chlorophenyl)-3-hydroxy-8-aza-bicyclo[3.2....) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University Curated by ChEMBL | Assay Description Binding affinity to human cloned dopamine D3 receptor by radioligand binding assay | Bioorg Med Chem Lett 16: 3219-23 (2006) Article DOI: 10.1016/j.bmcl.2006.03.057 BindingDB Entry DOI: 10.7270/Q2571BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50185473 (4-(3-(4-chlorophenyl)-3-hydroxy-8-aza-bicyclo[3.2....) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University Curated by ChEMBL | Assay Description Binding affinity to human cloned dopamine D4.4 receptor by radioligand binding assay | Bioorg Med Chem Lett 16: 3219-23 (2006) Article DOI: 10.1016/j.bmcl.2006.03.057 BindingDB Entry DOI: 10.7270/Q2571BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50185473 (4-(3-(4-chlorophenyl)-3-hydroxy-8-aza-bicyclo[3.2....) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University Curated by ChEMBL | Assay Description Binding affinity to human cloned 5HT1A receptor by radioligand binding assay | Bioorg Med Chem Lett 16: 3219-23 (2006) Article DOI: 10.1016/j.bmcl.2006.03.057 BindingDB Entry DOI: 10.7270/Q2571BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50185473 (4-(3-(4-chlorophenyl)-3-hydroxy-8-aza-bicyclo[3.2....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University Curated by ChEMBL | Assay Description Binding affinity to human cloned 5HT2A receptor by radioligand binding assay | Bioorg Med Chem Lett 16: 3219-23 (2006) Article DOI: 10.1016/j.bmcl.2006.03.057 BindingDB Entry DOI: 10.7270/Q2571BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50185473 (4-(3-(4-chlorophenyl)-3-hydroxy-8-aza-bicyclo[3.2....) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University Curated by ChEMBL | Assay Description Binding affinity to human cloned adrenergic alpha-1A receptor by radioligand binding assay | Bioorg Med Chem Lett 16: 3219-23 (2006) Article DOI: 10.1016/j.bmcl.2006.03.057 BindingDB Entry DOI: 10.7270/Q2571BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM50185473 (4-(3-(4-chlorophenyl)-3-hydroxy-8-aza-bicyclo[3.2....) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University Curated by ChEMBL | Assay Description Binding affinity to human cloned adrenergic alpha-1B receptor by radioligand binding assay | Bioorg Med Chem Lett 16: 3219-23 (2006) Article DOI: 10.1016/j.bmcl.2006.03.057 BindingDB Entry DOI: 10.7270/Q2571BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50185474 ((+)-4-(3-(4-chlorophenyl)-3-hydroxypyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University Curated by ChEMBL | Assay Description Binding affinity to human cloned dopamine D2 receptor by radioligand binding assay | Bioorg Med Chem Lett 16: 3219-23 (2006) Article DOI: 10.1016/j.bmcl.2006.03.057 BindingDB Entry DOI: 10.7270/Q2571BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50185474 ((+)-4-(3-(4-chlorophenyl)-3-hydroxypyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University Curated by ChEMBL | Assay Description Binding affinity to human cloned dopamine D2 receptor by radioligand binding assay | Bioorg Med Chem Lett 16: 3219-23 (2006) Article DOI: 10.1016/j.bmcl.2006.03.057 BindingDB Entry DOI: 10.7270/Q2571BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50185474 ((+)-4-(3-(4-chlorophenyl)-3-hydroxypyrrolidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University Curated by ChEMBL | Assay Description Binding affinity to human cloned 5HT2A receptor by radioligand binding assay | Bioorg Med Chem Lett 16: 3219-23 (2006) Article DOI: 10.1016/j.bmcl.2006.03.057 BindingDB Entry DOI: 10.7270/Q2571BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50185474 ((+)-4-(3-(4-chlorophenyl)-3-hydroxypyrrolidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University Curated by ChEMBL | Assay Description Binding affinity to human cloned 5HT2A receptor by radioligand binding assay | Bioorg Med Chem Lett 16: 3219-23 (2006) Article DOI: 10.1016/j.bmcl.2006.03.057 BindingDB Entry DOI: 10.7270/Q2571BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50185474 ((+)-4-(3-(4-chlorophenyl)-3-hydroxypyrrolidin-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University Curated by ChEMBL | Assay Description Binding affinity to human cloned adrenergic alpha-2C receptor by radioligand binding assay | Bioorg Med Chem Lett 16: 3219-23 (2006) Article DOI: 10.1016/j.bmcl.2006.03.057 BindingDB Entry DOI: 10.7270/Q2571BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50185474 ((+)-4-(3-(4-chlorophenyl)-3-hydroxypyrrolidin-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University Curated by ChEMBL | Assay Description Binding affinity to human cloned adrenergic alpha-2C receptor by radioligand binding assay | Bioorg Med Chem Lett 16: 3219-23 (2006) Article DOI: 10.1016/j.bmcl.2006.03.057 BindingDB Entry DOI: 10.7270/Q2571BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM50185474 ((+)-4-(3-(4-chlorophenyl)-3-hydroxypyrrolidin-1-yl...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University Curated by ChEMBL | Assay Description Binding affinity to human cloned adrenergic alpha-1B receptor by radioligand binding assay | Bioorg Med Chem Lett 16: 3219-23 (2006) Article DOI: 10.1016/j.bmcl.2006.03.057 BindingDB Entry DOI: 10.7270/Q2571BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM50185474 ((+)-4-(3-(4-chlorophenyl)-3-hydroxypyrrolidin-1-yl...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University Curated by ChEMBL | Assay Description Binding affinity to human cloned adrenergic alpha-1B receptor by radioligand binding assay | Bioorg Med Chem Lett 16: 3219-23 (2006) Article DOI: 10.1016/j.bmcl.2006.03.057 BindingDB Entry DOI: 10.7270/Q2571BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50185474 ((+)-4-(3-(4-chlorophenyl)-3-hydroxypyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University Curated by ChEMBL | Assay Description Binding affinity to human cloned adrenergic alpha-1A receptor by radioligand binding assay | Bioorg Med Chem Lett 16: 3219-23 (2006) Article DOI: 10.1016/j.bmcl.2006.03.057 BindingDB Entry DOI: 10.7270/Q2571BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50185474 ((+)-4-(3-(4-chlorophenyl)-3-hydroxypyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University Curated by ChEMBL | Assay Description Binding affinity to human cloned adrenergic alpha-1A receptor by radioligand binding assay | Bioorg Med Chem Lett 16: 3219-23 (2006) Article DOI: 10.1016/j.bmcl.2006.03.057 BindingDB Entry DOI: 10.7270/Q2571BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50185473 (4-(3-(4-chlorophenyl)-3-hydroxy-8-aza-bicyclo[3.2....) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University Curated by ChEMBL | Assay Description Binding affinity to human cloned adrenergic alpha-2C receptor by radioligand binding assay | Bioorg Med Chem Lett 16: 3219-23 (2006) Article DOI: 10.1016/j.bmcl.2006.03.057 BindingDB Entry DOI: 10.7270/Q2571BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (Homo sapiens (Human)) | BDBM50185473 (4-(3-(4-chlorophenyl)-3-hydroxy-8-aza-bicyclo[3.2....) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 516 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University Curated by ChEMBL | Assay Description Binding affinity to human cloned adrenergic alpha-2B receptor by radioligand binding assay | Bioorg Med Chem Lett 16: 3219-23 (2006) Article DOI: 10.1016/j.bmcl.2006.03.057 BindingDB Entry DOI: 10.7270/Q2571BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50185474 ((+)-4-(3-(4-chlorophenyl)-3-hydroxypyrrolidin-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 722 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University Curated by ChEMBL | Assay Description Binding affinity to human cloned 5HT1A receptor by radioligand binding assay | Bioorg Med Chem Lett 16: 3219-23 (2006) Article DOI: 10.1016/j.bmcl.2006.03.057 BindingDB Entry DOI: 10.7270/Q2571BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50185474 ((+)-4-(3-(4-chlorophenyl)-3-hydroxypyrrolidin-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 722 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University Curated by ChEMBL | Assay Description Binding affinity to human cloned 5HT1A receptor by radioligand binding assay | Bioorg Med Chem Lett 16: 3219-23 (2006) Article DOI: 10.1016/j.bmcl.2006.03.057 BindingDB Entry DOI: 10.7270/Q2571BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50185473 (4-(3-(4-chlorophenyl)-3-hydroxy-8-aza-bicyclo[3.2....) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 872 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University Curated by ChEMBL | Assay Description Binding affinity to human cloned 5HT2C receptor by radioligand binding assay | Bioorg Med Chem Lett 16: 3219-23 (2006) Article DOI: 10.1016/j.bmcl.2006.03.057 BindingDB Entry DOI: 10.7270/Q2571BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50185474 ((+)-4-(3-(4-chlorophenyl)-3-hydroxypyrrolidin-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University Curated by ChEMBL | Assay Description Binding affinity to human cloned dopamine D3 receptor by radioligand binding assay | Bioorg Med Chem Lett 16: 3219-23 (2006) Article DOI: 10.1016/j.bmcl.2006.03.057 BindingDB Entry DOI: 10.7270/Q2571BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50185474 ((+)-4-(3-(4-chlorophenyl)-3-hydroxypyrrolidin-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University Curated by ChEMBL | Assay Description Binding affinity to human cloned dopamine D3 receptor by radioligand binding assay | Bioorg Med Chem Lett 16: 3219-23 (2006) Article DOI: 10.1016/j.bmcl.2006.03.057 BindingDB Entry DOI: 10.7270/Q2571BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50185474 ((+)-4-(3-(4-chlorophenyl)-3-hydroxypyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University Curated by ChEMBL | Assay Description Binding affinity to human cloned histamine H1 receptor by radioligand binding assay | Bioorg Med Chem Lett 16: 3219-23 (2006) Article DOI: 10.1016/j.bmcl.2006.03.057 BindingDB Entry DOI: 10.7270/Q2571BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50185474 ((+)-4-(3-(4-chlorophenyl)-3-hydroxypyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University Curated by ChEMBL | Assay Description Binding affinity to human cloned histamine H1 receptor by radioligand binding assay | Bioorg Med Chem Lett 16: 3219-23 (2006) Article DOI: 10.1016/j.bmcl.2006.03.057 BindingDB Entry DOI: 10.7270/Q2571BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50185474 ((+)-4-(3-(4-chlorophenyl)-3-hydroxypyrrolidin-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University Curated by ChEMBL | Assay Description Binding affinity to human cloned adrenergic alpha-2A receptor by radioligand binding assay | Bioorg Med Chem Lett 16: 3219-23 (2006) Article DOI: 10.1016/j.bmcl.2006.03.057 BindingDB Entry DOI: 10.7270/Q2571BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50185474 ((+)-4-(3-(4-chlorophenyl)-3-hydroxypyrrolidin-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University Curated by ChEMBL | Assay Description Binding affinity to human cloned adrenergic alpha-2A receptor by radioligand binding assay | Bioorg Med Chem Lett 16: 3219-23 (2006) Article DOI: 10.1016/j.bmcl.2006.03.057 BindingDB Entry DOI: 10.7270/Q2571BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50185473 (4-(3-(4-chlorophenyl)-3-hydroxy-8-aza-bicyclo[3.2....) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University Curated by ChEMBL | Assay Description Binding affinity to human cloned adrenergic alpha-2A receptor by radioligand binding assay | Bioorg Med Chem Lett 16: 3219-23 (2006) Article DOI: 10.1016/j.bmcl.2006.03.057 BindingDB Entry DOI: 10.7270/Q2571BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50185474 ((+)-4-(3-(4-chlorophenyl)-3-hydroxypyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University Curated by ChEMBL | Assay Description Binding affinity to human cloned 5HT2C receptor by radioligand binding assay | Bioorg Med Chem Lett 16: 3219-23 (2006) Article DOI: 10.1016/j.bmcl.2006.03.057 BindingDB Entry DOI: 10.7270/Q2571BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50185474 ((+)-4-(3-(4-chlorophenyl)-3-hydroxypyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University Curated by ChEMBL | Assay Description Binding affinity to human cloned 5HT2C receptor by radioligand binding assay | Bioorg Med Chem Lett 16: 3219-23 (2006) Article DOI: 10.1016/j.bmcl.2006.03.057 BindingDB Entry DOI: 10.7270/Q2571BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (Homo sapiens (Human)) | BDBM50185474 ((+)-4-(3-(4-chlorophenyl)-3-hydroxypyrrolidin-1-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University Curated by ChEMBL | Assay Description Binding affinity to human cloned adrenergic alpha-2B receptor by radioligand binding assay | Bioorg Med Chem Lett 16: 3219-23 (2006) Article DOI: 10.1016/j.bmcl.2006.03.057 BindingDB Entry DOI: 10.7270/Q2571BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50185473 (4-(3-(4-chlorophenyl)-3-hydroxy-8-aza-bicyclo[3.2....) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University Curated by ChEMBL | Assay Description Binding affinity to human cloned histamine H1 receptor by radioligand binding assay | Bioorg Med Chem Lett 16: 3219-23 (2006) Article DOI: 10.1016/j.bmcl.2006.03.057 BindingDB Entry DOI: 10.7270/Q2571BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas [1-169,G12C,C118A] (Homo sapiens (Human)) | BDBM544213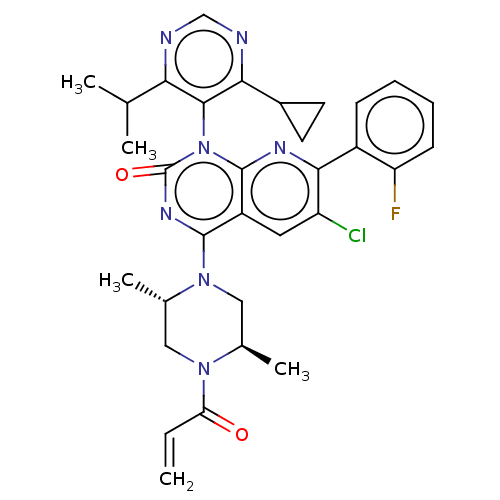 (6-Chloro-1-(4- cyclopropyl-6- isopropyl-pyrimidin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Purified GDP-bound KRAS protein (aa 1-169), containing both G12C and C118A amino acid substitutions and an N-terminal His-tag, was pre-incubated in a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2959MS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas [1-169,G12C,C118A] (Homo sapiens (Human)) | BDBM544157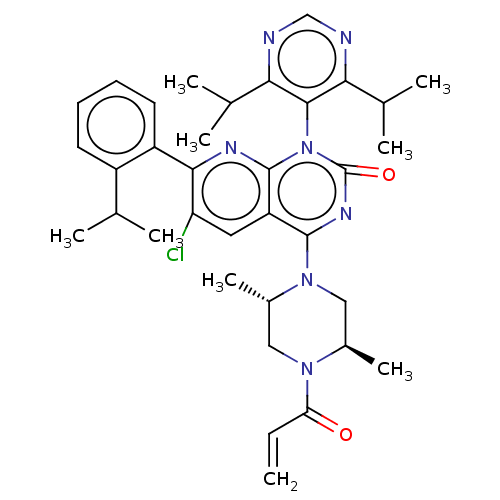 (6-Chloro-1-(4,6- diisopropylpyrimidin- 5-yl)-4-[(2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Purified GDP-bound KRAS protein (aa 1-169), containing both G12C and C118A amino acid substitutions and an N-terminal His-tag, was pre-incubated in a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2959MS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas [1-169,G12C,C118A] (Homo sapiens (Human)) | BDBM544213 (6-Chloro-1-(4- cyclopropyl-6- isopropyl-pyrimidin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Purified GDP-bound KRAS protein (aa 1-169), containing both G12C and C118A amino acid substitutions and an N-terminal His-tag, was pre-incubated in a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2959MS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas [1-169,G12C,C118A] (Homo sapiens (Human)) | BDBM544283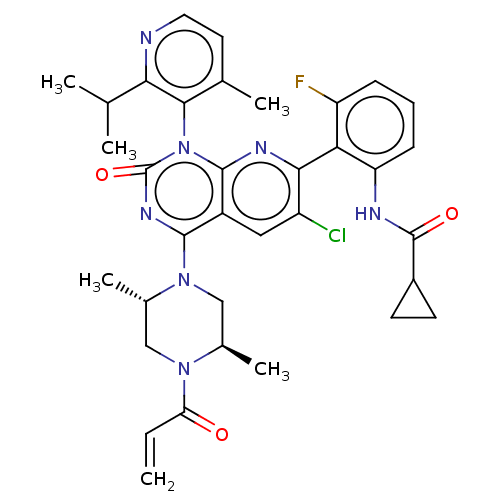 ((M)-N-[2-[6- Chloro-4- [(2S,5R)-2,5- dimethyl-4- p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Purified GDP-bound KRAS protein (aa 1-169), containing both G12C and C118A amino acid substitutions and an N-terminal His-tag, was pre-incubated in a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2959MS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas [1-169,G12C,C118A] (Homo sapiens (Human)) | BDBM544106 ((M)-6-Chloro- 7-(2,4- difluorophenyl)- 4-[(2S,5R)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Purified GDP-bound KRAS protein (aa 1-169), containing both G12C and C118A amino acid substitutions and an N-terminal His-tag, was pre-incubated in a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2959MS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas [1-169,G12C,C118A] (Homo sapiens (Human)) | BDBM544212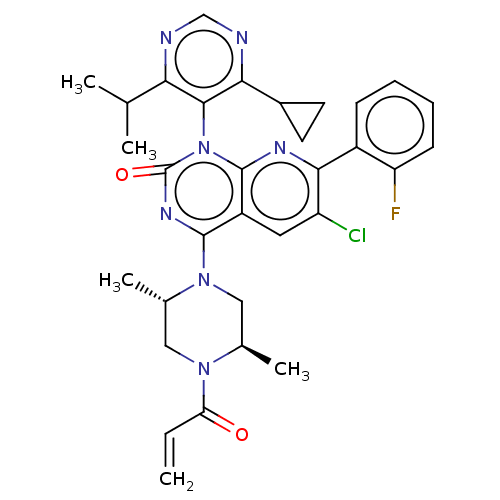 (US11285156, Ex.# 108-1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Purified GDP-bound KRAS protein (aa 1-169), containing both G12C and C118A amino acid substitutions and an N-terminal His-tag, was pre-incubated in a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2959MS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas [1-169,G12C,C118A] (Homo sapiens (Human)) | BDBM544107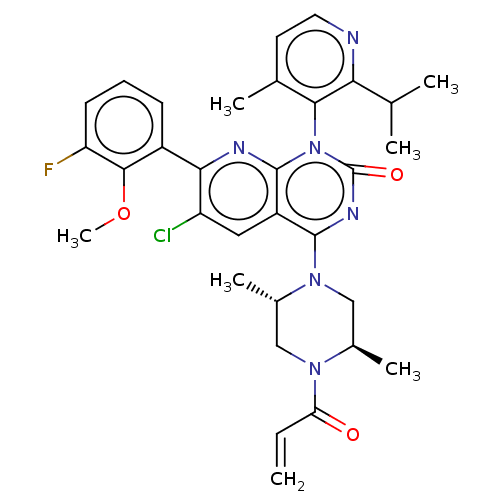 ((M)-6-Chloro- 4-[(2S,5R)-2,5- dimethyl-4- prop-2-e...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Purified GDP-bound KRAS protein (aa 1-169), containing both G12C and C118A amino acid substitutions and an N-terminal His-tag, was pre-incubated in a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2959MS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas [1-169,G12C,C118A] (Homo sapiens (Human)) | BDBM544382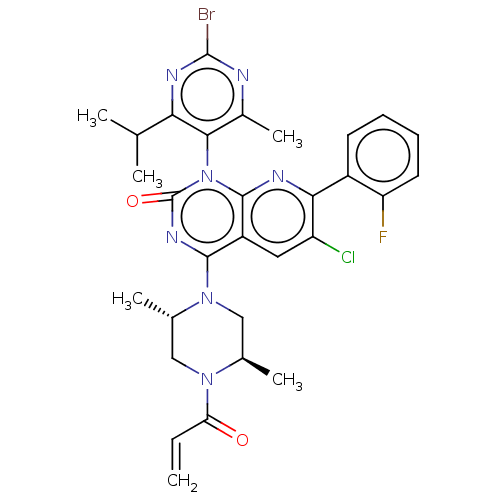 (4-((2S,5R)-4-Acryloyl-2,5-dimethylpiperazin-1-yl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Purified GDP-bound KRAS protein (aa 1-169), containing both G12C and C118A amino acid substitutions and an N-terminal His-tag, was pre-incubated in a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2959MS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas [1-169,G12D] () | BDBM550333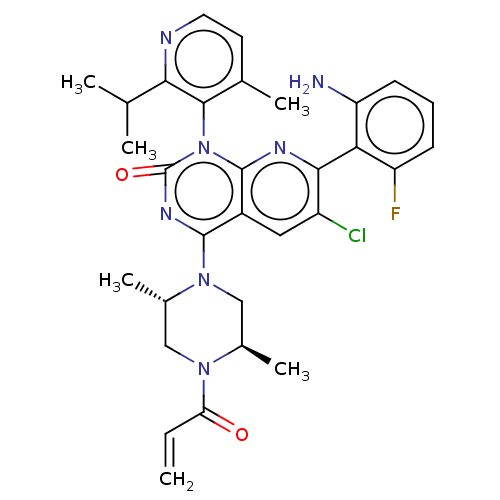 (4-((2S,5R,M)-4-Acryloyl-2,5-dimethylpiperazin-1-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following assay conditions were employed: Coupled Nucleotide Exchange Assay: Purified GDP-bound KRAS protein (aa 1-169), containing both G12C and... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CR5XJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas [1-169,G12C,C118A] (Homo sapiens (Human)) | BDBM544076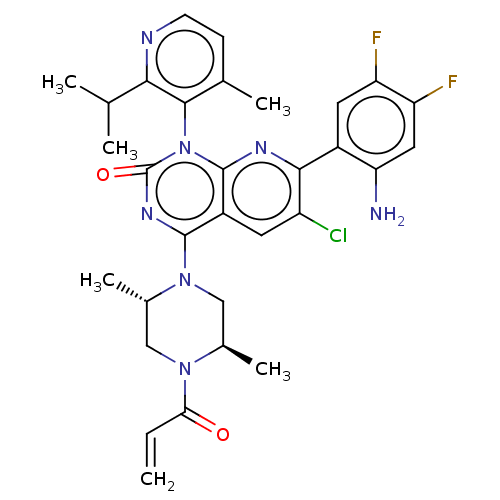 ((M)-7-(2- Amino-4,5- difluoro- phenyl)-6- chloro-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Purified GDP-bound KRAS protein (aa 1-169), containing both G12C and C118A amino acid substitutions and an N-terminal His-tag, was pre-incubated in a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2959MS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas [1-169,G12D] () | BDBM550345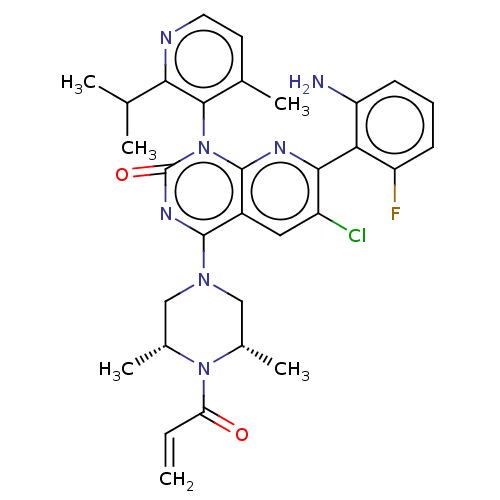 ((M)-4-(4-Acryloyl-cis-3,5-dimethylpiperazin-1-yl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following assay conditions were employed: Coupled Nucleotide Exchange Assay: Purified GDP-bound KRAS protein (aa 1-169), containing both G12C and... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CR5XJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas [1-169,G12C,C118A] (Homo sapiens (Human)) | BDBM544428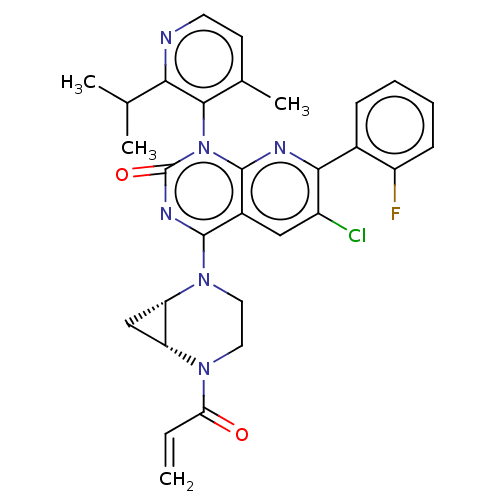 (US11285156, Ex.# 222-2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Purified GDP-bound KRAS protein (aa 1-169), containing both G12C and C118A amino acid substitutions and an N-terminal His-tag, was pre-incubated in a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2959MS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas [1-169,G12C,C118A] (Homo sapiens (Human)) | BDBM544046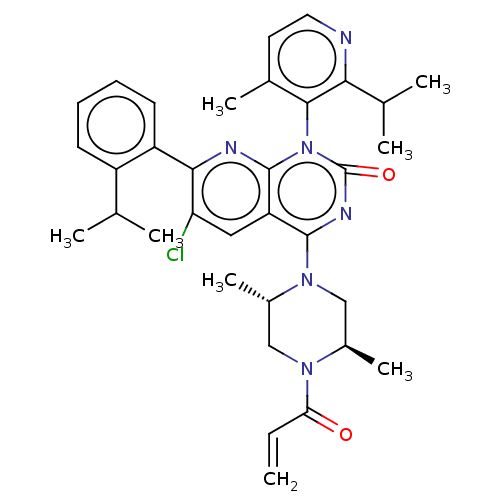 (US11285156, Ex.# 92-1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Purified GDP-bound KRAS protein (aa 1-169), containing both G12C and C118A amino acid substitutions and an N-terminal His-tag, was pre-incubated in a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2959MS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas [1-169,G12D] () | BDBM550344 ((M)-4-(4-Acryloyl-cis-3,5-dimethylpiperazin-1-yl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following assay conditions were employed: Coupled Nucleotide Exchange Assay: Purified GDP-bound KRAS protein (aa 1-169), containing both G12C and... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CR5XJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas [1-169,G12C,C118A] (Homo sapiens (Human)) | BDBM544057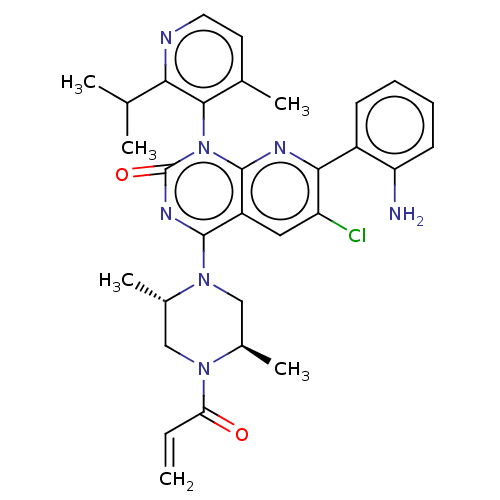 ((M)-7-(2- Aminophenyl)- 6-chloro-4- [(2S,5R)-2,5- ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Purified GDP-bound KRAS protein (aa 1-169), containing both G12C and C118A amino acid substitutions and an N-terminal His-tag, was pre-incubated in a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2959MS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 492 total ) | Next | Last >> |