 Found 442 hits with Last Name = 'green' and Initial = 'sj'
Found 442 hits with Last Name = 'green' and Initial = 'sj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-secretase 1
(Mus musculus (Mouse)) | BDBM400979
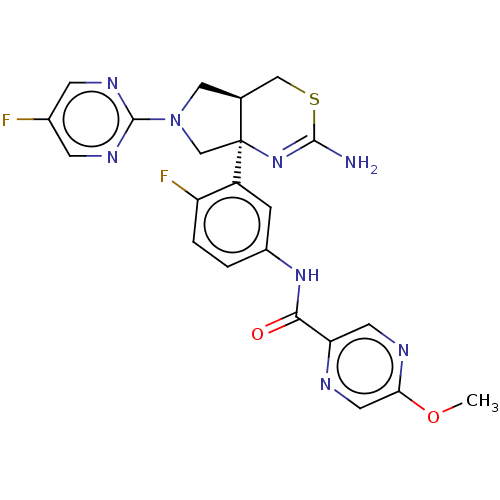
(US9999624, Compound 4)Show SMILES COc1cnc(cn1)C(=O)Nc1ccc(F)c(c1)[C@]12CN(C[C@H]1CSC(N)=N2)c1ncc(F)cn1 |r,c:29| Show InChI InChI=1S/C22H20F2N8O2S/c1-34-18-8-26-17(7-27-18)19(33)30-14-2-3-16(24)15(4-14)22-11-32(21-28-5-13(23)6-29-21)9-12(22)10-35-20(25)31-22/h2-8,12H,9-11H2,1H3,(H2,25,31)(H,30,33)/t12-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.275 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BACE1 in mouse primary cortical neuron assessed as reduction in Amyloid-beta level incubated for 24 hrs by sandwich ELISA assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00489
BindingDB Entry DOI: 10.7270/Q29W0KBW |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Mus musculus (Mouse)) | BDBM150693

(US8987254, 8 | US9999624, 9)Show SMILES NC1=N[C@]2(CN(C[C@H]2CS1)c1ncc(F)cn1)c1cc(NC(=O)c2ncc(F)cc2F)ccc1F |r,t:1| Show InChI InChI=1S/C22H17F4N7OS/c23-12-3-17(26)18(28-5-12)19(34)31-14-1-2-16(25)15(4-14)22-10-33(21-29-6-13(24)7-30-21)8-11(22)9-35-20(27)32-22/h1-7,11H,8-10H2,(H2,27,32)(H,31,34)/t11-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.309 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BACE1 in mouse primary cortical neuron assessed as reduction in Amyloid-beta level incubated for 24 hrs by sandwich ELISA assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00489
BindingDB Entry DOI: 10.7270/Q29W0KBW |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM150692
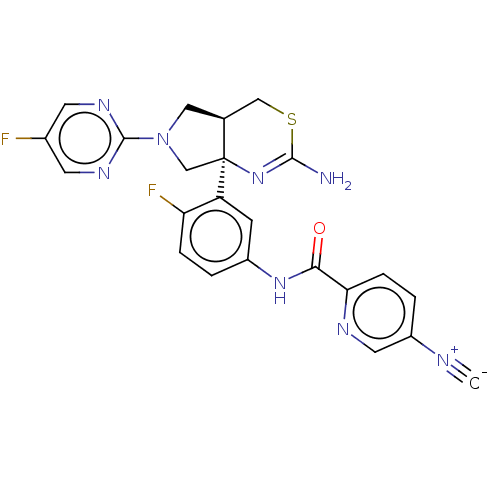
(US8987254, 7)Show SMILES NC1=N[C@]2(CN(C[C@H]2CS1)c1ncc(F)cn1)c1cc(NC(=O)c2ccc(cn2)[N+]#[C-])ccc1F |r,t:1| Show InChI InChI=1S/C23H18F2N8OS/c1-27-16-3-5-19(28-9-16)20(34)31-15-2-4-18(25)17(6-15)23-12-33(22-29-7-14(24)8-30-22)10-13(23)11-35-21(26)32-23/h2-9,13H,10-12H2,(H2,26,32)(H,31,34)/t13-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.358 | n/a | n/a | n/a | n/a | 4.6 | 25 |
Eli Lilly and Company
US Patent
| Assay Description
Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20x in KH2PO4 buffer. Ten uL of each dilution is ad... |
US Patent US8987254 (2015)
BindingDB Entry DOI: 10.7270/Q2FX786M |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM150688
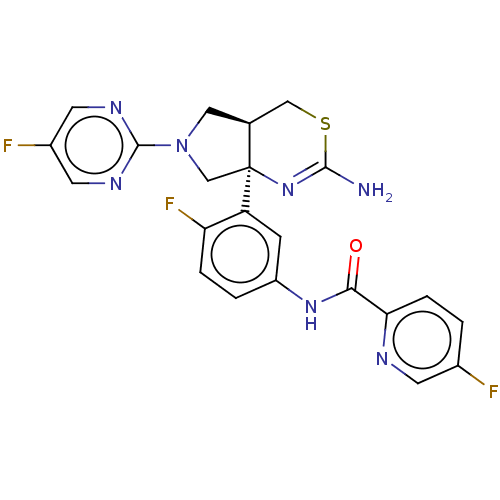
(US8987254, 3 | US9999624, 3)Show SMILES NC1=N[C@]2(CN(C[C@H]2CS1)c1ncc(F)cn1)c1cc(NC(=O)c2ccc(F)cn2)ccc1F |r,t:1| Show InChI InChI=1S/C22H18F3N7OS/c23-13-1-4-18(27-6-13)19(33)30-15-2-3-17(25)16(5-15)22-11-32(21-28-7-14(24)8-29-21)9-12(22)10-34-20(26)31-22/h1-8,12H,9-11H2,(H2,26,31)(H,30,33)/t12-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.388 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human BACE2 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00489
BindingDB Entry DOI: 10.7270/Q29W0KBW |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM150690
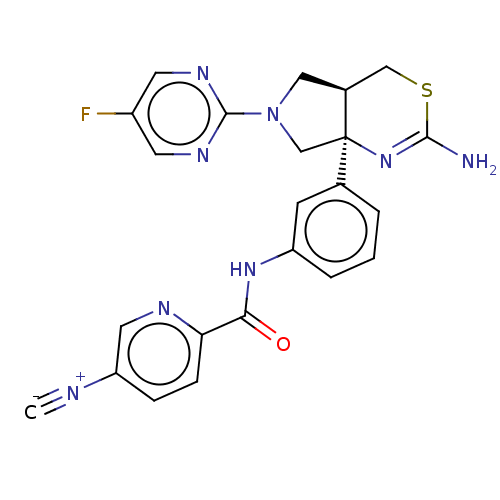
(US8987254, 5)Show SMILES NC1=N[C@]2(CN(C[C@H]2CS1)c1ncc(F)cn1)c1cccc(NC(=O)c2ccc(cn2)[N+]#[C-])c1 |r,t:1| Show InChI InChI=1S/C23H19FN8OS/c1-26-18-5-6-19(27-10-18)20(33)30-17-4-2-3-14(7-17)23-13-32(22-28-8-16(24)9-29-22)11-15(23)12-34-21(25)31-23/h2-10,15H,11-13H2,(H2,25,31)(H,30,33)/t15-,23+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | 4.6 | 25 |
Eli Lilly and Company
US Patent
| Assay Description
Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20x in KH2PO4 buffer. Ten uL of each dilution is ad... |
US Patent US8987254 (2015)
BindingDB Entry DOI: 10.7270/Q2FX786M |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Mus musculus (Mouse)) | BDBM150688

(US8987254, 3 | US9999624, 3)Show SMILES NC1=N[C@]2(CN(C[C@H]2CS1)c1ncc(F)cn1)c1cc(NC(=O)c2ccc(F)cn2)ccc1F |r,t:1| Show InChI InChI=1S/C22H18F3N7OS/c23-13-1-4-18(27-6-13)19(33)30-15-2-3-17(25)16(5-15)22-11-32(21-28-7-14(24)8-29-21)9-12(22)10-34-20(26)31-22/h1-8,12H,9-11H2,(H2,26,31)(H,30,33)/t12-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.481 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BACE1 in mouse primary cortical neuron assessed as reduction in Amyloid-beta level incubated for 24 hrs by sandwich ELISA assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00489
BindingDB Entry DOI: 10.7270/Q29W0KBW |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM150687
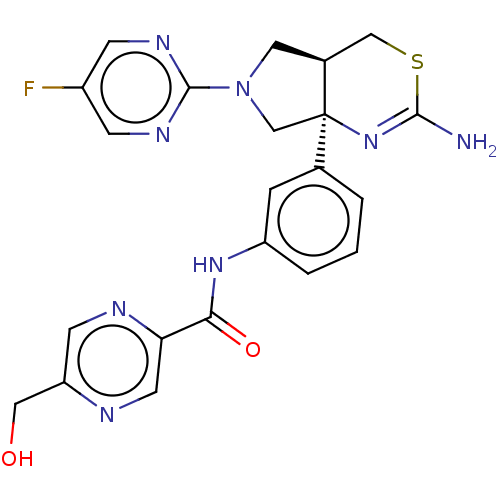
(US8987254, 2)Show SMILES NC1=N[C@]2(CN(C[C@H]2CS1)c1ncc(F)cn1)c1cccc(NC(=O)c2cnc(CO)cn2)c1 |r,t:1| Show InChI InChI=1S/C22H21FN8O2S/c23-15-5-27-21(28-6-15)31-9-14-11-34-20(24)30-22(14,12-31)13-2-1-3-16(4-13)29-19(33)18-8-25-17(10-32)7-26-18/h1-8,14,32H,9-12H2,(H2,24,30)(H,29,33)/t14-,22+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.482 | n/a | n/a | n/a | n/a | 4.6 | 25 |
Eli Lilly and Company
US Patent
| Assay Description
Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20x in KH2PO4 buffer. Ten uL of each dilution is ad... |
US Patent US8987254 (2015)
BindingDB Entry DOI: 10.7270/Q2FX786M |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM150688

(US8987254, 3 | US9999624, 3)Show SMILES NC1=N[C@]2(CN(C[C@H]2CS1)c1ncc(F)cn1)c1cc(NC(=O)c2ccc(F)cn2)ccc1F |r,t:1| Show InChI InChI=1S/C22H18F3N7OS/c23-13-1-4-18(27-6-13)19(33)30-15-2-3-17(25)16(5-15)22-11-32(21-28-7-14(24)8-29-21)9-12(22)10-34-20(26)31-22/h1-8,12H,9-11H2,(H2,26,31)(H,30,33)/t12-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.554 | n/a | n/a | n/a | n/a | 4.6 | 25 |
Eli Lilly and Company
US Patent
| Assay Description
Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20x in KH2PO4 buffer. Ten uL of each dilution is ad... |
US Patent US8987254 (2015)
BindingDB Entry DOI: 10.7270/Q2FX786M |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM150693

(US8987254, 8 | US9999624, 9)Show SMILES NC1=N[C@]2(CN(C[C@H]2CS1)c1ncc(F)cn1)c1cc(NC(=O)c2ncc(F)cc2F)ccc1F |r,t:1| Show InChI InChI=1S/C22H17F4N7OS/c23-12-3-17(26)18(28-5-12)19(34)31-14-1-2-16(25)15(4-14)22-10-33(21-29-6-13(24)7-30-21)8-11(22)9-35-20(27)32-22/h1-7,11H,8-10H2,(H2,27,32)(H,31,34)/t11-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.555 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human BACE2 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00489
BindingDB Entry DOI: 10.7270/Q29W0KBW |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM150689
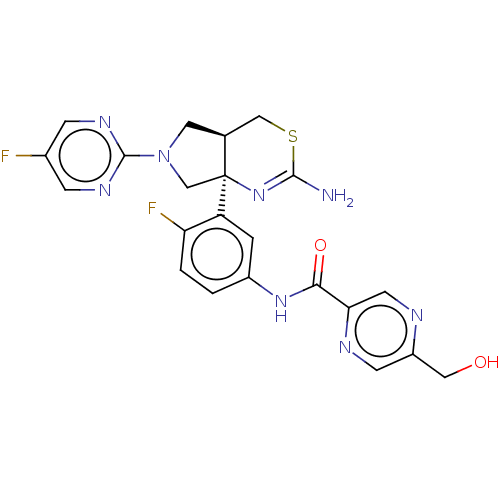
(US8987254, 4)Show SMILES NC1=N[C@]2(CN(C[C@H]2CS1)c1ncc(F)cn1)c1cc(NC(=O)c2cnc(CO)cn2)ccc1F |r,t:1| Show InChI InChI=1S/C22H20F2N8O2S/c23-13-4-28-21(29-5-13)32-8-12-10-35-20(25)31-22(12,11-32)16-3-14(1-2-17(16)24)30-19(34)18-7-26-15(9-33)6-27-18/h1-7,12,33H,8-11H2,(H2,25,31)(H,30,34)/t12-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.569 | n/a | n/a | n/a | n/a | 4.6 | 25 |
Eli Lilly and Company
US Patent
| Assay Description
Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20x in KH2PO4 buffer. Ten uL of each dilution is ad... |
US Patent US8987254 (2015)
BindingDB Entry DOI: 10.7270/Q2FX786M |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM150688

(US8987254, 3 | US9999624, 3)Show SMILES NC1=N[C@]2(CN(C[C@H]2CS1)c1ncc(F)cn1)c1cc(NC(=O)c2ccc(F)cn2)ccc1F |r,t:1| Show InChI InChI=1S/C22H18F3N7OS/c23-13-1-4-18(27-6-13)19(33)30-15-2-3-17(25)16(5-15)22-11-32(21-28-7-14(24)8-29-21)9-12(22)10-34-20(26)31-22/h1-8,12H,9-11H2,(H2,26,31)(H,30,33)/t12-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.603 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human BACE1 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate incubated for 8 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00489
BindingDB Entry DOI: 10.7270/Q29W0KBW |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM150686
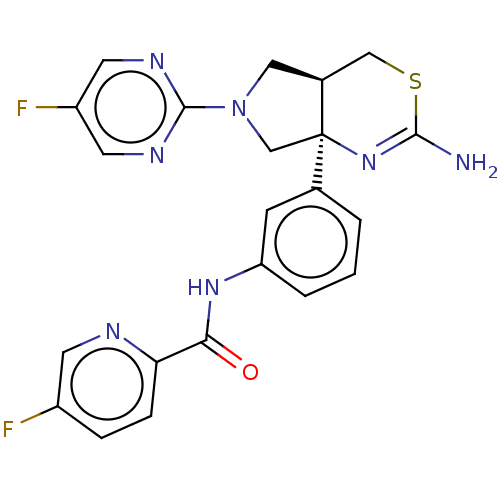
(US8987254, 1 | US9999624, 1)Show SMILES NC1=N[C@]2(CN(C[C@H]2CS1)c1ncc(F)cn1)c1cccc(NC(=O)c2ccc(F)cn2)c1 |r,t:1| Show InChI InChI=1S/C22H19F2N7OS/c23-15-4-5-18(26-7-15)19(32)29-17-3-1-2-13(6-17)22-12-31(21-27-8-16(24)9-28-21)10-14(22)11-33-20(25)30-22/h1-9,14H,10-12H2,(H2,25,30)(H,29,32)/t14-,22+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | 4.6 | 25 |
Eli Lilly and Company
US Patent
| Assay Description
Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20x in KH2PO4 buffer. Ten uL of each dilution is ad... |
US Patent US8987254 (2015)
BindingDB Entry DOI: 10.7270/Q2FX786M |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM400979

(US9999624, Compound 4)Show SMILES COc1cnc(cn1)C(=O)Nc1ccc(F)c(c1)[C@]12CN(C[C@H]1CSC(N)=N2)c1ncc(F)cn1 |r,c:29| Show InChI InChI=1S/C22H20F2N8O2S/c1-34-18-8-26-17(7-27-18)19(33)30-14-2-3-16(24)15(4-14)22-11-32(21-28-5-13(23)6-29-21)9-12(22)10-35-20(25)31-22/h2-8,12H,9-11H2,1H3,(H2,25,31)(H,30,33)/t12-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.615 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human BACE1 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate incubated for 8 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00489
BindingDB Entry DOI: 10.7270/Q29W0KBW |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM150693

(US8987254, 8 | US9999624, 9)Show SMILES NC1=N[C@]2(CN(C[C@H]2CS1)c1ncc(F)cn1)c1cc(NC(=O)c2ncc(F)cc2F)ccc1F |r,t:1| Show InChI InChI=1S/C22H17F4N7OS/c23-12-3-17(26)18(28-5-12)19(34)31-14-1-2-16(25)15(4-14)22-10-33(21-29-6-13(24)7-30-21)8-11(22)9-35-20(27)32-22/h1-7,11H,8-10H2,(H2,27,32)(H,31,34)/t11-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | 4.6 | 25 |
Eli Lilly and Company
US Patent
| Assay Description
Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20x in KH2PO4 buffer. Ten uL of each dilution is ad... |
US Patent US8987254 (2015)
BindingDB Entry DOI: 10.7270/Q2FX786M |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM150691
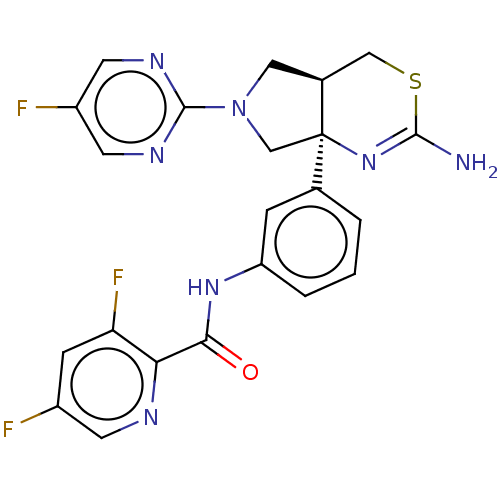
(US8987254, 6 | US9999624, 7)Show SMILES NC1=N[C@]2(CN(C[C@H]2CS1)c1ncc(F)cn1)c1cccc(NC(=O)c2ncc(F)cc2F)c1 |r,t:1| Show InChI InChI=1S/C22H18F3N7OS/c23-14-5-17(25)18(27-6-14)19(33)30-16-3-1-2-12(4-16)22-11-32(21-28-7-15(24)8-29-21)9-13(22)10-34-20(26)31-22/h1-8,13H,9-11H2,(H2,26,31)(H,30,33)/t13-,22+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.739 | n/a | n/a | n/a | n/a | 4.6 | 25 |
Eli Lilly and Company
US Patent
| Assay Description
Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20x in KH2PO4 buffer. Ten uL of each dilution is ad... |
US Patent US8987254 (2015)
BindingDB Entry DOI: 10.7270/Q2FX786M |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM150693

(US8987254, 8 | US9999624, 9)Show SMILES NC1=N[C@]2(CN(C[C@H]2CS1)c1ncc(F)cn1)c1cc(NC(=O)c2ncc(F)cc2F)ccc1F |r,t:1| Show InChI InChI=1S/C22H17F4N7OS/c23-12-3-17(26)18(28-5-12)19(34)31-14-1-2-16(25)15(4-14)22-10-33(21-29-6-13(24)7-30-21)8-11(22)9-35-20(27)32-22/h1-7,11H,8-10H2,(H2,27,32)(H,31,34)/t11-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human BACE1 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate incubated for 8 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00489
BindingDB Entry DOI: 10.7270/Q29W0KBW |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM400979

(US9999624, Compound 4)Show SMILES COc1cnc(cn1)C(=O)Nc1ccc(F)c(c1)[C@]12CN(C[C@H]1CSC(N)=N2)c1ncc(F)cn1 |r,c:29| Show InChI InChI=1S/C22H20F2N8O2S/c1-34-18-8-26-17(7-27-18)19(33)30-14-2-3-16(24)15(4-14)22-11-32(21-28-5-13(23)6-29-21)9-12(22)10-35-20(25)31-22/h2-8,12H,9-11H2,1H3,(H2,25,31)(H,30,33)/t12-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.871 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human BACE2 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00489
BindingDB Entry DOI: 10.7270/Q29W0KBW |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50012647
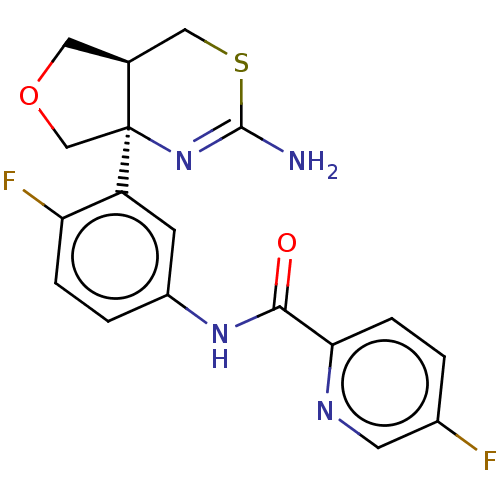
(CHEMBL2396989)Show SMILES [H][C@@]12COC[C@@]1(N=C(N)SC2)c1cc(NC(=O)c2ccc(F)cn2)ccc1F |r,t:7| Show InChI InChI=1S/C18H16F2N4O2S/c19-11-1-4-15(22-6-11)16(25)23-12-2-3-14(20)13(5-12)18-9-26-7-10(18)8-27-17(21)24-18/h1-6,10H,7-9H2,(H2,21,24)(H,23,25)/t10-,18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human BACE1 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate incubated for 8 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00489
BindingDB Entry DOI: 10.7270/Q29W0KBW |
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM639724

(3-[[6,7-Dichloro-3-(1H-pyrazol-4-yl)-1H-indol-4-yl...) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM639875

((S)—N-(5,6-Dichloro-8-(cyanomethoxy)-9-(1H-py...)Show SMILES OCC(=O)N[C@H]1CCn2c1c(-c1cn[nH]c1)c1c(OCC#N)cc(Cl)c(Cl)c21 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.38 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM639703

(N-[6, 7-Dichloro-3-(1H-pyrazol-4-yl)-1H-indol-4-yl...)Show SMILES FC(F)C(=O)Nc1cc(Cl)c(Cl)c2[nH]cc(-c3cn[nH]c3)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.56 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM639763

((S)—N-(5,6-Dichloro-8-(cyanomethoxy)-9-(1H-py...)Show SMILES CC(=O)N[C@H]1CCn2c1c(-c1cn[nH]c1)c1c(OCC#N)cc(Cl)c(Cl)c21 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.76 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM639676

(3-[[6,7-Dichloro-3-(1H-pyrazol-4-yl)-1H-indol-4-yl...) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4.33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM639869
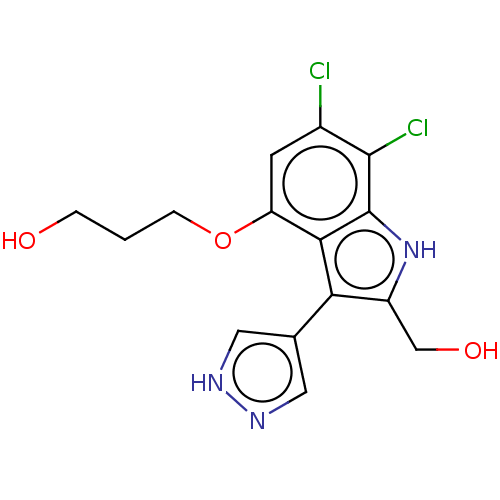
(3-((6,7-Dichloro-2-(hydroxymethyl)-3-(1H-pyrazol-4...)Show SMILES OCCCOc1cc(Cl)c(Cl)c2[nH]c(CO)c(-c3cn[nH]c3)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4.74 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM639655
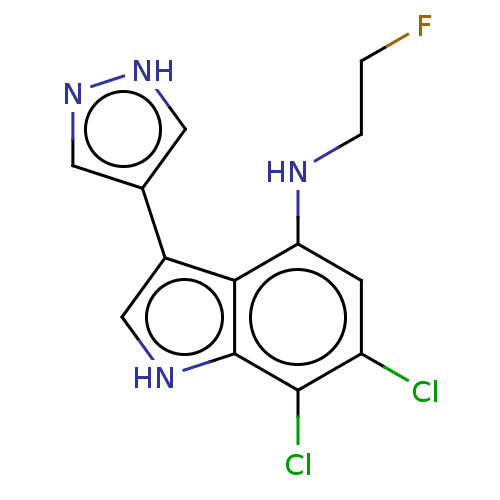
(6,7-Dichloro-N-(2-fluoroethyl)-3-(1H-pyrazol-4-yl)...) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4.91 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM639685
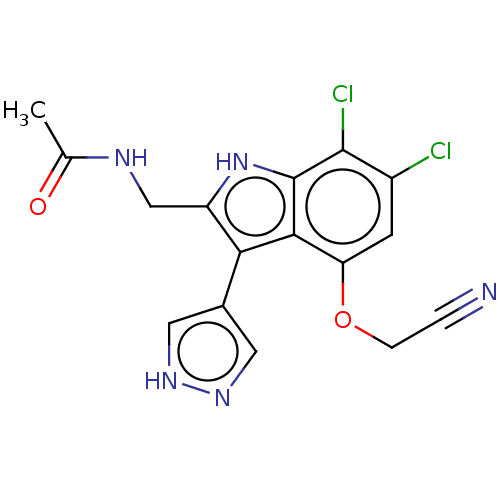
(N-((6,7-Dichloro-4-(cyanomethoxy)-3-(1H-pyrazol-4-...)Show SMILES CC(=O)NCc1[nH]c2c(Cl)c(Cl)cc(OCC#N)c2c1-c1cn[nH]c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5.11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM639781

(3-[[6,7-Dichloro-3-(1H-pyrazol-4-yl)-1H-indazol-4-...) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50540172
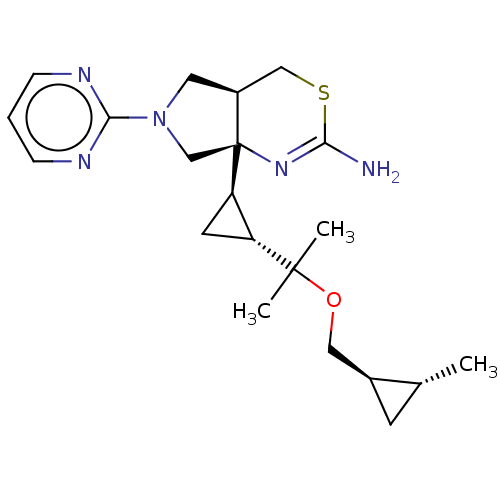
(CHEMBL4637426)Show SMILES [H][C@]1(C[C@H]1C(C)(C)OC[C@@H]1C[C@H]1C)[C@]12CN(C[C@@]1([H])CSC(N)=N2)c1ncccn1 |r,c:25| Show InChI InChI=1S/C21H31N5OS/c1-13-7-14(13)10-27-20(2,3)16-8-17(16)21-12-26(19-23-5-4-6-24-19)9-15(21)11-28-18(22)25-21/h4-6,13-17H,7-12H2,1-3H3,(H2,22,25)/t13-,14+,15+,16-,17-,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human BACE2 |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115194
BindingDB Entry DOI: 10.7270/Q2B56P8K |
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM639702
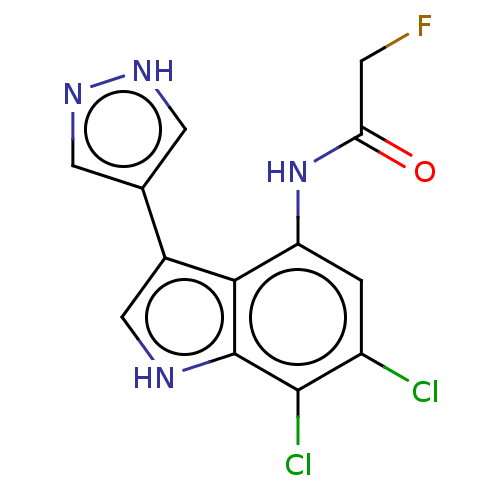
(N-(6,7-Dichloro-3-(1H-pyrazol-4-yl)-1H-indol-4-yl)...)Show SMILES FCC(=O)Nc1cc(Cl)c(Cl)c2[nH]cc(-c3cn[nH]c3)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5.67 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM639874
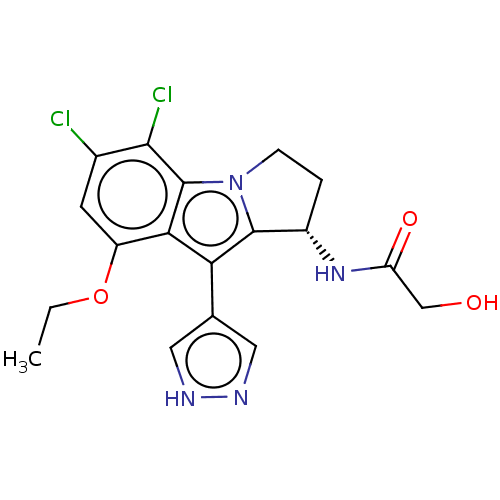
((S)—N-(5,6-Dichloro-8-ethoxy-9-(1H-pyrazol-4-...)Show SMILES CCOc1cc(Cl)c(Cl)c2n3CC[C@H](NC(=O)CO)c3c(-c3cn[nH]c3)c12 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 6.14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM158179

(US9029367, 3)Show SMILES NC1=N[C@]2(COC[C@@]2(F)CS1)c1cc(NC(=O)c2ccc(cn2)[N+]#[C-])ccc1F |r,t:1| Show InChI InChI=1S/C19H15F2N5O2S/c1-23-12-3-5-15(24-7-12)16(27)25-11-2-4-14(20)13(6-11)19-9-28-8-18(19,21)10-29-17(22)26-19/h2-7H,8-10H2,(H2,22,26)(H,25,27)/t18-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6.66 | n/a | n/a | n/a | n/a | 4.6 | 25 |
Eli Lilly and Company
US Patent
| Assay Description
Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20x in KH2PO4 buffer. Ten uL of each dilution is ad... |
US Patent US9029367 (2015)
BindingDB Entry DOI: 10.7270/Q21Z4344 |
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM639734
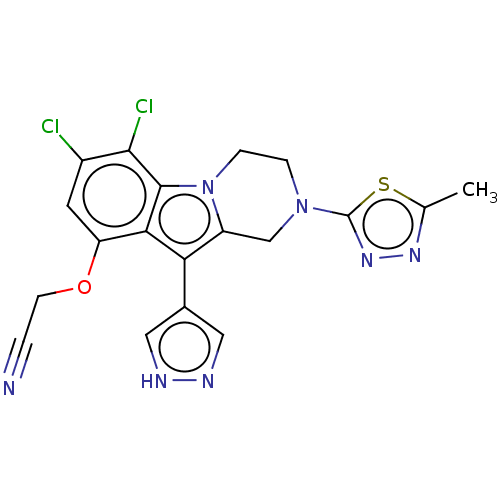
(2-((6,7-Dichloro-2-(5-methyl-1,3,4-thiadiazol-2-yl...)Show SMILES Cc1nnc(s1)N1CCn2c(C1)c(-c1cn[nH]c1)c1c(OCC#N)cc(Cl)c(Cl)c21 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 6.77 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM639879
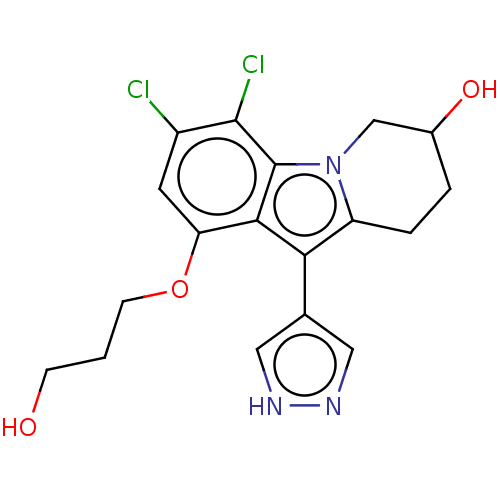
(3,4-Dichloro-1-(3-hydroxypropoxy)-10-(1H-pyrazol-4...)Show SMILES OCCCOc1cc(Cl)c(Cl)c2n3CC(O)CCc3c(-c3cn[nH]c3)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 6.95 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM639810
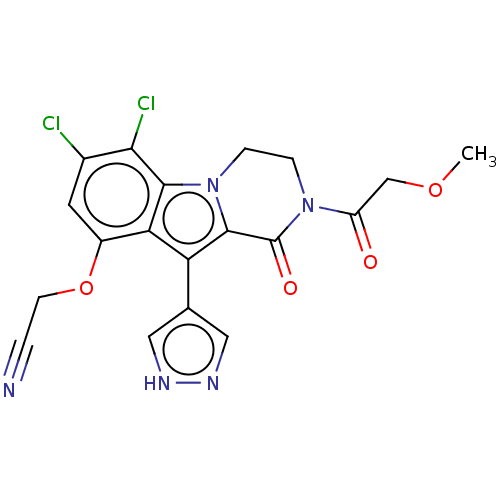
(2-((6,7-Dichloro-2-(2-methoxyacetyl)-10-(1H-pyrazo...)Show SMILES COCC(=O)N1CCn2c(c(-c3cn[nH]c3)c3c(OCC#N)cc(Cl)c(Cl)c23)C1=O | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM639768
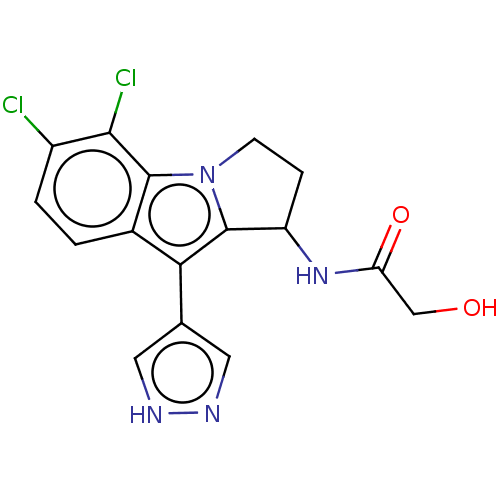
(N-(5,6-Dichloro-9-(1H-pyrazol-4-yl)-2,3-dihydro-1H...)Show SMILES OCC(=O)NC1CCn2c1c(-c1cn[nH]c1)c1ccc(Cl)c(Cl)c21 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 7.69 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM639870
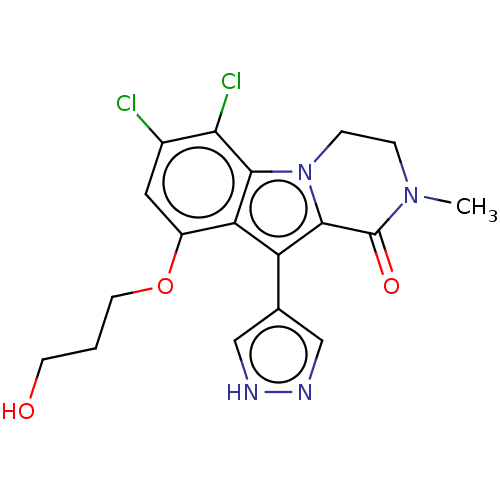
(6,7-Dichloro-9-(3-hydroxypropoxy)-2-methyl-10-(1H-...)Show SMILES CN1CCn2c(c(-c3cn[nH]c3)c3c(OCCCO)cc(Cl)c(Cl)c23)C1=O | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 7.89 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM639811
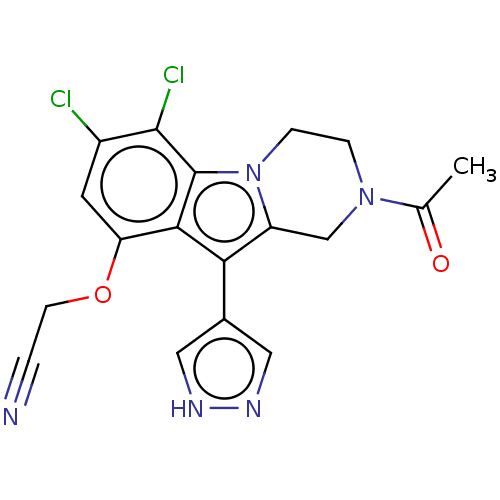
(US20230391786, Example 280)Show SMILES CC(=O)N1CCn2c(C1)c(-c1cn[nH]c1)c1c(OCC#N)cc(Cl)c(Cl)c21 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM639812
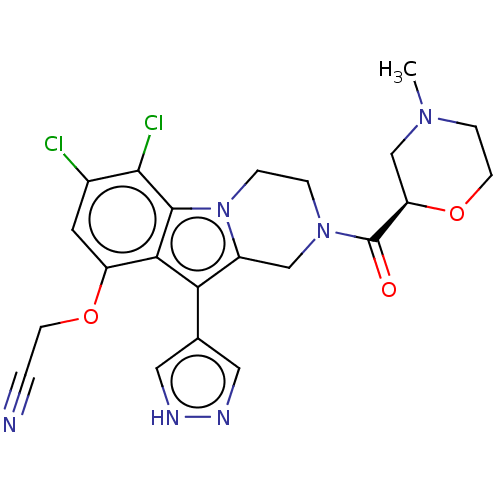
(US20230391786, Example 281)Show SMILES CN1CCO[C@H](C1)C(=O)N1CCn2c(C1)c(-c1cn[nH]c1)c1c(OCC#N)cc(Cl)c(Cl)c21 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM639728

(22-[6, 7-Dichloro-1-[1-(2-hydroxy ethyl)triazol-4-...)Show SMILES OCCn1cc(nn1)-n1cc(-c2cn[nH]c2)c2c(OCC#N)cc(Cl)c(Cl)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 8.93 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM639813
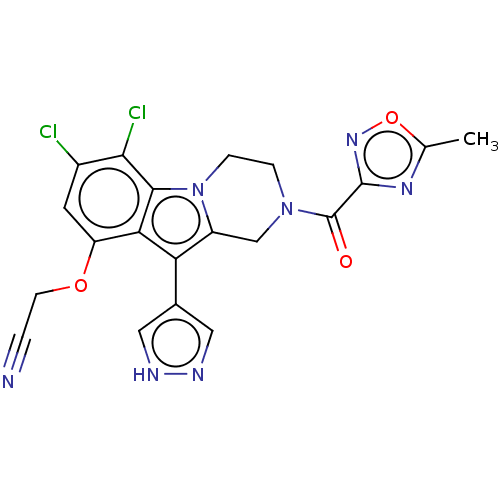
(US20230391786, Example 282)Show SMILES Cc1nc(no1)C(=O)N1CCn2c(C1)c(-c1cn[nH]c1)c1c(OCC#N)cc(Cl)c(Cl)c21 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 8.96 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM639775
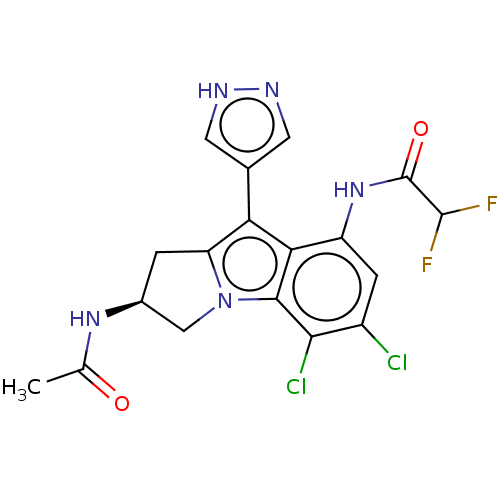
((S)—N-(2-Acetamido-5,6-dichloro-9-(1H-pyrazol...)Show SMILES CC(=O)N[C@H]1Cc2c(-c3cn[nH]c3)c3c(NC(=O)C(F)F)cc(Cl)c(Cl)c3n2C1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50540172

(CHEMBL4637426)Show SMILES [H][C@]1(C[C@H]1C(C)(C)OC[C@@H]1C[C@H]1C)[C@]12CN(C[C@@]1([H])CSC(N)=N2)c1ncccn1 |r,c:25| Show InChI InChI=1S/C21H31N5OS/c1-13-7-14(13)10-27-20(2,3)16-8-17(16)21-12-26(19-23-5-4-6-24-19)9-15(21)11-28-18(22)25-21/h4-6,13-17H,7-12H2,1-3H3,(H2,22,25)/t13-,14+,15+,16-,17-,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human BACE1 (1 to 460 residues) expressed in HEK293 cells using mcaFRET peptide as substrate after 20 hrs by FRET assay |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115194
BindingDB Entry DOI: 10.7270/Q2B56P8K |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50540172

(CHEMBL4637426)Show SMILES [H][C@]1(C[C@H]1C(C)(C)OC[C@@H]1C[C@H]1C)[C@]12CN(C[C@@]1([H])CSC(N)=N2)c1ncccn1 |r,c:25| Show InChI InChI=1S/C21H31N5OS/c1-13-7-14(13)10-27-20(2,3)16-8-17(16)21-12-26(19-23-5-4-6-24-19)9-15(21)11-28-18(22)25-21/h4-6,13-17H,7-12H2,1-3H3,(H2,22,25)/t13-,14+,15+,16-,17-,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human BACE1 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate incubated for 8 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00489
BindingDB Entry DOI: 10.7270/Q29W0KBW |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50012647

(CHEMBL2396989)Show SMILES [H][C@@]12COC[C@@]1(N=C(N)SC2)c1cc(NC(=O)c2ccc(F)cn2)ccc1F |r,t:7| Show InChI InChI=1S/C18H16F2N4O2S/c19-11-1-4-15(22-6-11)16(25)23-12-2-3-14(20)13(5-12)18-9-26-7-10(18)8-27-17(21)24-18/h1-6,10H,7-9H2,(H2,21,24)(H,23,25)/t10-,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human BACE2 |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115194
BindingDB Entry DOI: 10.7270/Q2B56P8K |
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM639871
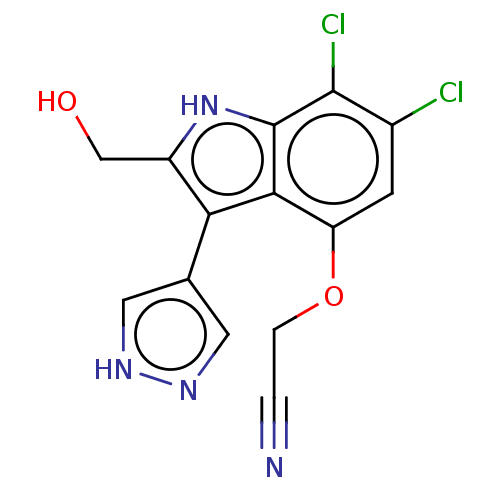
(2-((6,7-Dichloro-2-(hydroxymethyl)-3-(1H-pyrazol-4...)Show SMILES OCc1[nH]c2c(Cl)c(Cl)cc(OCC#N)c2c1-c1cn[nH]c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 10.2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM639657
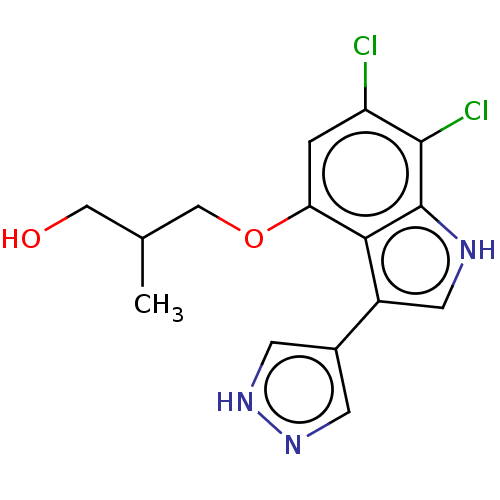
(3-((6,7-Dichloro-3-(1H-pyrazol-4-yl)-1H-indol-4-yl...)Show SMILES CC(CO)COc1cc(Cl)c(Cl)c2[nH]cc(-c3cn[nH]c3)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 10.3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM639718

(3-[[6,7-Dichloro-3-(1H-pyrazol-4-yl)-1H-indol-4-yl...)Show SMILES OCC(F)CNc1cc(Cl)c(Cl)c2[nH]cc(-c3cn[nH]c3)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 10.4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM639814
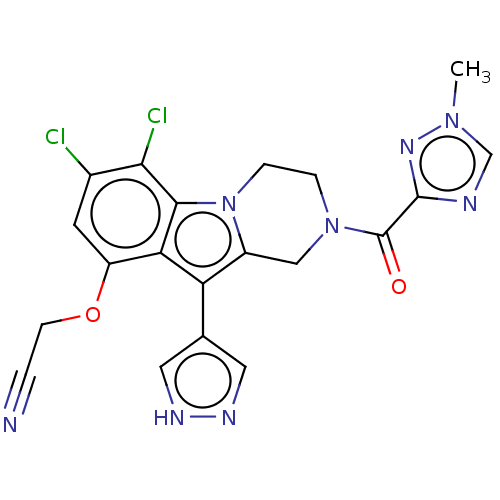
(US20230391786, Example 283)Show SMILES Cn1cnc(n1)C(=O)N1CCn2c(C1)c(-c1cn[nH]c1)c1c(OCC#N)cc(Cl)c(Cl)c21 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 10.6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Mus musculus (Mouse)) | BDBM50012647

(CHEMBL2396989)Show SMILES [H][C@@]12COC[C@@]1(N=C(N)SC2)c1cc(NC(=O)c2ccc(F)cn2)ccc1F |r,t:7| Show InChI InChI=1S/C18H16F2N4O2S/c19-11-1-4-15(22-6-11)16(25)23-12-2-3-14(20)13(5-12)18-9-26-7-10(18)8-27-17(21)24-18/h1-6,10H,7-9H2,(H2,21,24)(H,23,25)/t10-,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BACE1 in mouse primary cortical neuron assessed as reduction in Amyloid-beta level incubated for 24 hrs by sandwich ELISA assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00489
BindingDB Entry DOI: 10.7270/Q29W0KBW |
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM639729
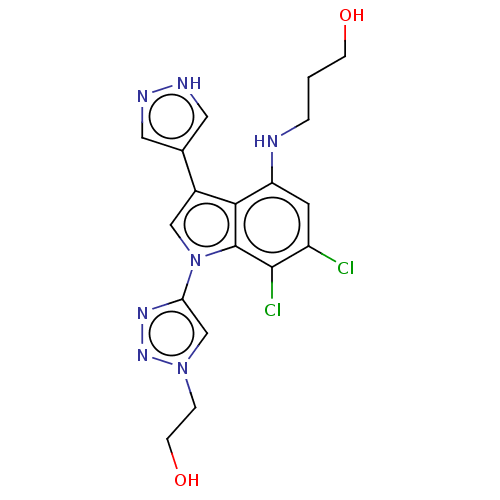
(3-[[6,7-Dichloro-1-[1-(2-hydroxyethyl)triazol-4-yl...)Show SMILES OCCCNc1cc(Cl)c(Cl)c2n(cc(-c3cn[nH]c3)c12)-c1cn(CCO)nn1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data