 Found 1105 hits with Last Name = 'greiner' and Initial = 'i'
Found 1105 hits with Last Name = 'greiner' and Initial = 'i' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50382290

(CARIPRAZINE HYDROCHLORIDE | RGH-188 HCL)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc(Cl)c2Cl)CC1 |r,wU:6.5,wD:9.9,(.89,-12.18,;2.22,-12.95,;2.22,-14.49,;3.56,-12.18,;3.56,-10.64,;4.89,-12.95,;6.22,-12.18,;6.22,-10.64,;7.55,-9.86,;8.88,-10.64,;10.22,-9.87,;11.55,-10.65,;12.88,-9.88,;14.21,-10.66,;15.54,-9.9,;15.55,-8.36,;14.22,-7.58,;12.88,-8.35,;16.89,-7.6,;18.25,-8.33,;19.56,-7.51,;19.51,-5.97,;18.16,-5.25,;18.11,-3.71,;16.84,-6.06,;15.49,-5.33,;8.88,-12.18,;7.55,-12.94,)| Show InChI InChI=1S/C21H32Cl2N4O/c1-25(2)21(28)24-17-8-6-16(7-9-17)10-11-26-12-14-27(15-13-26)19-5-3-4-18(22)20(19)23/h3-5,16-17H,6-15H2,1-2H3,(H,24,28)/t16-,17- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Binding affinity to human dopamine D3 receptor |
Bioorg Med Chem Lett 22: 3437-40 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.104
BindingDB Entry DOI: 10.7270/Q2ZW1MZN |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50583877
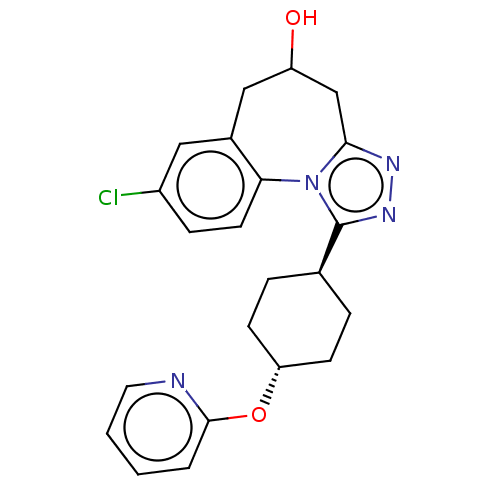
(CHEMBL5070261)Show SMILES OC1Cc2nnc([C@H]3CC[C@@H](CC3)Oc3ccccn3)n2-c2ccc(Cl)cc2C1 |r,wU:7.6,wD:10.13,(84.69,-14.49,;83.74,-13.39,;84.35,-12.06,;83.71,-10.78,;84.49,-9.44,;83.46,-8.29,;82.05,-8.91,;80.71,-8.14,;79.38,-8.9,;78.04,-8.14,;78.04,-6.6,;79.38,-5.81,;80.71,-6.6,;76.69,-5.82,;75.36,-6.6,;74.02,-5.82,;72.68,-6.6,;72.68,-8.15,;74.04,-8.92,;75.37,-8.14,;82.19,-10.46,;81.05,-11.4,;79.72,-10.7,;78.44,-11.49,;78.5,-13,;77.18,-13.83,;79.83,-13.71,;81.1,-12.91,;82.34,-13.81,)| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-arginine-vasopressin from human V1A receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00863
BindingDB Entry DOI: 10.7270/Q2GM8C6F |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50583879
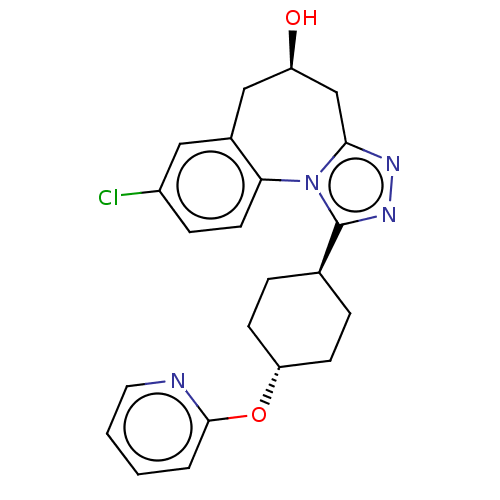
(CHEMBL5070075)Show SMILES O[C@H]1Cc2nnc([C@H]3CC[C@@H](CC3)Oc3ccccn3)n2-c2ccc(Cl)cc2C1 |r,wU:7.6,wD:10.13,1.0,(28.94,-32.53,;27.98,-31.43,;28.59,-30.1,;27.96,-28.82,;28.74,-27.48,;27.7,-26.33,;26.29,-26.96,;24.95,-26.18,;23.62,-26.95,;22.28,-26.18,;22.28,-24.64,;23.62,-23.85,;24.95,-24.64,;20.94,-23.86,;19.61,-24.64,;18.26,-23.87,;16.92,-24.65,;16.93,-26.19,;18.28,-26.96,;19.61,-26.18,;26.44,-28.5,;25.29,-29.44,;23.96,-28.74,;22.69,-29.53,;22.74,-31.04,;21.43,-31.87,;24.07,-31.75,;25.34,-30.95,;26.58,-31.85,)| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-arginine-vasopressin from human V1A receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00863
BindingDB Entry DOI: 10.7270/Q2GM8C6F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50615419
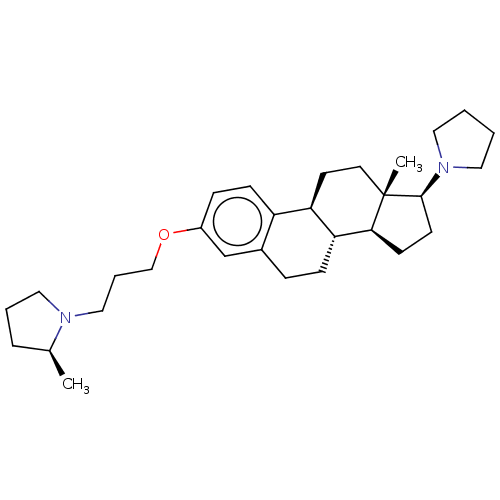
(CHEMBL5285270)Show SMILES [H][C@@]12CC[C@H](N3CCCC3)[C@@]1(C)CC[C@]1([H])c3ccc(OCCCN4CCC[C@@H]4C)cc3CC[C@@]21[H] |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50583878
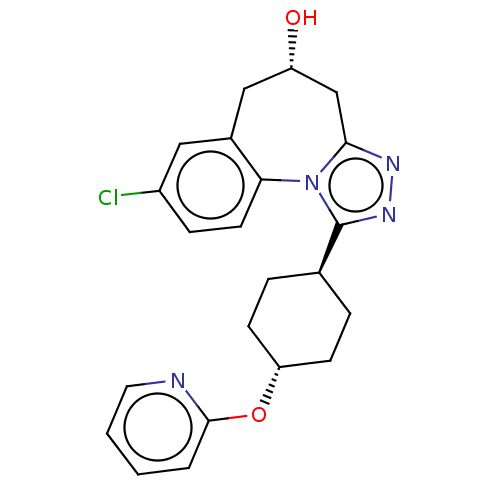
(CHEMBL5074709)Show SMILES O[C@@H]1Cc2nnc([C@H]3CC[C@@H](CC3)Oc3ccccn3)n2-c2ccc(Cl)cc2C1 |r,wU:7.6,1.0,wD:10.13,(13.29,-32.25,;12.34,-31.15,;12.94,-29.82,;12.31,-28.53,;13.09,-27.19,;12.06,-26.04,;10.65,-26.67,;9.3,-25.9,;7.97,-26.66,;6.64,-25.9,;6.64,-24.35,;7.97,-23.57,;9.3,-24.35,;5.29,-23.58,;3.96,-24.36,;2.61,-23.58,;1.27,-24.36,;1.28,-25.91,;2.63,-26.67,;3.97,-25.9,;10.79,-28.21,;9.65,-29.16,;8.32,-28.45,;7.04,-29.24,;7.09,-30.76,;5.78,-31.58,;8.42,-31.46,;9.69,-30.66,;10.94,-31.56,)| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-arginine-vasopressin from human V1A receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00863
BindingDB Entry DOI: 10.7270/Q2GM8C6F |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50583881
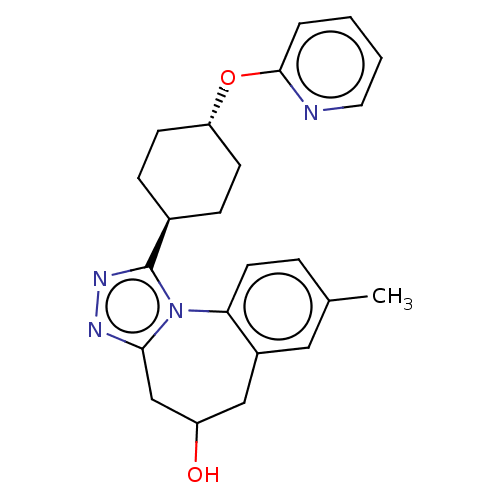
(CHEMBL5081323)Show SMILES Cc1ccc-2c(CC(O)Cc3nnc([C@H]4CC[C@@H](CC4)Oc4ccccn4)n-23)c1 |r,wU:14.13,wD:17.20,(55.33,-32.61,;56.65,-31.79,;56.59,-30.27,;57.87,-29.48,;59.2,-30.19,;59.25,-31.7,;60.49,-32.59,;61.89,-32.18,;62.84,-33.28,;62.5,-30.85,;61.86,-29.57,;62.64,-28.22,;61.61,-27.07,;60.2,-27.7,;58.86,-26.93,;57.53,-27.69,;56.19,-26.93,;56.19,-25.38,;57.53,-24.6,;58.86,-25.38,;54.84,-24.61,;53.51,-25.39,;52.17,-24.61,;50.83,-25.39,;50.83,-26.94,;52.19,-27.7,;53.52,-26.93,;60.35,-29.24,;57.98,-32.49,)| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-arginine-vasopressin from human V1A receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00863
BindingDB Entry DOI: 10.7270/Q2GM8C6F |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50536755
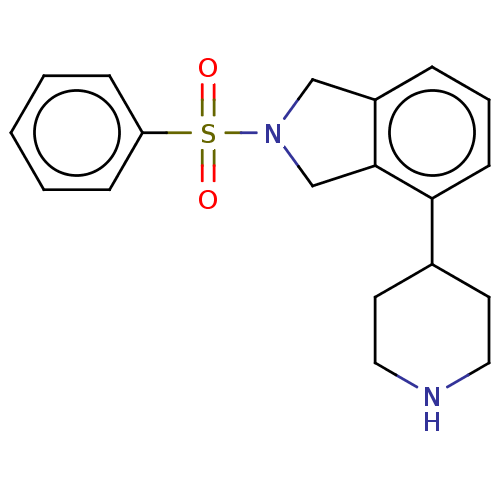
(CHEMBL4555868)Show InChI InChI=1S/C19H22N2O2S/c22-24(23,17-6-2-1-3-7-17)21-13-16-5-4-8-18(19(16)14-21)15-9-11-20-12-10-15/h1-8,15,20H,9-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5-HT6 receptor expressed in CHO-K1 cells incubated for 3 hrs by scintillation counting analysis |
Bioorg Med Chem Lett 26: 4211-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.055
BindingDB Entry DOI: 10.7270/Q2D50RGF |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50382312
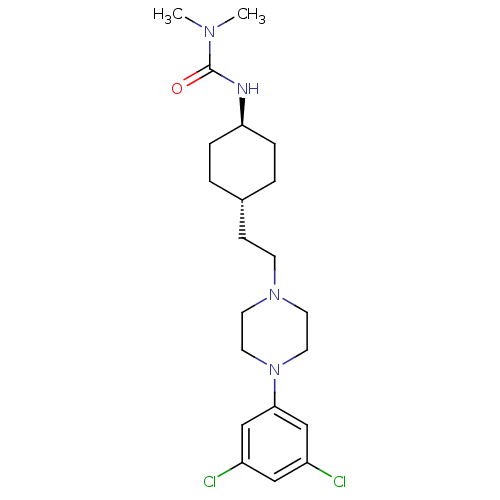
(CHEMBL2024677)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cc(Cl)cc(Cl)c2)CC1 |r,wU:6.5,wD:9.9,(6.35,-23.87,;7.68,-24.65,;7.68,-26.19,;9.02,-23.88,;9.02,-22.34,;10.35,-24.65,;11.68,-23.88,;11.68,-22.34,;13.01,-21.56,;14.34,-22.34,;15.68,-21.57,;17.01,-22.35,;18.34,-21.58,;19.67,-22.36,;21,-21.6,;21.01,-20.06,;19.68,-19.28,;18.34,-20.04,;22.35,-19.3,;23.68,-20.08,;25.02,-19.32,;26.35,-20.1,;25.03,-17.78,;23.69,-17,;23.7,-15.46,;22.36,-17.76,;14.34,-23.88,;13.01,-24.64,)| Show InChI InChI=1S/C21H32Cl2N4O/c1-25(2)21(28)24-19-5-3-16(4-6-19)7-8-26-9-11-27(12-10-26)20-14-17(22)13-18(23)15-20/h13-16,19H,3-12H2,1-2H3,(H,24,28)/t16-,19- | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from rat recombinant dopamine D3 receptor expressed in Sf9 cells |
Bioorg Med Chem Lett 22: 3437-40 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.104
BindingDB Entry DOI: 10.7270/Q2ZW1MZN |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-5/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50605870
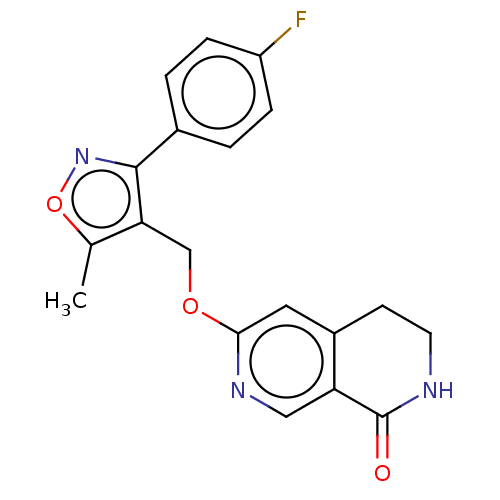
(CHEMBL5196475) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00414
BindingDB Entry DOI: 10.7270/Q2DV1Q01 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50615418
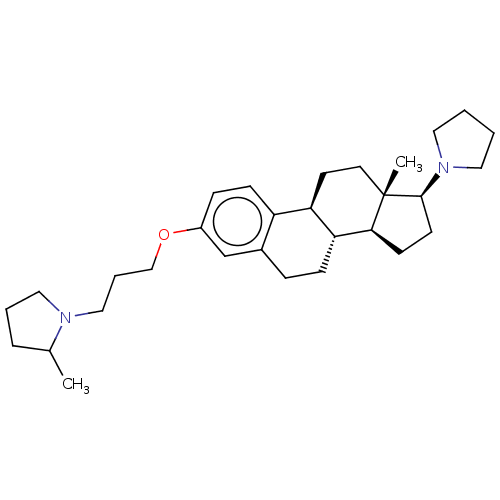
(CHEMBL5270265)Show SMILES [H][C@@]12CC[C@H](N3CCCC3)[C@@]1(C)CC[C@]1([H])c3ccc(OCCCN4CCCC4C)cc3CC[C@@]21[H] |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50525810
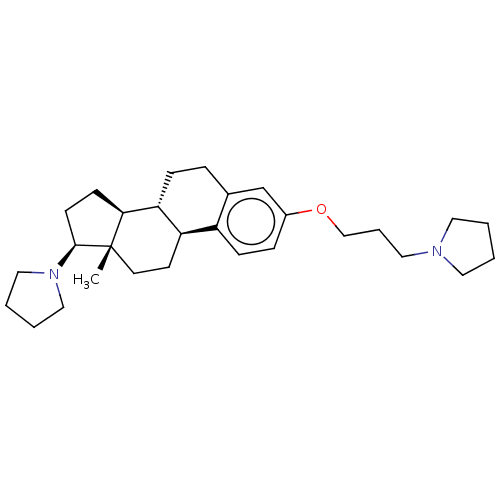
(CHEMBL4451680)Show SMILES [H][C@@]12CC[C@H](N3CCCC3)[C@@]1(C)CC[C@]1([H])c3ccc(OCCCN4CCCC4)cc3CC[C@@]21[H] |r| Show InChI InChI=1S/C29H44N2O/c1-29-14-13-25-24-10-8-23(32-20-6-17-30-15-2-3-16-30)21-22(24)7-9-26(25)27(29)11-12-28(29)31-18-4-5-19-31/h8,10,21,25-28H,2-7,9,11-20H2,1H3/t25-,26-,27+,28+,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Works of Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyl- N-alpha methylhistamine dihydrochloride from human recombinant H3 receptor expressed in CHOK1 cells measured after 30 min... |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126643
BindingDB Entry DOI: 10.7270/Q22F7RW4 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50615421
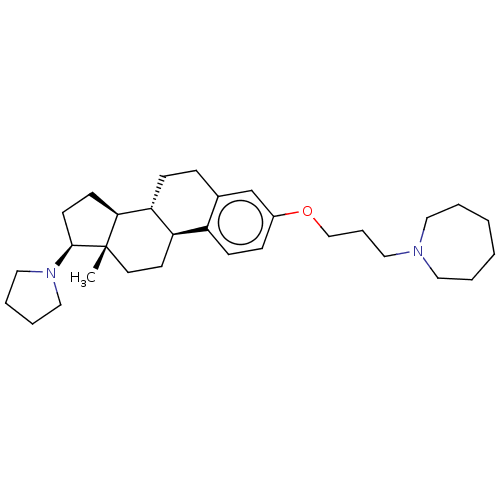
(CHEMBL5288889)Show SMILES [H][C@@]12CC[C@H](N3CCCC3)[C@@]1(C)CC[C@]1([H])c3ccc(OCCCN4CCCCCC4)cc3CC[C@@]21[H] |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50615420

(CHEMBL5274183)Show SMILES [H][C@@]12CC[C@H](N3CCCC3)[C@@]1(C)CC[C@]1([H])c3ccc(OCCCN4CCNC(=O)C4)cc3CC[C@@]21[H] |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50525810

(CHEMBL4451680)Show SMILES [H][C@@]12CC[C@H](N3CCCC3)[C@@]1(C)CC[C@]1([H])c3ccc(OCCCN4CCCC4)cc3CC[C@@]21[H] |r| Show InChI InChI=1S/C29H44N2O/c1-29-14-13-25-24-10-8-23(32-20-6-17-30-15-2-3-16-30)21-22(24)7-9-26(25)27(29)11-12-28(29)31-18-4-5-19-31/h8,10,21,25-28H,2-7,9,11-20H2,1H3/t25-,26-,27+,28+,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50382310
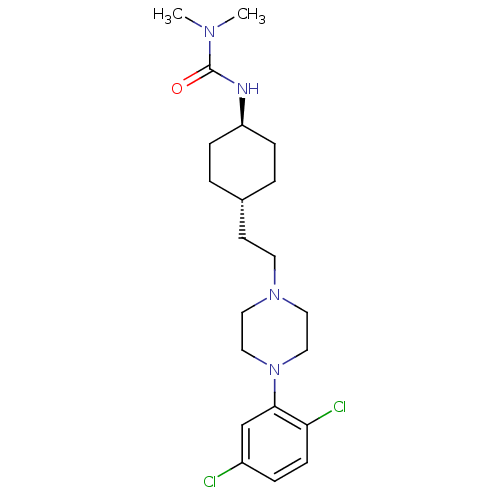
(CHEMBL2024675)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cc(Cl)ccc2Cl)CC1 |r,wU:6.5,wD:9.9,(23.12,-11.63,;24.45,-12.4,;24.45,-13.94,;25.79,-11.64,;25.79,-10.1,;27.12,-12.41,;28.45,-11.64,;28.45,-10.1,;29.78,-9.32,;31.11,-10.1,;32.45,-9.33,;33.78,-10.1,;35.11,-9.34,;36.44,-10.12,;37.77,-9.36,;37.78,-7.82,;36.45,-7.04,;35.11,-7.8,;39.12,-7.06,;39.13,-5.52,;40.46,-4.76,;40.46,-3.22,;41.8,-5.54,;41.79,-7.08,;40.45,-7.84,;40.44,-9.38,;31.11,-11.64,;29.78,-12.4,)| Show InChI InChI=1S/C21H32Cl2N4O/c1-25(2)21(28)24-18-6-3-16(4-7-18)9-10-26-11-13-27(14-12-26)20-15-17(22)5-8-19(20)23/h5,8,15-16,18H,3-4,6-7,9-14H2,1-2H3,(H,24,28)/t16-,18- | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from rat recombinant dopamine D3 receptor expressed in Sf9 cells |
Bioorg Med Chem Lett 22: 3437-40 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.104
BindingDB Entry DOI: 10.7270/Q2ZW1MZN |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50546439
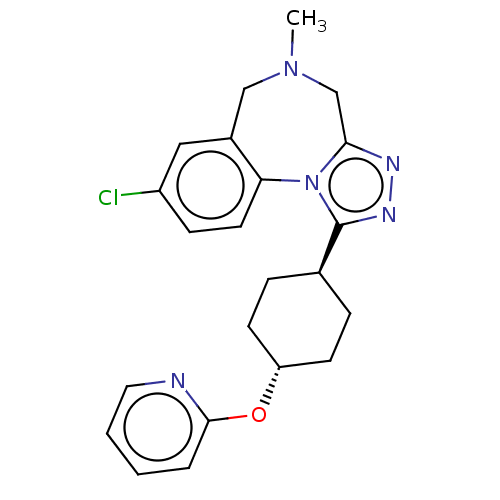
(Balovaptan | RG-7314 | RO-5285119 | RO5285119 | Rg...)Show SMILES CN1Cc2nnc([C@H]3CC[C@@H](CC3)Oc3ccccn3)n2-c2ccc(Cl)cc2C1 |r,wU:7.6,wD:10.13,(-.51,-6.75,;-1.47,-5.54,;-.79,-4.16,;-1.45,-2.76,;-.65,-1.45,;-1.66,-.28,;-3.08,-.88,;-4.39,-.07,;-5.74,-.79,;-7.05,.02,;-7,1.56,;-5.65,2.28,;-4.34,1.47,;-8.31,2.37,;-9.67,1.64,;-9.72,.1,;-11.08,-.63,;-12.38,.19,;-12.34,1.72,;-10.98,2.45,;-2.95,-2.41,;-4.16,-3.37,;-5.49,-2.59,;-6.83,-3.35,;-6.83,-4.89,;-8.17,-5.65,;-5.51,-5.67,;-4.17,-4.91,;-2.97,-5.87,)| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-arginine-vasopressin from human V1A receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00863
BindingDB Entry DOI: 10.7270/Q2GM8C6F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50615409
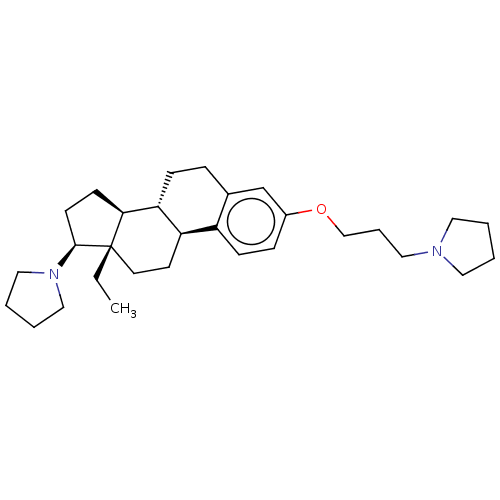
(CHEMBL5285176)Show SMILES [H][C@@]12CC[C@H](N3CCCC3)[C@@]1(CC)CC[C@]1([H])c3ccc(OCCCN4CCCC4)cc3CC[C@@]21[H] |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50615412
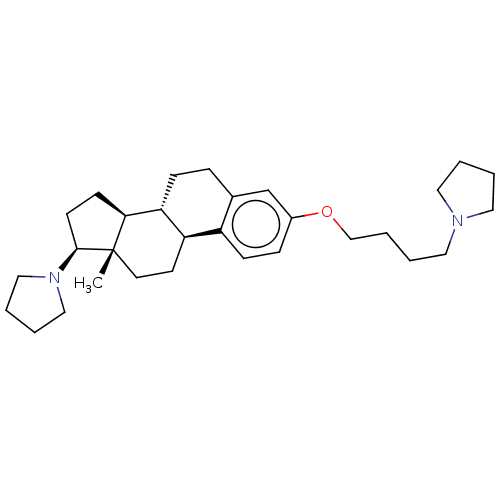
(CHEMBL5273254)Show SMILES [H][C@@]12CC[C@H](N3CCCC3)[C@@]1(C)CC[C@]1([H])c3ccc(OCCCCN4CCCC4)cc3CC[C@@]21[H] |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50546436
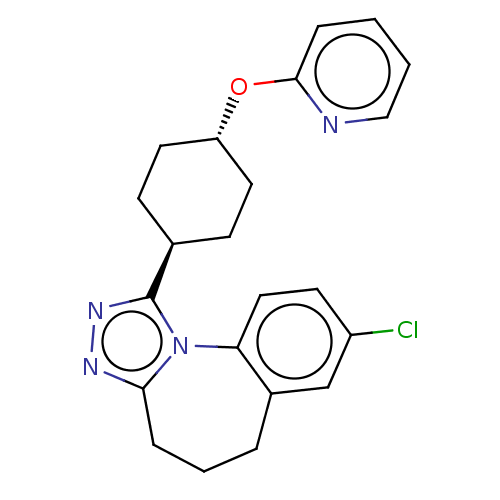
(CHEMBL4799793)Show SMILES Clc1ccc-2c(CCCc3nnc([C@H]4CC[C@@H](CC4)Oc4ccccn4)n-23)c1 |r,wU:13.12,wD:16.19,(20.56,-28.4,;21.91,-29.12,;23.23,-28.31,;24.58,-29.05,;24.62,-30.59,;23.31,-31.39,;23.13,-32.92,;24.2,-34.02,;25.73,-33.86,;26.56,-32.56,;28.09,-32.56,;28.56,-31.1,;27.32,-30.2,;27.31,-28.66,;25.98,-27.88,;25.99,-26.34,;27.34,-25.57,;28.67,-26.35,;28.66,-27.9,;27.34,-24.04,;26.01,-23.27,;26.02,-21.74,;24.69,-20.97,;23.36,-21.74,;23.36,-23.28,;24.69,-24.04,;26.07,-31.1,;21.96,-30.66,)| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-arginine-vasopressin from human V1A receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00863
BindingDB Entry DOI: 10.7270/Q2GM8C6F |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50382290

(CARIPRAZINE HYDROCHLORIDE | RGH-188 HCL)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc(Cl)c2Cl)CC1 |r,wU:6.5,wD:9.9,(.89,-12.18,;2.22,-12.95,;2.22,-14.49,;3.56,-12.18,;3.56,-10.64,;4.89,-12.95,;6.22,-12.18,;6.22,-10.64,;7.55,-9.86,;8.88,-10.64,;10.22,-9.87,;11.55,-10.65,;12.88,-9.88,;14.21,-10.66,;15.54,-9.9,;15.55,-8.36,;14.22,-7.58,;12.88,-8.35,;16.89,-7.6,;18.25,-8.33,;19.56,-7.51,;19.51,-5.97,;18.16,-5.25,;18.11,-3.71,;16.84,-6.06,;15.49,-5.33,;8.88,-12.18,;7.55,-12.94,)| Show InChI InChI=1S/C21H32Cl2N4O/c1-25(2)21(28)24-17-8-6-16(7-9-17)10-11-26-12-14-27(15-13-26)19-5-3-4-18(22)20(19)23/h3-5,16-17H,6-15H2,1-2H3,(H,24,28)/t16-,17- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Binding affinity to human dopamine D2S receptor |
Bioorg Med Chem Lett 22: 3437-40 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.104
BindingDB Entry DOI: 10.7270/Q2ZW1MZN |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50615423

(CHEMBL5281542)Show SMILES [H][C@@]12CC[C@H](N3CCCC3)[C@@]1(C)CC[C@]1([H])c3ccc(OCc4ccncc4)cc3CC[C@@]21[H] |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM35723

(CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(C(=O)N2CCCC(O)c3cc(Cl)ccc23)c(C)c1 Show InChI InChI=1S/C26H25ClN2O3/c1-16-6-3-4-7-20(16)25(31)28-19-10-11-21(17(2)14-19)26(32)29-13-5-8-24(30)22-15-18(27)9-12-23(22)29/h3-4,6-7,9-12,14-15,24,30H,5,8,13H2,1-2H3,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-arginine-vasopressin from human V2 receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindin... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00863
BindingDB Entry DOI: 10.7270/Q2GM8C6F |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50066519
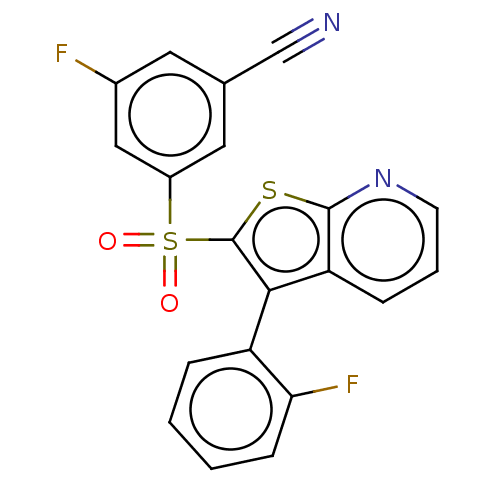
(CHEMBL3403102)Show SMILES Fc1cc(cc(c1)S(=O)(=O)c1sc2ncccc2c1-c1ccccc1F)C#N |(8.48,2.75,;7.25,2.74,;6.46,4.06,;4.92,4.05,;4.17,2.7,;4.95,1.38,;6.49,1.4,;4.2,.04,;4.82,-1.02,;5.43,.05,;2.66,.02,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;2.98,-5.69,;4.09,-4.62,;3.72,-3.12,;4.61,-2.27,;4.14,5.37,;3.51,6.43,)| Show InChI InChI=1S/C20H10F2N2O2S2/c21-13-8-12(11-23)9-14(10-13)28(25,26)20-18(15-4-1-2-6-17(15)22)16-5-3-7-24-19(16)27-20/h1-10H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-M-MPEP from rat cerebrocortical mGluR5 receptor |
Bioorg Med Chem Lett 25: 1724-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.073
BindingDB Entry DOI: 10.7270/Q2JS9S47 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50615410
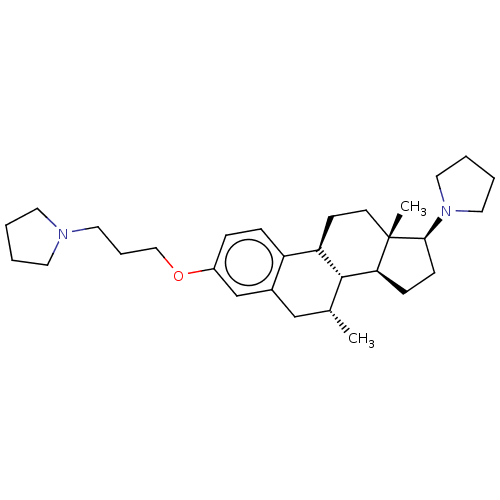
(CHEMBL5279080)Show SMILES [H][C@@]12CC[C@H](N3CCCC3)[C@@]1(C)CC[C@]1([H])c3ccc(OCCCN4CCCC4)cc3C[C@@H](C)[C@@]21[H] |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50615413
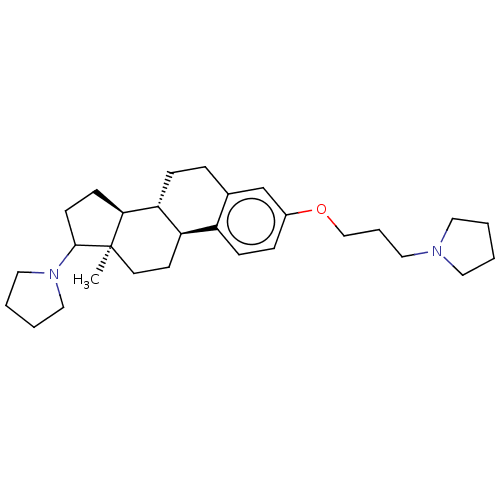
(CHEMBL5267299)Show SMILES [H][C@@]12CCC(N3CCCC3)[C@]1(C)CC[C@]1([H])c3ccc(OCCCN4CCCC4)cc3CC[C@@]21[H] |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50066730
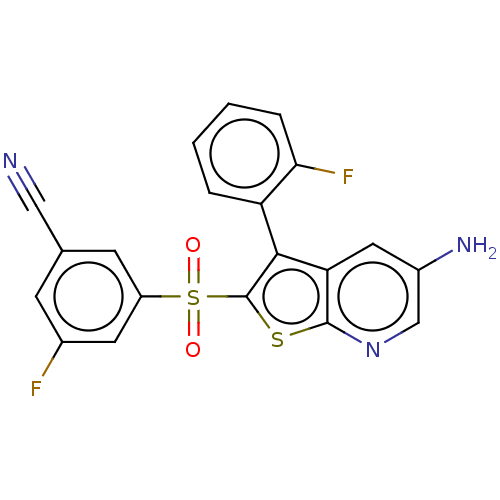
(CHEMBL3403126)Show SMILES Nc1cnc2sc(c(-c3ccccc3F)c2c1)S(=O)(=O)c1cc(F)cc(c1)C#N |(-3.45,-1.38,;-2.38,-.77,;-2.38,.77,;-1.03,1.55,;.3,.77,;1.76,1.24,;2.66,.02,;1.76,-1.24,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;2.98,-5.69,;4.09,-4.62,;3.72,-3.12,;4.61,-2.27,;.3,-.77,;-1.03,-1.55,;4.2,.04,;4.82,-1.02,;5.43,.05,;4.95,1.38,;6.49,1.4,;7.25,2.74,;8.48,2.75,;6.46,4.06,;4.92,4.05,;4.17,2.7,;4.14,5.37,;3.51,6.43,)| Show InChI InChI=1S/C20H11F2N3O2S2/c21-12-5-11(9-23)6-14(7-12)29(26,27)20-18(15-3-1-2-4-17(15)22)16-8-13(24)10-25-19(16)28-20/h1-8,10H,24H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-M-MPEP from rat cerebrocortical mGluR5 receptor |
Bioorg Med Chem Lett 25: 1724-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.073
BindingDB Entry DOI: 10.7270/Q2JS9S47 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50382306

(CHEMBL2024522)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2ccccc2Cl)CC1 |r,wU:6.5,wD:9.9,(5.86,-.97,;7.19,-1.74,;7.19,-3.28,;8.53,-.97,;8.53,.57,;9.86,-1.74,;11.19,-.97,;11.19,.57,;12.52,1.35,;13.85,.57,;15.19,1.33,;16.52,.56,;17.85,1.33,;19.18,.55,;20.51,1.31,;20.52,2.85,;19.19,3.62,;17.85,2.86,;21.86,3.61,;21.87,5.14,;23.2,5.91,;24.54,5.13,;24.53,3.58,;23.19,2.83,;23.18,1.29,;13.85,-.97,;12.52,-1.73,)| Show InChI InChI=1S/C21H33ClN4O/c1-24(2)21(27)23-18-9-7-17(8-10-18)11-12-25-13-15-26(16-14-25)20-6-4-3-5-19(20)22/h3-6,17-18H,7-16H2,1-2H3,(H,23,27)/t17-,18- | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from rat recombinant dopamine D3 receptor expressed in Sf9 cells |
Bioorg Med Chem Lett 22: 3437-40 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.104
BindingDB Entry DOI: 10.7270/Q2ZW1MZN |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50615425
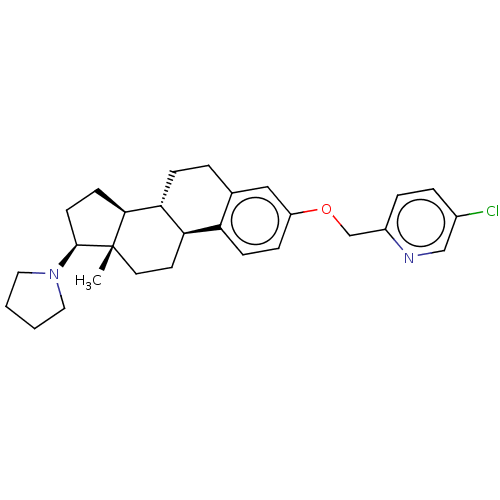
(CHEMBL5271387)Show SMILES [H][C@@]12CC[C@H](N3CCCC3)[C@@]1(C)CC[C@]1([H])c3ccc(OCc4ccc(Cl)cn4)cc3CC[C@@]21[H] |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50066735
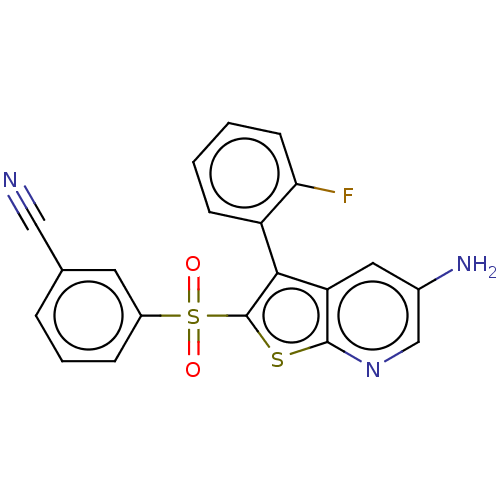
(CHEMBL3403121)Show SMILES Nc1cnc2sc(c(-c3ccccc3F)c2c1)S(=O)(=O)c1cccc(c1)C#N |(-3.45,-1.38,;-2.38,-.77,;-2.38,.77,;-1.03,1.55,;.3,.77,;1.76,1.24,;2.66,.02,;1.76,-1.24,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;2.98,-5.69,;4.09,-4.62,;3.72,-3.12,;4.61,-2.27,;.3,-.77,;-1.03,-1.55,;4.2,.04,;4.82,-1.02,;5.43,.05,;4.95,1.38,;4.17,2.7,;4.92,4.05,;6.46,4.06,;7.25,2.74,;6.49,1.4,;8.79,2.75,;10.02,2.77,)| Show InChI InChI=1S/C20H12FN3O2S2/c21-17-7-2-1-6-15(17)18-16-9-13(23)11-24-19(16)27-20(18)28(25,26)14-5-3-4-12(8-14)10-22/h1-9,11H,23H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-M-MPEP from rat cerebrocortical mGluR5 receptor |
Bioorg Med Chem Lett 25: 1724-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.073
BindingDB Entry DOI: 10.7270/Q2JS9S47 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50251027
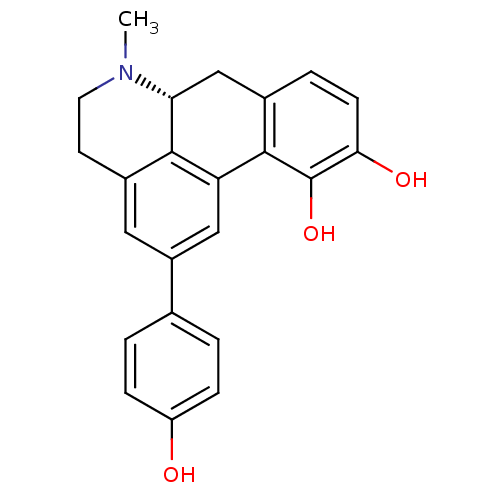
((R)-(-)-2-(4-Hydroxyphenyl)-apomorphine hydrochlor...)Show SMILES CN1CCc2cc(cc-3c2[C@H]1Cc1ccc(O)c(O)c-31)-c1ccc(O)cc1 |r| Show InChI InChI=1S/C23H21NO3/c1-24-9-8-15-10-16(13-2-5-17(25)6-3-13)11-18-21(15)19(24)12-14-4-7-20(26)23(27)22(14)18/h2-7,10-11,19,25-27H,8-9,12H2,1H3/t19-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from rat recombinant dopamine D3 receptor expressed in SF9 cells |
Bioorg Med Chem 16: 3773-9 (2008)
Article DOI: 10.1016/j.bmc.2008.01.057
BindingDB Entry DOI: 10.7270/Q2959HB7 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50615411

(CHEMBL5283960)Show SMILES [H][C@@]12CC[C@H](N3CCCC3)[C@@]1(C)CC[C@]1([H])c3ccc(OCCN4CCCC4)cc3CC[C@@]21[H] |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50066731
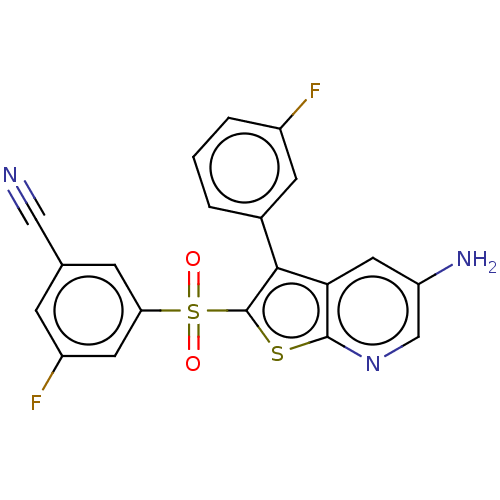
(CHEMBL3403125)Show SMILES Nc1cnc2sc(c(-c3cccc(F)c3)c2c1)S(=O)(=O)c1cc(F)cc(c1)C#N Show InChI InChI=1S/C20H11F2N3O2S2/c21-13-3-1-2-12(6-13)18-17-8-15(24)10-25-19(17)28-20(18)29(26,27)16-5-11(9-23)4-14(22)7-16/h1-8,10H,24H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-M-MPEP from rat cerebrocortical mGluR5 receptor |
Bioorg Med Chem Lett 25: 1724-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.073
BindingDB Entry DOI: 10.7270/Q2JS9S47 |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-5/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50605882
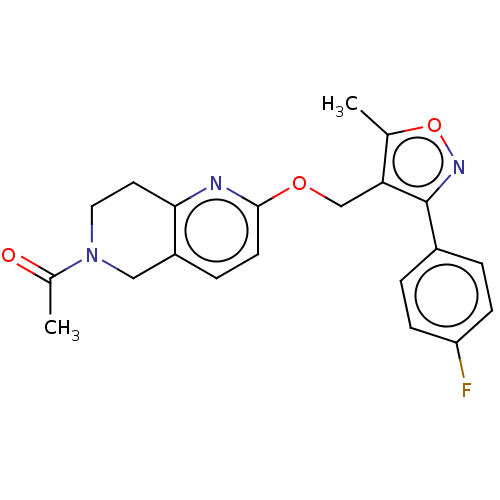
(CHEMBL5202280)Show SMILES CC(=O)N1CCc2nc(OCc3c(C)onc3-c3ccc(F)cc3)ccc2C1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00414
BindingDB Entry DOI: 10.7270/Q2DV1Q01 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50536759
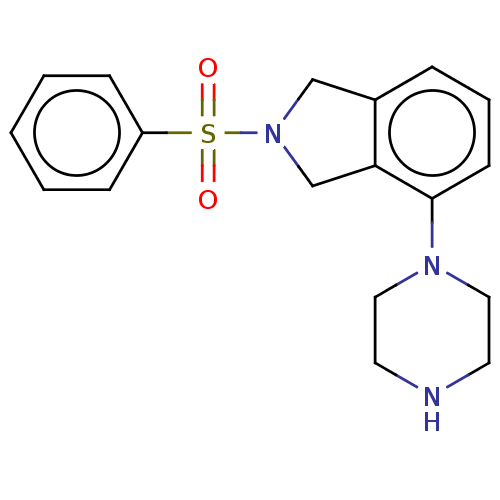
(CHEMBL4527051)Show InChI InChI=1S/C18H21N3O2S/c22-24(23,16-6-2-1-3-7-16)21-13-15-5-4-8-18(17(15)14-21)20-11-9-19-10-12-20/h1-8,19H,9-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5-HT6 receptor expressed in CHO-K1 cells incubated for 3 hrs by scintillation counting analysis |
Bioorg Med Chem Lett 26: 4211-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.055
BindingDB Entry DOI: 10.7270/Q2D50RGF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50525813
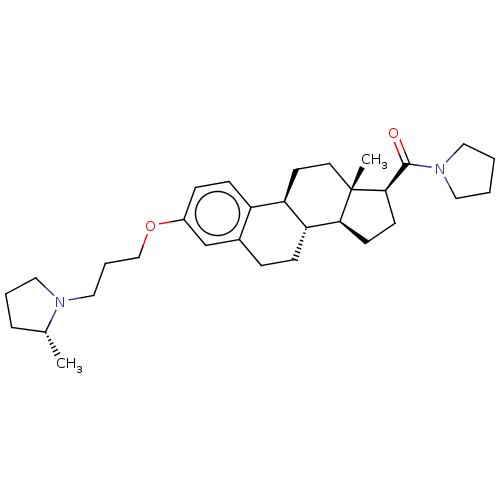
(CHEMBL4579337)Show SMILES [H][C@@]12CC[C@H](C(=O)N3CCCC3)[C@@]1(C)CC[C@]1([H])c3ccc(OCCCN4CCC[C@H]4C)cc3CC[C@@]21[H] |r| Show InChI InChI=1S/C31H46N2O2/c1-22-7-5-18-32(22)19-6-20-35-24-9-11-25-23(21-24)8-10-27-26(25)14-15-31(2)28(27)12-13-29(31)30(34)33-16-3-4-17-33/h9,11,21-22,26-29H,3-8,10,12-20H2,1-2H3/t22-,26-,27-,28+,29-,31+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Works of Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyl- N-alpha methylhistamine dihydrochloride from human recombinant H3 receptor expressed in CHOK1 cells measured after 30 min... |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126643
BindingDB Entry DOI: 10.7270/Q22F7RW4 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50066366
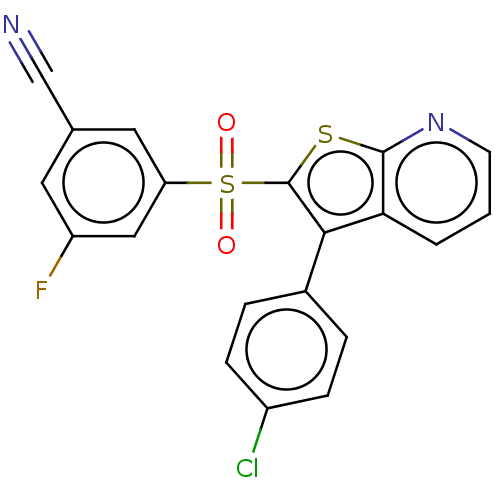
(CHEMBL3401592)Show SMILES Fc1cc(cc(c1)S(=O)(=O)c1sc2ncccc2c1-c1ccc(Cl)cc1)C#N Show InChI InChI=1S/C20H10ClFN2O2S2/c21-14-5-3-13(4-6-14)18-17-2-1-7-24-19(17)27-20(18)28(25,26)16-9-12(11-23)8-15(22)10-16/h1-10H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-M-MPEP from rat cerebrocortical mGluR5 receptor |
Bioorg Med Chem Lett 25: 1724-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.073
BindingDB Entry DOI: 10.7270/Q2JS9S47 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50615421

(CHEMBL5288889)Show SMILES [H][C@@]12CC[C@H](N3CCCC3)[C@@]1(C)CC[C@]1([H])c3ccc(OCCCN4CCCCCC4)cc3CC[C@@]21[H] |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50615408
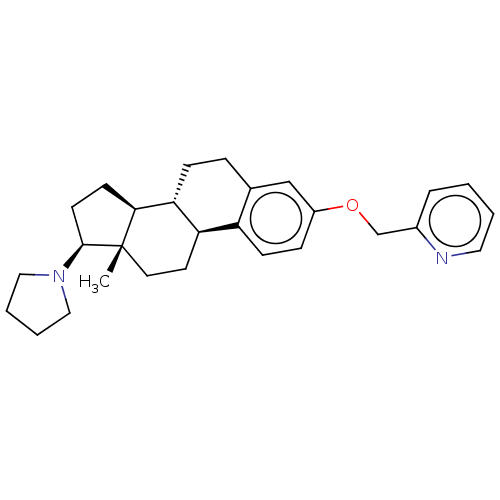
(CHEMBL5278683)Show SMILES [H][C@@]12CC[C@H](N3CCCC3)[C@@]1(C)CC[C@]1([H])c3ccc(OCc4ccccn4)cc3CC[C@@]21[H] |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50066734
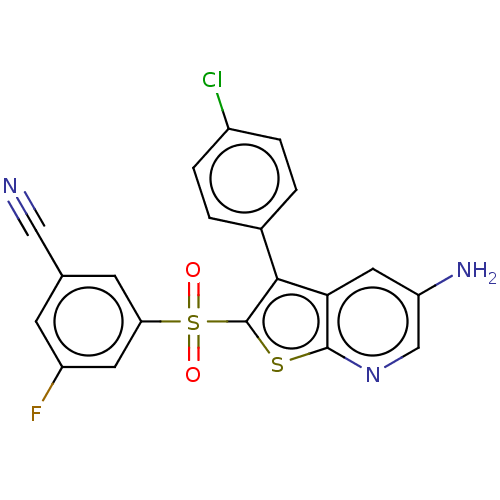
(CHEMBL3403122)Show SMILES Nc1cnc2sc(c(-c3ccc(Cl)cc3)c2c1)S(=O)(=O)c1cc(F)cc(c1)C#N Show InChI InChI=1S/C20H11ClFN3O2S2/c21-13-3-1-12(2-4-13)18-17-8-15(24)10-25-19(17)28-20(18)29(26,27)16-6-11(9-23)5-14(22)7-16/h1-8,10H,24H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-M-MPEP from rat cerebrocortical mGluR5 receptor |
Bioorg Med Chem Lett 25: 1724-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.073
BindingDB Entry DOI: 10.7270/Q2JS9S47 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50066520
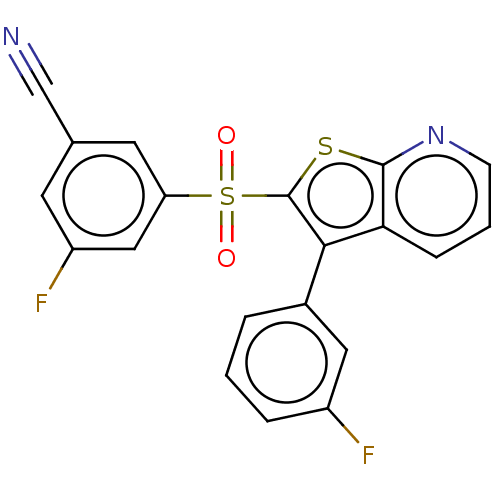
(CHEMBL3403101)Show SMILES Fc1cccc(c1)-c1c(sc2ncccc12)S(=O)(=O)c1cc(F)cc(c1)C#N Show InChI InChI=1S/C20H10F2N2O2S2/c21-14-4-1-3-13(9-14)18-17-5-2-6-24-19(17)27-20(18)28(25,26)16-8-12(11-23)7-15(22)10-16/h1-10H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-M-MPEP from rat cerebrocortical mGluR5 receptor |
Bioorg Med Chem Lett 25: 1724-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.073
BindingDB Entry DOI: 10.7270/Q2JS9S47 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50536757
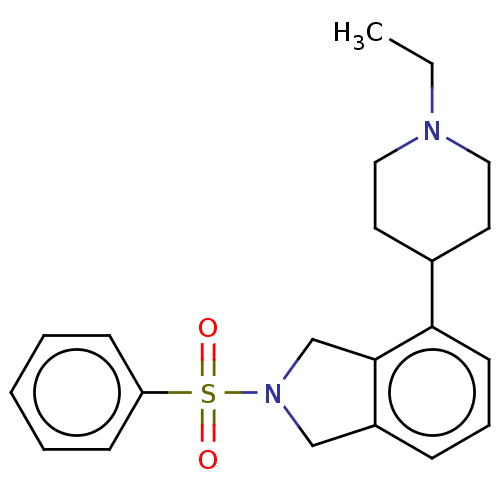
(CHEMBL4548434)Show SMILES CCN1CCC(CC1)c1cccc2CN(Cc12)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C21H26N2O2S/c1-2-22-13-11-17(12-14-22)20-10-6-7-18-15-23(16-21(18)20)26(24,25)19-8-4-3-5-9-19/h3-10,17H,2,11-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5-HT6 receptor expressed in CHO-K1 cells incubated for 3 hrs by scintillation counting analysis |
Bioorg Med Chem Lett 26: 4211-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.055
BindingDB Entry DOI: 10.7270/Q2D50RGF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50525810

(CHEMBL4451680)Show SMILES [H][C@@]12CC[C@H](N3CCCC3)[C@@]1(C)CC[C@]1([H])c3ccc(OCCCN4CCCC4)cc3CC[C@@]21[H] |r| Show InChI InChI=1S/C29H44N2O/c1-29-14-13-25-24-10-8-23(32-20-6-17-30-15-2-3-16-30)21-22(24)7-9-26(25)27(29)11-12-28(29)31-18-4-5-19-31/h8,10,21,25-28H,2-7,9,11-20H2,1H3/t25-,26-,27+,28+,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Works of Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyl- N-alpha methylhistamine dihydrochloride from Sprague-Dawley rat cerebral cortex H3 receptor measured after 30 mins by sci... |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126643
BindingDB Entry DOI: 10.7270/Q22F7RW4 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50525810

(CHEMBL4451680)Show SMILES [H][C@@]12CC[C@H](N3CCCC3)[C@@]1(C)CC[C@]1([H])c3ccc(OCCCN4CCCC4)cc3CC[C@@]21[H] |r| Show InChI InChI=1S/C29H44N2O/c1-29-14-13-25-24-10-8-23(32-20-6-17-30-15-2-3-16-30)21-22(24)7-9-26(25)27(29)11-12-28(29)31-18-4-5-19-31/h8,10,21,25-28H,2-7,9,11-20H2,1H3/t25-,26-,27+,28+,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50066728
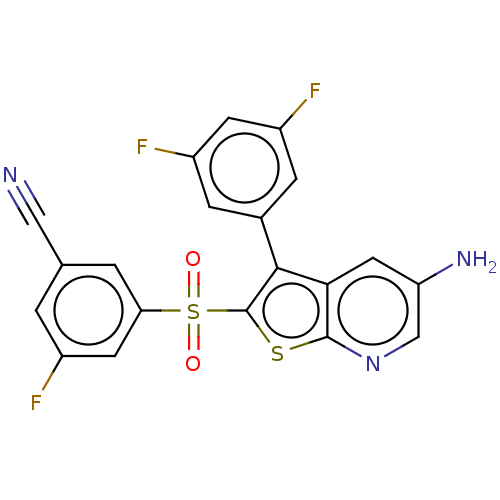
(CHEMBL3403128)Show SMILES Nc1cnc2sc(c(-c3cc(F)cc(F)c3)c2c1)S(=O)(=O)c1cc(F)cc(c1)C#N Show InChI InChI=1S/C20H10F3N3O2S2/c21-12-1-10(8-24)2-16(6-12)30(27,28)20-18(11-3-13(22)5-14(23)4-11)17-7-15(25)9-26-19(17)29-20/h1-7,9H,25H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-M-MPEP from rat cerebrocortical mGluR5 receptor |
Bioorg Med Chem Lett 25: 1724-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.073
BindingDB Entry DOI: 10.7270/Q2JS9S47 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50066521
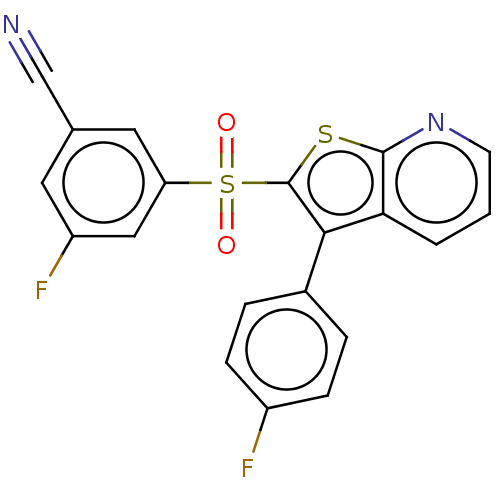
(CHEMBL3403100)Show SMILES Fc1ccc(cc1)-c1c(sc2ncccc12)S(=O)(=O)c1cc(F)cc(c1)C#N Show InChI InChI=1S/C20H10F2N2O2S2/c21-14-5-3-13(4-6-14)18-17-2-1-7-24-19(17)27-20(18)28(25,26)16-9-12(11-23)8-15(22)10-16/h1-10H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-M-MPEP from rat cerebrocortical mGluR5 receptor |
Bioorg Med Chem Lett 25: 1724-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.073
BindingDB Entry DOI: 10.7270/Q2JS9S47 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50066671
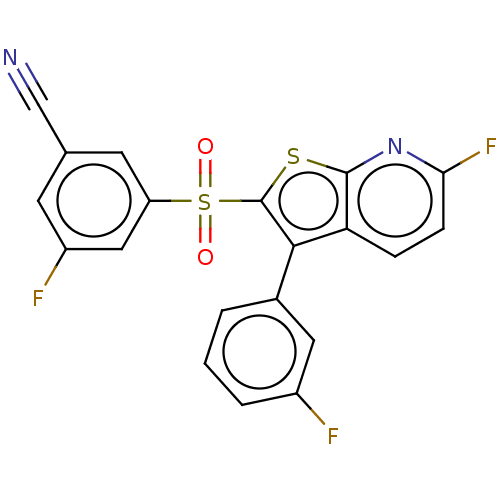
(CHEMBL3403146)Show SMILES Fc1cccc(c1)-c1c(sc2nc(F)ccc12)S(=O)(=O)c1cc(F)cc(c1)C#N Show InChI InChI=1S/C20H9F3N2O2S2/c21-13-3-1-2-12(8-13)18-16-4-5-17(23)25-19(16)28-20(18)29(26,27)15-7-11(10-24)6-14(22)9-15/h1-9H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-M-MPEP from rat cerebrocortical mGluR5 receptor |
Bioorg Med Chem Lett 25: 1724-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.073
BindingDB Entry DOI: 10.7270/Q2JS9S47 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50536760
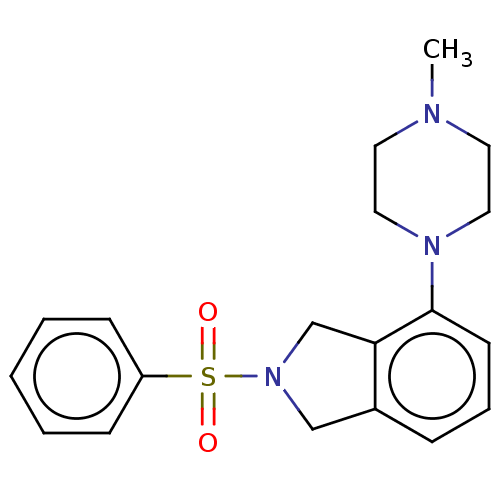
(CHEMBL4572260)Show SMILES CN1CCN(CC1)c1cccc2CN(Cc12)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C19H23N3O2S/c1-20-10-12-21(13-11-20)19-9-5-6-16-14-22(15-18(16)19)25(23,24)17-7-3-2-4-8-17/h2-9H,10-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5-HT6 receptor expressed in CHO-K1 cells incubated for 3 hrs by scintillation counting analysis |
Bioorg Med Chem Lett 26: 4211-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.055
BindingDB Entry DOI: 10.7270/Q2D50RGF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50536728
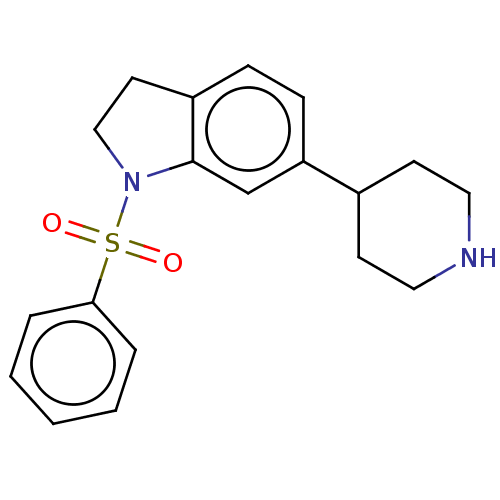
(CHEMBL4551858)Show InChI InChI=1S/C19H22N2O2S/c22-24(23,18-4-2-1-3-5-18)21-13-10-16-6-7-17(14-19(16)21)15-8-11-20-12-9-15/h1-7,14-15,20H,8-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5-HT6 receptor expressed in CHO-K1 cells incubated for 3 hrs by scintillation counting analysis |
Bioorg Med Chem Lett 26: 4211-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.055
BindingDB Entry DOI: 10.7270/Q2D50RGF |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-5/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50605891
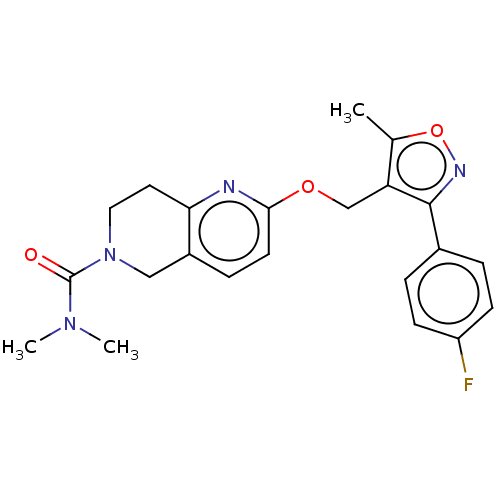
(CHEMBL5174560)Show SMILES CN(C)C(=O)N1CCc2nc(OCc3c(C)onc3-c3ccc(F)cc3)ccc2C1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00414
BindingDB Entry DOI: 10.7270/Q2DV1Q01 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50066729
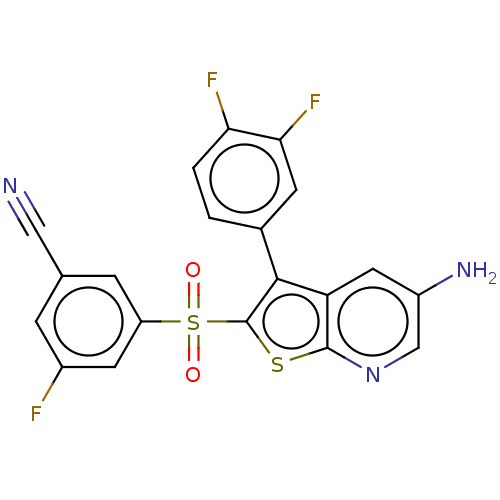
(CHEMBL3403127)Show SMILES Nc1cnc2sc(c(-c3ccc(F)c(F)c3)c2c1)S(=O)(=O)c1cc(F)cc(c1)C#N Show InChI InChI=1S/C20H10F3N3O2S2/c21-12-3-10(8-24)4-14(6-12)30(27,28)20-18(11-1-2-16(22)17(23)5-11)15-7-13(25)9-26-19(15)29-20/h1-7,9H,25H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-M-MPEP from rat cerebrocortical mGluR5 receptor |
Bioorg Med Chem Lett 25: 1724-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.073
BindingDB Entry DOI: 10.7270/Q2JS9S47 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data