 Found 56 hits with Last Name = 'guan' and Initial = 'w'
Found 56 hits with Last Name = 'guan' and Initial = 'w' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50132351
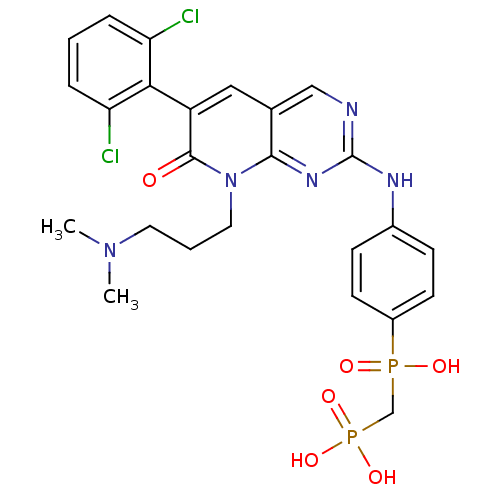
(({4-[6-(2,6-Dichloro-phenyl)-8-(3-dimethylamino-pr...)Show SMILES CN(C)CCCn1c2nc(Nc3ccc(cc3)P(O)(=O)CP(O)(O)=O)ncc2cc(-c2c(Cl)cccc2Cl)c1=O |(3.9,-7.22,;3.9,-5.68,;2.57,-4.91,;5.23,-4.91,;5.23,-3.37,;6.56,-2.59,;6.56,-1.05,;5.21,-.28,;3.88,-1.05,;2.55,-.29,;1.22,-1.06,;-.11,-.29,;-.11,1.25,;-1.44,2.01,;-2.78,1.23,;-2.77,-.31,;-1.44,-1.07,;-4.12,2,;-4.89,.67,;-3.35,3.34,;-5.45,2.77,;-5.45,4.31,;-6.99,4.32,;-5.86,5.8,;-4.12,5.09,;2.55,1.26,;3.88,2.03,;5.21,1.26,;6.54,2.03,;7.89,1.25,;9.22,2.02,;9.22,3.57,;7.89,4.33,;10.55,4.36,;11.88,3.57,;11.88,2.02,;10.55,1.25,;10.55,-.29,;7.89,-.28,;9.22,-1.05,)| Show InChI InChI=1S/C25H27Cl2N5O6P2/c1-31(2)11-4-12-32-23-16(13-19(24(32)33)22-20(26)5-3-6-21(22)27)14-28-25(30-23)29-17-7-9-18(10-8-17)39(34,35)15-40(36,37)38/h3,5-10,13-14H,4,11-12,15H2,1-2H3,(H,34,35)(H,28,29,30)(H2,36,37,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Src protein tryrosine kinase |
Bioorg Med Chem Lett 13: 3071-4 (2003)
BindingDB Entry DOI: 10.7270/Q21J995T |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Mus musculus) | BDBM50509436
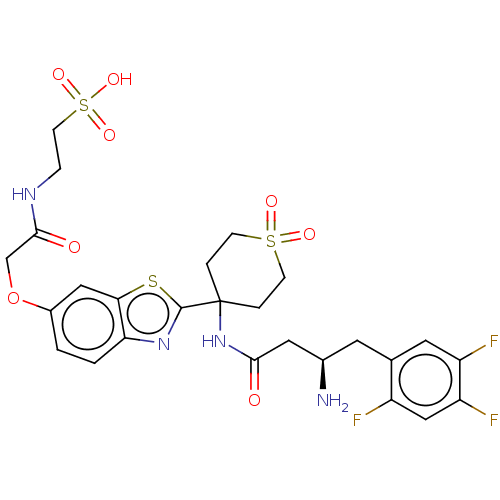
(CHEMBL4463027)Show SMILES OC(=O)C(F)(F)F.N[C@@H](CC(=O)NC1(CCS(=O)(=O)CC1)c1nc2ccc(OCC(=O)NCCS(O)(=O)=O)cc2s1)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C26H29F3N4O8S3.C2HF3O2/c27-18-13-20(29)19(28)10-15(18)9-16(30)11-23(34)33-26(3-6-43(36,37)7-4-26)25-32-21-2-1-17(12-22(21)42-25)41-14-24(35)31-5-8-44(38,39)40;3-2(4,5)1(6)7/h1-2,10,12-13,16H,3-9,11,14,30H2,(H,31,35)(H,33,34)(H,38,39,40);(H,6,7)/t16-;/m1./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in C57BL/6 mouse serum pre-incubated for 5 mins followed by Gly-Pro-7-AMC substrate addition by fluorescence based assay |
J Med Chem 62: 10919-10925 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01649
BindingDB Entry DOI: 10.7270/Q2PN98X6 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Mus musculus) | BDBM50509435
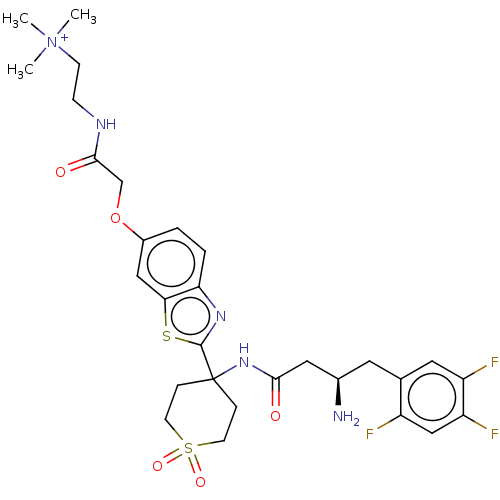
(CHEMBL4593371)Show SMILES OC(=O)C(F)(F)F.[O-]C(=O)C(F)(F)F.C[N+](C)(C)CCNC(=O)COc1ccc2nc(sc2c1)C1(CCS(=O)(=O)CC1)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C29H36F3N5O5S2.2C2HF3O2/c1-37(2,3)9-8-34-27(39)17-42-20-4-5-24-25(15-20)43-28(35-24)29(6-10-44(40,41)11-7-29)36-26(38)14-19(33)12-18-13-22(31)23(32)16-21(18)30;2*3-2(4,5)1(6)7/h4-5,13,15-16,19H,6-12,14,17,33H2,1-3H3,(H-,34,36,38,39);2*(H,6,7)/t19-;;/m1../s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in C57BL/6 mouse serum pre-incubated for 5 mins followed by Gly-Pro-7-AMC substrate addition by fluorescence based assay |
J Med Chem 62: 10919-10925 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01649
BindingDB Entry DOI: 10.7270/Q2PN98X6 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Mus musculus) | BDBM50509441
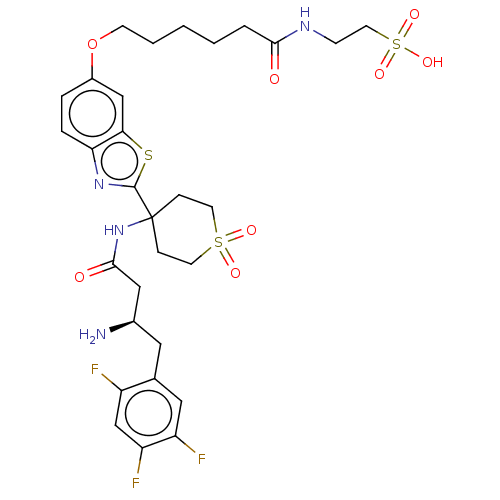
(CHEMBL4452661)Show SMILES OC(=O)C(F)(F)F.N[C@@H](CC(=O)NC1(CCS(=O)(=O)CC1)c1nc2ccc(OCCCCCC(=O)NCCS(O)(=O)=O)cc2s1)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C30H37F3N4O8S3.C2HF3O2/c31-22-18-24(33)23(32)15-19(22)14-20(34)16-28(39)37-30(7-11-47(40,41)12-8-30)29-36-25-6-5-21(17-26(25)46-29)45-10-3-1-2-4-27(38)35-9-13-48(42,43)44;3-2(4,5)1(6)7/h5-6,15,17-18,20H,1-4,7-14,16,34H2,(H,35,38)(H,37,39)(H,42,43,44);(H,6,7)/t20-;/m1./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in C57BL/6 mouse serum pre-incubated for 5 mins followed by Gly-Pro-7-AMC substrate addition by fluorescence based assay |
J Med Chem 62: 10919-10925 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01649
BindingDB Entry DOI: 10.7270/Q2PN98X6 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50132348
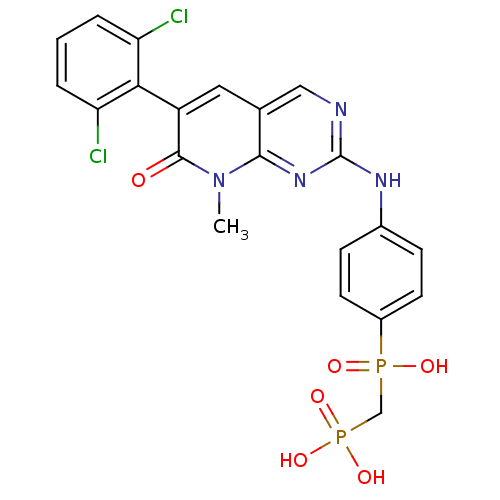
(({4-[6-(2,6-Dichloro-phenyl)-8-methyl-7-oxo-7,8-di...)Show SMILES Cn1c2nc(Nc3ccc(cc3)P(O)(=O)CP(O)(O)=O)ncc2cc(-c2c(Cl)cccc2Cl)c1=O |(3.94,-2.74,;3.93,-1.2,;2.6,-.43,;1.27,-1.2,;-.06,-.43,;-1.39,-1.21,;-2.73,-.45,;-2.73,1.1,;-4.06,1.86,;-5.41,1.09,;-5.38,-.47,;-4.05,-1.22,;-6.74,1.86,;-7.53,.53,;-5.97,3.19,;-8.09,2.61,;-9.4,1.82,;-10.19,3.17,;-10.91,1.42,;-9.02,.34,;-.07,1.11,;1.27,1.88,;2.6,1.11,;3.93,1.88,;5.26,1.11,;6.59,1.88,;6.59,3.42,;5.26,4.19,;7.92,4.19,;9.26,3.42,;9.26,1.88,;7.92,1.11,;7.92,-.43,;5.26,-.43,;6.6,-1.2,)| Show InChI InChI=1S/C21H18Cl2N4O6P2/c1-27-19-12(9-15(20(27)28)18-16(22)3-2-4-17(18)23)10-24-21(26-19)25-13-5-7-14(8-6-13)34(29,30)11-35(31,32)33/h2-10H,11H2,1H3,(H,29,30)(H,24,25,26)(H2,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Src protein tryrosine kinase |
Bioorg Med Chem Lett 13: 3071-4 (2003)
BindingDB Entry DOI: 10.7270/Q21J995T |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Mus musculus) | BDBM50509440
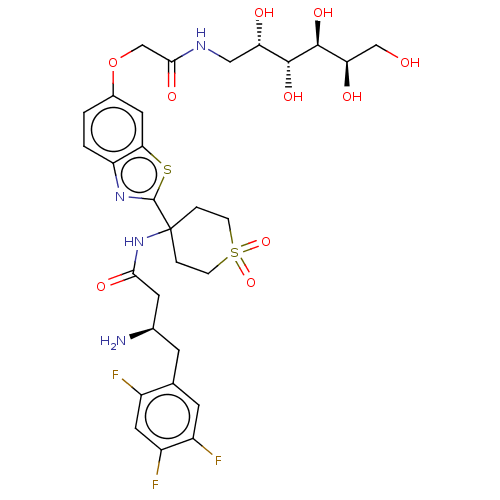
(CHEMBL4533178)Show SMILES OC(=O)C(F)(F)F.N[C@@H](CC(=O)NC1(CCS(=O)(=O)CC1)c1nc2ccc(OCC(=O)NC[C@H](O)[C@@H](O)[C@@H](O)[C@H](O)CO)cc2s1)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C30H37F3N4O10S2.C2HF3O2/c31-18-11-20(33)19(32)8-15(18)7-16(34)9-25(41)37-30(3-5-49(45,46)6-4-30)29-36-21-2-1-17(10-24(21)48-29)47-14-26(42)35-12-22(39)27(43)28(44)23(40)13-38;3-2(4,5)1(6)7/h1-2,8,10-11,16,22-23,27-28,38-40,43-44H,3-7,9,12-14,34H2,(H,35,42)(H,37,41);(H,6,7)/t16-,22+,23-,27-,28+;/m1./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in C57BL/6 mouse serum pre-incubated for 5 mins followed by Gly-Pro-7-AMC substrate addition by fluorescence based assay |
J Med Chem 62: 10919-10925 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01649
BindingDB Entry DOI: 10.7270/Q2PN98X6 |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50512267
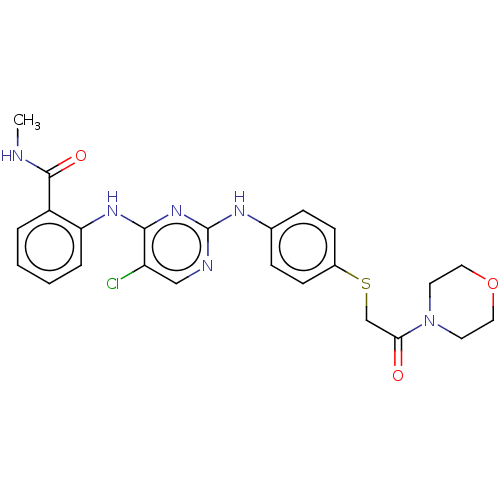
(CHEMBL4471571)Show SMILES CNC(=O)c1ccccc1Nc1nc(Nc2ccc(SCC(=O)N3CCOCC3)cc2)ncc1Cl Show InChI InChI=1S/C24H25ClN6O3S/c1-26-23(33)18-4-2-3-5-20(18)29-22-19(25)14-27-24(30-22)28-16-6-8-17(9-7-16)35-15-21(32)31-10-12-34-13-11-31/h2-9,14H,10-13,15H2,1H3,(H,26,33)(H2,27,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
The First Affiliated Hospital of Dalian Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His-tagged FAK (393 to 698 residues) expressed in baculovirus infected Sf9 insect cells using Poly (4:1 Gl... |
Eur J Med Chem 172: 154-162 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.004
BindingDB Entry DOI: 10.7270/Q23T9MK9 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Mus musculus) | BDBM50509434

(CHEMBL4443285)Show SMILES OC(=O)C(F)(F)F.[O-]C(=O)C(F)(F)F.C[N+](C)(C)CCNC(=O)CCCCCOc1ccc2nc(sc2c1)C1(CCS(=O)(=O)CC1)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C33H44F3N5O5S2.2C2HF3O2/c1-41(2,3)13-12-38-30(42)7-5-4-6-14-46-24-8-9-28-29(20-24)47-32(39-28)33(10-15-48(44,45)16-11-33)40-31(43)19-23(37)17-22-18-26(35)27(36)21-25(22)34;2*3-2(4,5)1(6)7/h8-9,18,20-21,23H,4-7,10-17,19,37H2,1-3H3,(H-,38,40,42,43);2*(H,6,7)/t23-;;/m1../s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in C57BL/6 mouse serum pre-incubated for 5 mins followed by Gly-Pro-7-AMC substrate addition by fluorescence based assay |
J Med Chem 62: 10919-10925 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01649
BindingDB Entry DOI: 10.7270/Q2PN98X6 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Mus musculus) | BDBM50509433
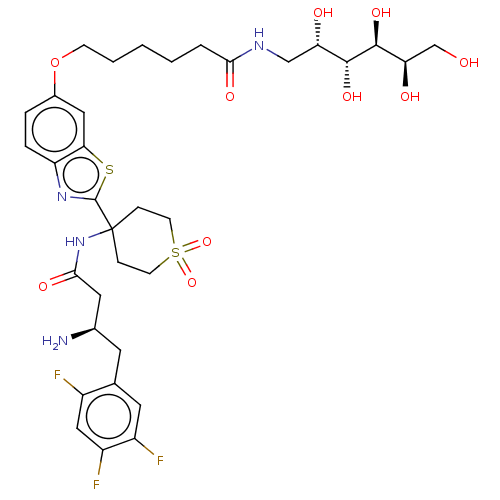
(CHEMBL4532312)Show SMILES OC(=O)C(F)(F)F.N[C@@H](CC(=O)NC1(CCS(=O)(=O)CC1)c1nc2ccc(OCCCCCC(=O)NC[C@H](O)[C@@H](O)[C@@H](O)[C@H](O)CO)cc2s1)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C34H45F3N4O10S2.C2HF3O2/c35-22-16-24(37)23(36)13-19(22)12-20(38)14-30(46)41-34(7-10-53(49,50)11-8-34)33-40-25-6-5-21(15-28(25)52-33)51-9-3-1-2-4-29(45)39-17-26(43)31(47)32(48)27(44)18-42;3-2(4,5)1(6)7/h5-6,13,15-16,20,26-27,31-32,42-44,47-48H,1-4,7-12,14,17-18,38H2,(H,39,45)(H,41,46);(H,6,7)/t20-,26+,27-,31-,32+;/m1./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in C57BL/6 mouse serum pre-incubated for 5 mins followed by Gly-Pro-7-AMC substrate addition by fluorescence based assay |
J Med Chem 62: 10919-10925 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01649
BindingDB Entry DOI: 10.7270/Q2PN98X6 |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50512265
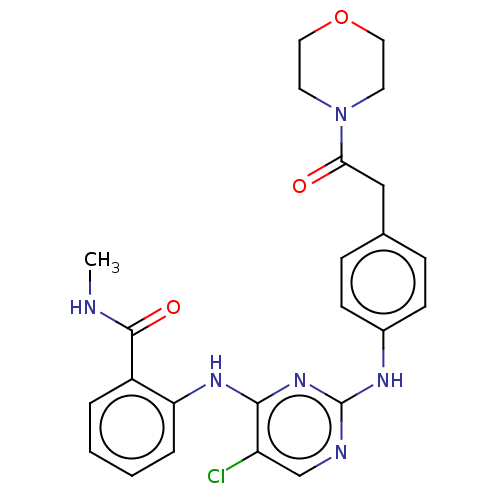
(CHEMBL4587135)Show SMILES CNC(=O)c1ccccc1Nc1nc(Nc2ccc(CC(=O)N3CCOCC3)cc2)ncc1Cl Show InChI InChI=1S/C24H25ClN6O3/c1-26-23(33)18-4-2-3-5-20(18)29-22-19(25)15-27-24(30-22)28-17-8-6-16(7-9-17)14-21(32)31-10-12-34-13-11-31/h2-9,15H,10-14H2,1H3,(H,26,33)(H2,27,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
The First Affiliated Hospital of Dalian Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His-tagged FAK (393 to 698 residues) expressed in baculovirus infected Sf9 insect cells using Poly (4:1 Gl... |
Eur J Med Chem 172: 154-162 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.004
BindingDB Entry DOI: 10.7270/Q23T9MK9 |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50334594
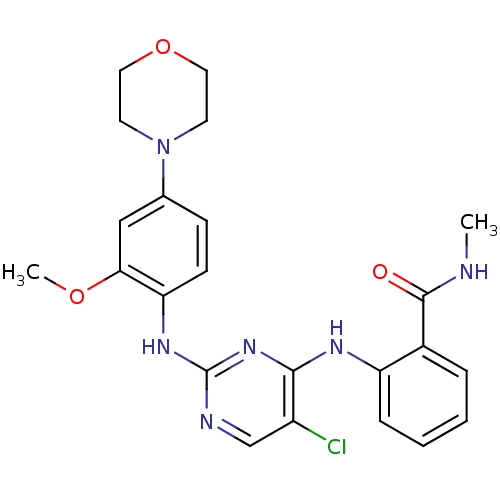
(2-(5-chloro-2-(2-methoxy-4-morpholinophenylamino)p...)Show SMILES CNC(=O)c1ccccc1Nc1nc(Nc2ccc(cc2OC)N2CCOCC2)ncc1Cl Show InChI InChI=1S/C23H25ClN6O3/c1-25-22(31)16-5-3-4-6-18(16)27-21-17(24)14-26-23(29-21)28-19-8-7-15(13-20(19)32-2)30-9-11-33-12-10-30/h3-8,13-14H,9-12H2,1-2H3,(H,25,31)(H2,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
The First Affiliated Hospital of Dalian Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His-tagged FAK (393 to 698 residues) expressed in baculovirus infected Sf9 insect cells using Poly (4:1 Gl... |
Eur J Med Chem 172: 154-162 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.004
BindingDB Entry DOI: 10.7270/Q23T9MK9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50512263
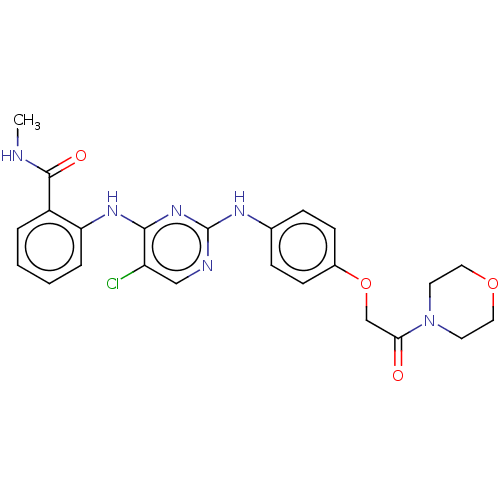
(CHEMBL4580829)Show SMILES CNC(=O)c1ccccc1Nc1nc(Nc2ccc(OCC(=O)N3CCOCC3)cc2)ncc1Cl Show InChI InChI=1S/C24H25ClN6O4/c1-26-23(33)18-4-2-3-5-20(18)29-22-19(25)14-27-24(30-22)28-16-6-8-17(9-7-16)35-15-21(32)31-10-12-34-13-11-31/h2-9,14H,10-13,15H2,1H3,(H,26,33)(H2,27,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
The First Affiliated Hospital of Dalian Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His-tagged FAK (393 to 698 residues) expressed in baculovirus infected Sf9 insect cells using Poly (4:1 Gl... |
Eur J Med Chem 172: 154-162 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.004
BindingDB Entry DOI: 10.7270/Q23T9MK9 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Mus musculus) | BDBM11162
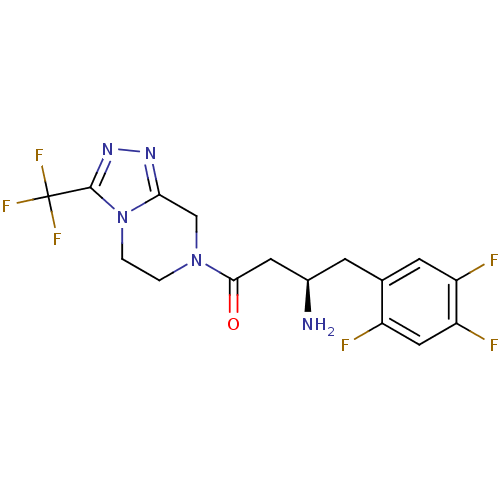
((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...)Show SMILES N[C@@H](CC(=O)N1CCn2c(C1)nnc2C(F)(F)F)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C16H15F6N5O/c17-10-6-12(19)11(18)4-8(10)3-9(23)5-14(28)26-1-2-27-13(7-26)24-25-15(27)16(20,21)22/h4,6,9H,1-3,5,7,23H2/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in C57BL/6 mouse serum pre-incubated for 5 mins followed by Gly-Pro-7-AMC substrate addition by fluorescence based assay |
J Med Chem 62: 10919-10925 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01649
BindingDB Entry DOI: 10.7270/Q2PN98X6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM3071

(2-aminopyrido[2,3-d]pyrimidin-7(8H)-one 38 | 2-ani...)Show SMILES Cn1c2nc(Nc3ccccc3)ncc2cc(-c2c(Cl)cccc2Cl)c1=O |(-2.82,-1.97,;-2.82,-.43,;-4.15,.34,;-5.48,-.43,;-6.82,.34,;-8.15,-.43,;-8.15,-1.97,;-6.81,-2.74,;-6.81,-4.28,;-8.14,-5.05,;-9.48,-4.29,;-9.48,-2.75,;-6.82,1.88,;-5.48,2.65,;-4.15,1.88,;-2.82,2.65,;-1.48,1.88,;-.15,2.65,;-.15,4.19,;-1.48,4.96,;1.18,4.96,;2.52,4.19,;2.52,2.65,;1.18,1.88,;1.18,.34,;-1.48,.34,;-.15,-.43,)| Show InChI InChI=1S/C20H14Cl2N4O/c1-26-18-12(11-23-20(25-18)24-13-6-3-2-4-7-13)10-14(19(26)27)17-15(21)8-5-9-16(17)22/h2-11H,1H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Src protein tryrosine kinase |
Bioorg Med Chem Lett 13: 3071-4 (2003)
BindingDB Entry DOI: 10.7270/Q21J995T |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50512266
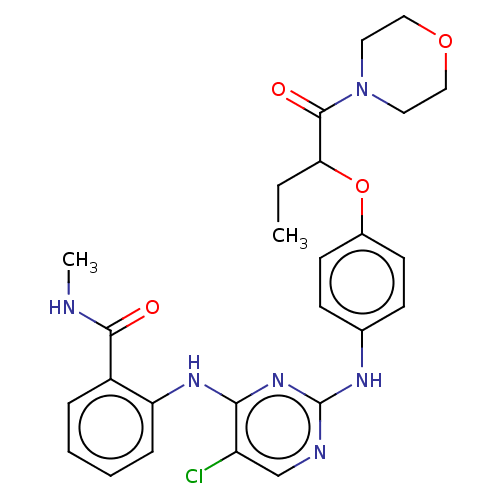
(CHEMBL4461676)Show SMILES CCC(Oc1ccc(Nc2ncc(Cl)c(Nc3ccccc3C(=O)NC)n2)cc1)C(=O)N1CCOCC1 Show InChI InChI=1S/C26H29ClN6O4/c1-3-22(25(35)33-12-14-36-15-13-33)37-18-10-8-17(9-11-18)30-26-29-16-20(27)23(32-26)31-21-7-5-4-6-19(21)24(34)28-2/h4-11,16,22H,3,12-15H2,1-2H3,(H,28,34)(H2,29,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
The First Affiliated Hospital of Dalian Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His-tagged FAK (393 to 698 residues) expressed in baculovirus infected Sf9 insect cells using Poly (4:1 Gl... |
Eur J Med Chem 172: 154-162 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.004
BindingDB Entry DOI: 10.7270/Q23T9MK9 |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50512268
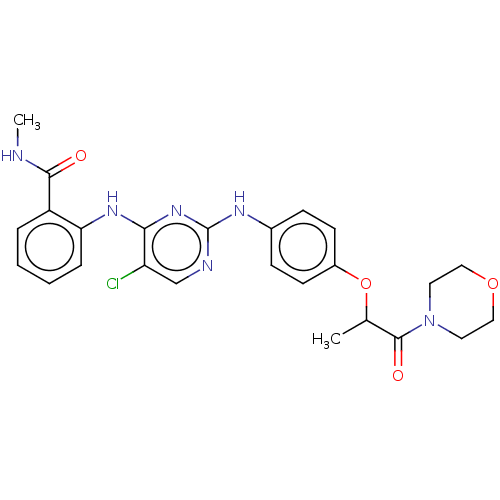
(CHEMBL4444509)Show SMILES CNC(=O)c1ccccc1Nc1nc(Nc2ccc(OC(C)C(=O)N3CCOCC3)cc2)ncc1Cl Show InChI InChI=1S/C25H27ClN6O4/c1-16(24(34)32-11-13-35-14-12-32)36-18-9-7-17(8-10-18)29-25-28-15-20(26)22(31-25)30-21-6-4-3-5-19(21)23(33)27-2/h3-10,15-16H,11-14H2,1-2H3,(H,27,33)(H2,28,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
The First Affiliated Hospital of Dalian Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His-tagged FAK (393 to 698 residues) expressed in baculovirus infected Sf9 insect cells using Poly (4:1 Gl... |
Eur J Med Chem 172: 154-162 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.004
BindingDB Entry DOI: 10.7270/Q23T9MK9 |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50512264
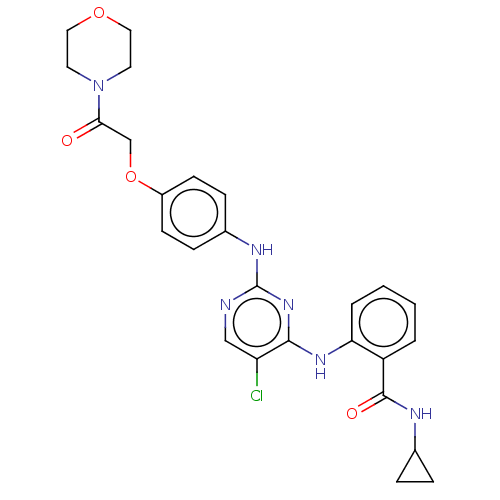
(CHEMBL4452825)Show SMILES Clc1cnc(Nc2ccc(OCC(=O)N3CCOCC3)cc2)nc1Nc1ccccc1C(=O)NC1CC1 Show InChI InChI=1S/C26H27ClN6O4/c27-21-15-28-26(32-24(21)31-22-4-2-1-3-20(22)25(35)29-17-5-6-17)30-18-7-9-19(10-8-18)37-16-23(34)33-11-13-36-14-12-33/h1-4,7-10,15,17H,5-6,11-14,16H2,(H,29,35)(H2,28,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
The First Affiliated Hospital of Dalian Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His-tagged FAK (393 to 698 residues) expressed in baculovirus infected Sf9 insect cells using Poly (4:1 Gl... |
Eur J Med Chem 172: 154-162 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.004
BindingDB Entry DOI: 10.7270/Q23T9MK9 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50132352
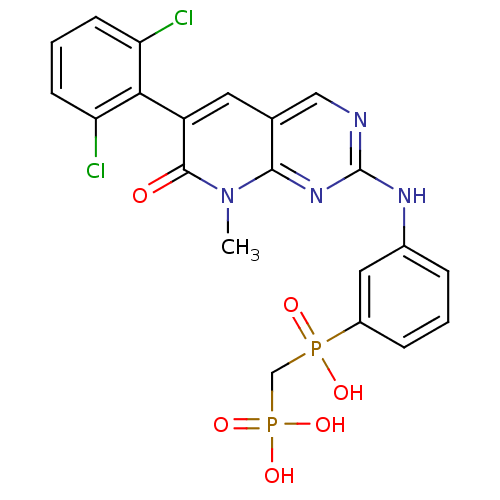
(({3-[6-(2,6-Dichloro-phenyl)-8-methyl-7-oxo-7,8-di...)Show SMILES Cn1c2nc(Nc3cccc(c3)P(O)(=O)CP(O)(O)=O)ncc2cc(-c2c(Cl)cccc2Cl)c1=O |(2.81,-5.78,;2.81,-4.24,;1.47,-3.47,;.14,-4.24,;-1.19,-3.48,;-2.53,-4.26,;-3.87,-3.49,;-5.19,-4.26,;-6.52,-3.52,;-6.54,-1.96,;-5.21,-1.18,;-3.87,-1.95,;-5.21,.37,;-6.75,.39,;-3.66,.37,;-5.21,1.91,;-6.55,2.68,;-5.78,4.01,;-7.64,3.78,;-7.66,1.61,;-1.21,-1.93,;.14,-1.15,;1.47,-1.93,;2.81,-1.15,;4.15,-1.94,;5.48,-1.17,;6.81,-1.94,;6.81,-3.48,;8.14,-1.17,;8.14,.39,;6.81,1.16,;5.48,.37,;4.15,1.15,;4.15,-3.47,;5.48,-4.24,)| Show InChI InChI=1S/C21H18Cl2N4O6P2/c1-27-19-12(8-15(20(27)28)18-16(22)6-3-7-17(18)23)10-24-21(26-19)25-13-4-2-5-14(9-13)34(29,30)11-35(31,32)33/h2-10H,11H2,1H3,(H,29,30)(H,24,25,26)(H2,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Src protein tryrosine kinase |
Bioorg Med Chem Lett 13: 3071-4 (2003)
BindingDB Entry DOI: 10.7270/Q21J995T |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Mus musculus) | BDBM50509437
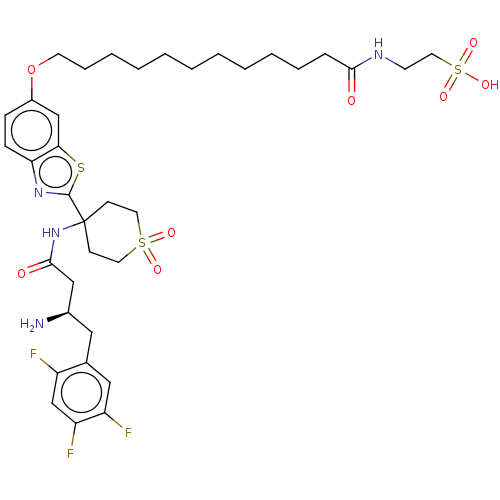
(CHEMBL4445546)Show SMILES OC(=O)C(F)(F)F.N[C@@H](CC(=O)NC1(CCS(=O)(=O)CC1)c1nc2ccc(OCCCCCCCCCCCC(=O)NCCS(O)(=O)=O)cc2s1)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C36H49F3N4O8S3.C2HF3O2/c37-28-24-30(39)29(38)21-25(28)20-26(40)22-34(45)43-36(13-17-53(46,47)18-14-36)35-42-31-12-11-27(23-32(31)52-35)51-16-9-7-5-3-1-2-4-6-8-10-33(44)41-15-19-54(48,49)50;3-2(4,5)1(6)7/h11-12,21,23-24,26H,1-10,13-20,22,40H2,(H,41,44)(H,43,45)(H,48,49,50);(H,6,7)/t26-;/m1./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in C57BL/6 mouse serum pre-incubated for 5 mins followed by Gly-Pro-7-AMC substrate addition by fluorescence based assay |
J Med Chem 62: 10919-10925 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01649
BindingDB Entry DOI: 10.7270/Q2PN98X6 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Mus musculus) | BDBM50509439
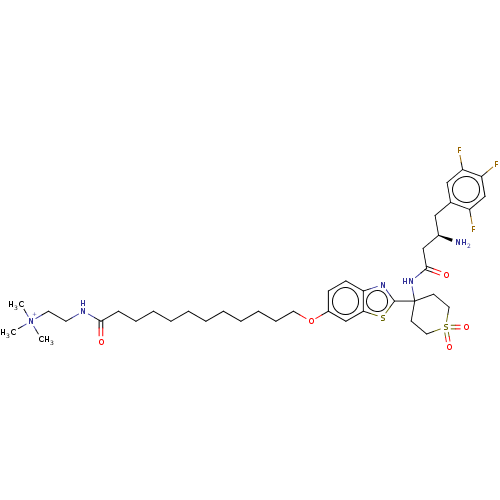
(CHEMBL4442618)Show SMILES OC(=O)C(F)(F)F.[O-]C(=O)C(F)(F)F.C[N+](C)(C)CCNC(=O)CCCCCCCCCCCOc1ccc2nc(sc2c1)C1(CCS(=O)(=O)CC1)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C39H56F3N5O5S2.2C2HF3O2/c1-47(2,3)19-18-44-36(48)13-11-9-7-5-4-6-8-10-12-20-52-30-14-15-34-35(26-30)53-38(45-34)39(16-21-54(50,51)22-17-39)46-37(49)25-29(43)23-28-24-32(41)33(42)27-31(28)40;2*3-2(4,5)1(6)7/h14-15,24,26-27,29H,4-13,16-23,25,43H2,1-3H3,(H-,44,46,48,49);2*(H,6,7)/t29-;;/m1../s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in C57BL/6 mouse serum pre-incubated for 5 mins followed by Gly-Pro-7-AMC substrate addition by fluorescence based assay |
J Med Chem 62: 10919-10925 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01649
BindingDB Entry DOI: 10.7270/Q2PN98X6 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Mus musculus) | BDBM50509438
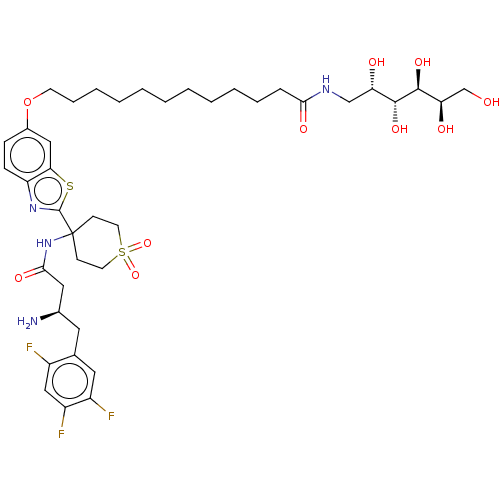
(CHEMBL4584732)Show SMILES OC(=O)C(F)(F)F.N[C@@H](CC(=O)NC1(CCS(=O)(=O)CC1)c1nc2ccc(OCCCCCCCCCCCC(=O)NC[C@H](O)[C@@H](O)[C@@H](O)[C@H](O)CO)cc2s1)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C40H57F3N4O10S2.C2HF3O2/c41-28-22-30(43)29(42)19-25(28)18-26(44)20-36(52)47-40(13-16-59(55,56)17-14-40)39-46-31-12-11-27(21-34(31)58-39)57-15-9-7-5-3-1-2-4-6-8-10-35(51)45-23-32(49)37(53)38(54)33(50)24-48;3-2(4,5)1(6)7/h11-12,19,21-22,26,32-33,37-38,48-50,53-54H,1-10,13-18,20,23-24,44H2,(H,45,51)(H,47,52);(H,6,7)/t26-,32+,33-,37-,38+;/m1./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 168 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in C57BL/6 mouse serum pre-incubated for 5 mins followed by Gly-Pro-7-AMC substrate addition by fluorescence based assay |
J Med Chem 62: 10919-10925 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01649
BindingDB Entry DOI: 10.7270/Q2PN98X6 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50132353
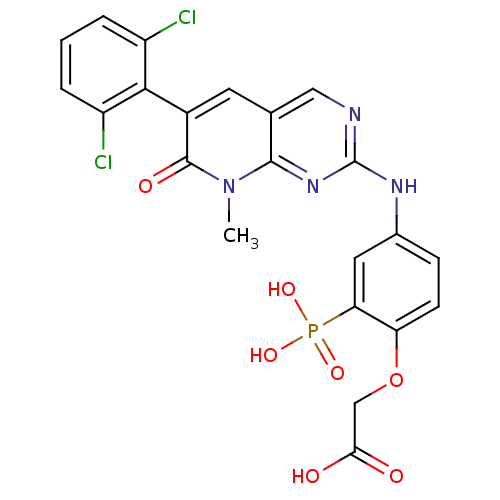
(CHEMBL102801 | {4-[6-(2,6-Dichloro-phenyl)-8-methy...)Show SMILES Cn1c2nc(Nc3ccc(OCC(O)=O)c(c3)P(O)(O)=O)ncc2cc(-c2c(Cl)cccc2Cl)c1=O |(4.7,-3.27,;4.67,-1.73,;3.34,-.96,;2.01,-1.73,;.68,-.96,;-.65,-1.73,;-1.98,-.98,;-1.98,.58,;-3.31,1.33,;-4.64,.55,;-5.99,1.32,;-5.99,2.86,;-7.33,3.63,;-8.67,2.85,;-7.34,5.17,;-4.64,-.99,;-3.31,-1.75,;-5.97,-1.77,;-7.3,-2.57,;-7.46,-.86,;-5.3,-3.15,;.68,.58,;2.01,1.35,;3.34,.58,;4.67,1.35,;6.02,.58,;7.35,1.35,;7.35,2.89,;6.02,3.66,;8.68,3.66,;10.02,2.89,;10.02,1.35,;8.68,.58,;8.68,-.96,;6.02,-.96,;7.36,-1.73,)| Show InChI InChI=1S/C22H17Cl2N4O7P/c1-28-20-11(7-13(21(28)31)19-14(23)3-2-4-15(19)24)9-25-22(27-20)26-12-5-6-16(35-10-18(29)30)17(8-12)36(32,33)34/h2-9H,10H2,1H3,(H,29,30)(H,25,26,27)(H2,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Src protein tryrosine kinase |
Bioorg Med Chem Lett 13: 3071-4 (2003)
BindingDB Entry DOI: 10.7270/Q21J995T |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50132350

(({4-[6-(2,6-Dichloro-phenyl)-8-methyl-7-oxo-7,8-di...)Show SMILES Cn1c2nc(Nc3ccc(CP(O)(=O)CP(O)(O)=O)cc3)ncc2cc(-c2c(Cl)cccc2Cl)c1=O |(5.26,-5.02,;5.25,-3.48,;3.9,-2.71,;2.57,-3.48,;1.24,-2.71,;-.09,-3.5,;-1.42,-2.74,;-1.42,-1.19,;-2.75,-.43,;-4.08,-1.2,;-5.43,-.43,;-5.43,1.11,;-6.97,1.16,;-3.89,1.09,;-5.41,2.64,;-6.74,3.42,;-5.94,4.76,;-7.81,4.54,;-7.86,2.37,;-4.08,-2.75,;-2.75,-3.51,;1.24,-1.17,;2.57,-.4,;3.9,-1.17,;5.23,-.4,;6.58,-1.17,;7.91,-.4,;9.24,-1.17,;9.24,-2.71,;10.58,-.4,;10.58,1.14,;9.24,1.91,;7.91,1.14,;6.58,1.91,;6.58,-2.71,;7.92,-3.48,)| Show InChI InChI=1S/C22H20Cl2N4O6P2/c1-28-20-14(9-16(21(28)29)19-17(23)3-2-4-18(19)24)10-25-22(27-20)26-15-7-5-13(6-8-15)11-35(30,31)12-36(32,33)34/h2-10H,11-12H2,1H3,(H,30,31)(H,25,26,27)(H2,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Src protein tryrosine kinase |
Bioorg Med Chem Lett 13: 3071-4 (2003)
BindingDB Entry DOI: 10.7270/Q21J995T |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50132349
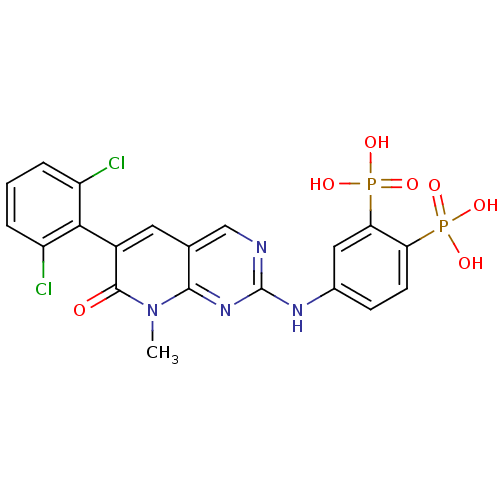
(CHEMBL320374 | {5-[6-(2,6-Dichloro-phenyl)-8-methy...)Show SMILES Cn1c2nc(Nc3ccc(c(c3)P(O)(O)=O)P(O)(O)=O)ncc2cc(-c2c(Cl)cccc2Cl)c1=O |(3.82,-1.69,;3.82,-.15,;2.48,.62,;1.15,-.15,;-.19,.61,;-1.52,-.17,;-2.87,.6,;-2.87,2.14,;-4.2,2.91,;-5.53,2.13,;-5.52,.58,;-4.19,-.17,;-6.85,-.19,;-8.2,-.99,;-8.36,.72,;-6.19,-1.57,;-6.87,2.9,;-6.07,4.25,;-7.94,4.02,;-7.99,1.84,;-.2,2.17,;1.15,2.94,;2.48,2.17,;3.81,2.94,;5.15,2.15,;6.48,2.92,;6.48,4.48,;5.15,5.24,;7.82,5.25,;9.15,4.48,;9.15,2.92,;7.82,2.15,;7.82,.61,;5.15,.62,;6.48,-.15,)| Show InChI InChI=1S/C20H16Cl2N4O7P2/c1-26-18-10(7-12(19(26)27)17-13(21)3-2-4-14(17)22)9-23-20(25-18)24-11-5-6-15(34(28,29)30)16(8-11)35(31,32)33/h2-9H,1H3,(H,23,24,25)(H2,28,29,30)(H2,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Src protein tryrosine kinase |
Bioorg Med Chem Lett 13: 3071-4 (2003)
BindingDB Entry DOI: 10.7270/Q21J995T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50103784
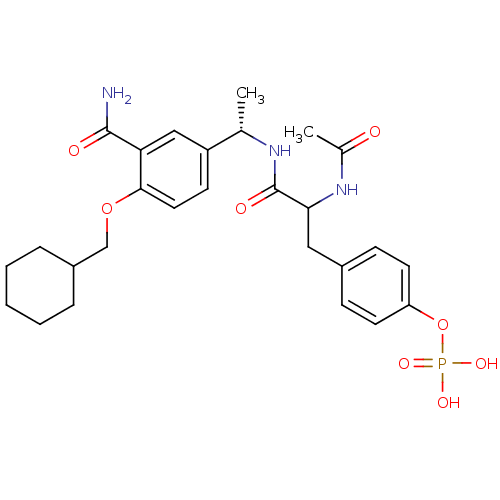
(CHEMBL77638 | Phosphoric acid mono-(4-{2-acetylami...)Show SMILES C[C@H](NC(=O)C(Cc1ccc(OP(O)(O)=O)cc1)NC(C)=O)c1ccc(OCC2CCCCC2)c(c1)C(N)=O Show InChI InChI=1S/C27H36N3O8P/c1-17(21-10-13-25(23(15-21)26(28)32)37-16-20-6-4-3-5-7-20)29-27(33)24(30-18(2)31)14-19-8-11-22(12-9-19)38-39(34,35)36/h8-13,15,17,20,24H,3-7,14,16H2,1-2H3,(H2,28,32)(H,29,33)(H,30,31)(H2,34,35,36)/t17-,24?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Yes SH2 domain |
Bioorg Med Chem Lett 11: 2319-23 (2001)
BindingDB Entry DOI: 10.7270/Q2513XHJ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50103784

(CHEMBL77638 | Phosphoric acid mono-(4-{2-acetylami...)Show SMILES C[C@H](NC(=O)C(Cc1ccc(OP(O)(O)=O)cc1)NC(C)=O)c1ccc(OCC2CCCCC2)c(c1)C(N)=O Show InChI InChI=1S/C27H36N3O8P/c1-17(21-10-13-25(23(15-21)26(28)32)37-16-20-6-4-3-5-7-20)29-27(33)24(30-18(2)31)14-19-8-11-22(12-9-19)38-39(34,35)36/h8-13,15,17,20,24H,3-7,14,16H2,1-2H3,(H2,28,32)(H,29,33)(H,30,31)(H2,34,35,36)/t17-,24?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Src SH2 domain |
Bioorg Med Chem Lett 11: 2319-23 (2001)
BindingDB Entry DOI: 10.7270/Q2513XHJ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50103781
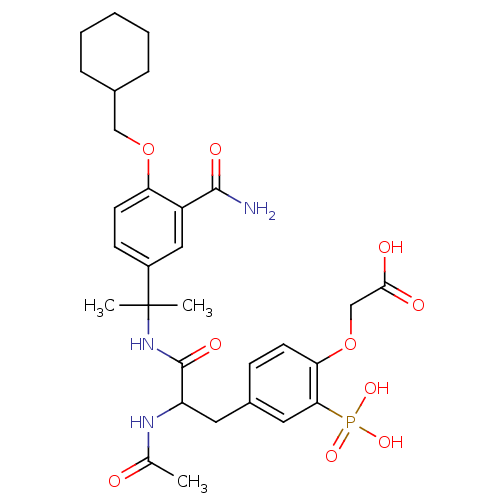
((4-{2-Acetylamino-2-[1-(3-carbamoyl-4-cyclohexylme...)Show SMILES CC(=O)NC(Cc1ccc(OCC(O)=O)c(c1)P(O)(O)=O)C(=O)NC(C)(C)c1ccc(OCC2CCCCC2)c(c1)C(N)=O Show InChI InChI=1S/C30H40N3O10P/c1-18(34)32-23(13-20-9-11-25(43-17-27(35)36)26(14-20)44(39,40)41)29(38)33-30(2,3)21-10-12-24(22(15-21)28(31)37)42-16-19-7-5-4-6-8-19/h9-12,14-15,19,23H,4-8,13,16-17H2,1-3H3,(H2,31,37)(H,32,34)(H,33,38)(H,35,36)(H2,39,40,41) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Src SH2 domain |
Bioorg Med Chem Lett 11: 2319-23 (2001)
BindingDB Entry DOI: 10.7270/Q2513XHJ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50103782
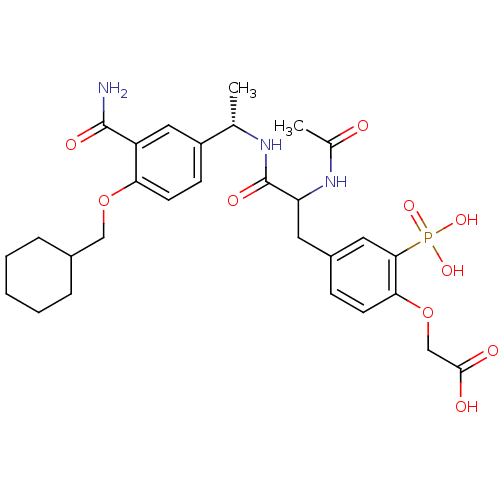
((4-{2-Acetylamino-2-[(S)-1-(3-carbamoyl-4-cyclohex...)Show SMILES C[C@H](NC(=O)C(Cc1ccc(OCC(O)=O)c(c1)P(O)(O)=O)NC(C)=O)c1ccc(OCC2CCCCC2)c(c1)C(N)=O Show InChI InChI=1S/C29H38N3O10P/c1-17(21-9-11-24(22(14-21)28(30)36)41-15-19-6-4-3-5-7-19)31-29(37)23(32-18(2)33)12-20-8-10-25(42-16-27(34)35)26(13-20)43(38,39)40/h8-11,13-14,17,19,23H,3-7,12,15-16H2,1-2H3,(H2,30,36)(H,31,37)(H,32,33)(H,34,35)(H2,38,39,40)/t17-,23?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Src SH2 domain |
Bioorg Med Chem Lett 11: 2319-23 (2001)
BindingDB Entry DOI: 10.7270/Q2513XHJ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50103780
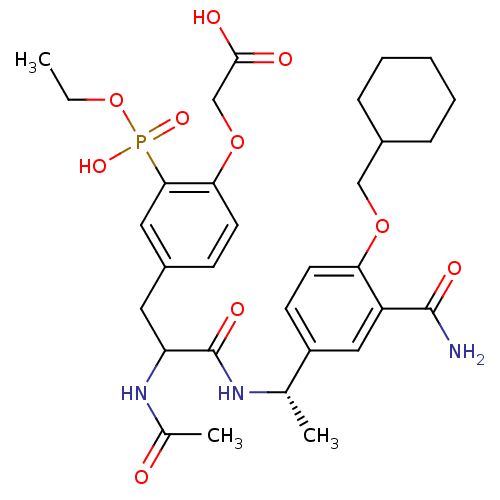
(CHEMBL308734 | [4-{2-Acetylamino-2-[(S)-1-(3-carba...)Show SMILES CCOP(O)(=O)c1cc(CC(NC(C)=O)C(=O)N[C@@H](C)c2ccc(OCC3CCCCC3)c(c2)C(N)=O)ccc1OCC(O)=O Show InChI InChI=1S/C31H42N3O10P/c1-4-44-45(40,41)28-15-22(10-12-27(28)43-18-29(36)37)14-25(34-20(3)35)31(39)33-19(2)23-11-13-26(24(16-23)30(32)38)42-17-21-8-6-5-7-9-21/h10-13,15-16,19,21,25H,4-9,14,17-18H2,1-3H3,(H2,32,38)(H,33,39)(H,34,35)(H,36,37)(H,40,41)/t19-,25?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Src SH2 domain |
Bioorg Med Chem Lett 11: 2319-23 (2001)
BindingDB Entry DOI: 10.7270/Q2513XHJ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50103786
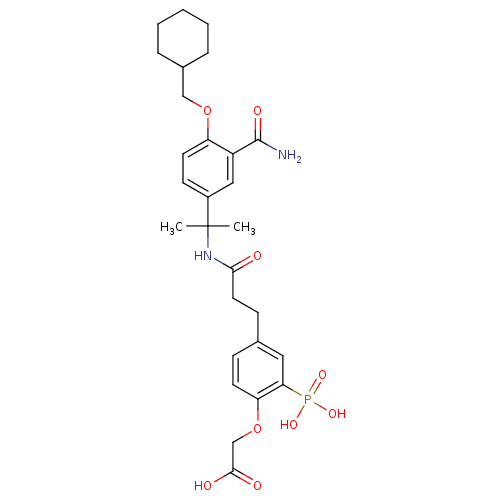
((4-{2-[1-(3-Carbamoyl-4-cyclohexylmethoxy-phenyl)-...)Show SMILES CC(C)(NC(=O)CCc1ccc(OCC(O)=O)c(c1)P(O)(O)=O)c1ccc(OCC2CCCCC2)c(c1)C(N)=O Show InChI InChI=1S/C28H37N2O9P/c1-28(2,20-10-12-22(21(15-20)27(29)34)38-16-19-6-4-3-5-7-19)30-25(31)13-9-18-8-11-23(39-17-26(32)33)24(14-18)40(35,36)37/h8,10-12,14-15,19H,3-7,9,13,16-17H2,1-2H3,(H2,29,34)(H,30,31)(H,32,33)(H2,35,36,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Src SH2 domain |
Bioorg Med Chem Lett 11: 2319-23 (2001)
BindingDB Entry DOI: 10.7270/Q2513XHJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50103781

((4-{2-Acetylamino-2-[1-(3-carbamoyl-4-cyclohexylme...)Show SMILES CC(=O)NC(Cc1ccc(OCC(O)=O)c(c1)P(O)(O)=O)C(=O)NC(C)(C)c1ccc(OCC2CCCCC2)c(c1)C(N)=O Show InChI InChI=1S/C30H40N3O10P/c1-18(34)32-23(13-20-9-11-25(43-17-27(35)36)26(14-20)44(39,40)41)29(38)33-30(2,3)21-10-12-24(22(15-21)28(31)37)42-16-19-7-5-4-6-8-19/h9-12,14-15,19,23H,4-8,13,16-17H2,1-3H3,(H2,31,37)(H,32,34)(H,33,38)(H,35,36)(H2,39,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Yes SH2 domain |
Bioorg Med Chem Lett 11: 2319-23 (2001)
BindingDB Entry DOI: 10.7270/Q2513XHJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50103782

((4-{2-Acetylamino-2-[(S)-1-(3-carbamoyl-4-cyclohex...)Show SMILES C[C@H](NC(=O)C(Cc1ccc(OCC(O)=O)c(c1)P(O)(O)=O)NC(C)=O)c1ccc(OCC2CCCCC2)c(c1)C(N)=O Show InChI InChI=1S/C29H38N3O10P/c1-17(21-9-11-24(22(14-21)28(30)36)41-15-19-6-4-3-5-7-19)31-29(37)23(32-18(2)33)12-20-8-10-25(42-16-27(34)35)26(13-20)43(38,39)40/h8-11,13-14,17,19,23H,3-7,12,15-16H2,1-2H3,(H2,30,36)(H,31,37)(H,32,33)(H,34,35)(H2,38,39,40)/t17-,23?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Yes SH2 domain |
Bioorg Med Chem Lett 11: 2319-23 (2001)
BindingDB Entry DOI: 10.7270/Q2513XHJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50103780

(CHEMBL308734 | [4-{2-Acetylamino-2-[(S)-1-(3-carba...)Show SMILES CCOP(O)(=O)c1cc(CC(NC(C)=O)C(=O)N[C@@H](C)c2ccc(OCC3CCCCC3)c(c2)C(N)=O)ccc1OCC(O)=O Show InChI InChI=1S/C31H42N3O10P/c1-4-44-45(40,41)28-15-22(10-12-27(28)43-18-29(36)37)14-25(34-20(3)35)31(39)33-19(2)23-11-13-26(24(16-23)30(32)38)42-17-21-8-6-5-7-9-21/h10-13,15-16,19,21,25H,4-9,14,17-18H2,1-3H3,(H2,32,38)(H,33,39)(H,34,35)(H,36,37)(H,40,41)/t19-,25?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.56E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Yes SH2 domain |
Bioorg Med Chem Lett 11: 2319-23 (2001)
BindingDB Entry DOI: 10.7270/Q2513XHJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50103786

((4-{2-[1-(3-Carbamoyl-4-cyclohexylmethoxy-phenyl)-...)Show SMILES CC(C)(NC(=O)CCc1ccc(OCC(O)=O)c(c1)P(O)(O)=O)c1ccc(OCC2CCCCC2)c(c1)C(N)=O Show InChI InChI=1S/C28H37N2O9P/c1-28(2,20-10-12-22(21(15-20)27(29)34)38-16-19-6-4-3-5-7-19)30-25(31)13-9-18-8-11-23(39-17-26(32)33)24(14-18)40(35,36)37/h8,10-12,14-15,19H,3-7,9,13,16-17H2,1-2H3,(H2,29,34)(H,30,31)(H,32,33)(H2,35,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.03E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Yes SH2 domain |
Bioorg Med Chem Lett 11: 2319-23 (2001)
BindingDB Entry DOI: 10.7270/Q2513XHJ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50103787

((4-{2-Acetylamino-2-[(S)-1-(3-carbamoyl-4-cyclohex...)Show SMILES C[C@H](NC(=O)C(Cc1ccc(OCC(O)=O)cc1)NC(C)=O)c1ccc(OCC2CCCCC2)c(c1)C(N)=O Show InChI InChI=1S/C29H37N3O7/c1-18(22-10-13-26(24(15-22)28(30)36)39-16-21-6-4-3-5-7-21)31-29(37)25(32-19(2)33)14-20-8-11-23(12-9-20)38-17-27(34)35/h8-13,15,18,21,25H,3-7,14,16-17H2,1-2H3,(H2,30,36)(H,31,37)(H,32,33)(H,34,35)/t18-,25?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Src SH2 domain |
Bioorg Med Chem Lett 11: 2319-23 (2001)
BindingDB Entry DOI: 10.7270/Q2513XHJ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50103785
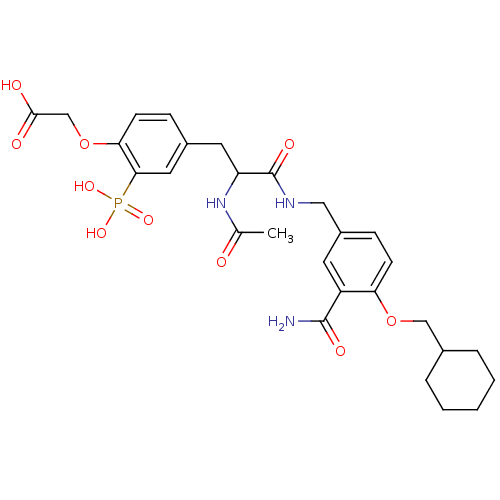
(CHEMBL263721 | {4-[2-Acetylamino-2-(3-carbamoyl-4-...)Show SMILES CC(=O)NC(Cc1ccc(OCC(O)=O)c(c1)P(O)(O)=O)C(=O)NCc1ccc(OCC2CCCCC2)c(c1)C(N)=O Show InChI InChI=1S/C28H36N3O10P/c1-17(32)31-22(12-19-7-10-24(41-16-26(33)34)25(13-19)42(37,38)39)28(36)30-14-20-8-9-23(21(11-20)27(29)35)40-15-18-5-3-2-4-6-18/h7-11,13,18,22H,2-6,12,14-16H2,1H3,(H2,29,35)(H,30,36)(H,31,32)(H,33,34)(H2,37,38,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.78E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Src SH2 domain |
Bioorg Med Chem Lett 11: 2319-23 (2001)
BindingDB Entry DOI: 10.7270/Q2513XHJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM50103784

(CHEMBL77638 | Phosphoric acid mono-(4-{2-acetylami...)Show SMILES C[C@H](NC(=O)C(Cc1ccc(OP(O)(O)=O)cc1)NC(C)=O)c1ccc(OCC2CCCCC2)c(c1)C(N)=O Show InChI InChI=1S/C27H36N3O8P/c1-17(21-10-13-25(23(15-21)26(28)32)37-16-20-6-4-3-5-7-20)29-27(33)24(30-18(2)31)14-19-8-11-22(12-9-19)38-39(34,35)36/h8-13,15,17,20,24H,3-7,14,16H2,1-2H3,(H2,28,32)(H,29,33)(H,30,31)(H2,34,35,36)/t17-,24?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.41E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Zap70 protein kinase |
Bioorg Med Chem Lett 11: 2319-23 (2001)
BindingDB Entry DOI: 10.7270/Q2513XHJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50103787

((4-{2-Acetylamino-2-[(S)-1-(3-carbamoyl-4-cyclohex...)Show SMILES C[C@H](NC(=O)C(Cc1ccc(OCC(O)=O)cc1)NC(C)=O)c1ccc(OCC2CCCCC2)c(c1)C(N)=O Show InChI InChI=1S/C29H37N3O7/c1-18(22-10-13-26(24(15-22)28(30)36)39-16-21-6-4-3-5-7-21)31-29(37)25(32-19(2)33)14-20-8-11-23(12-9-20)38-17-27(34)35/h8-13,15,18,21,25H,3-7,14,16-17H2,1-2H3,(H2,30,36)(H,31,37)(H,32,33)(H,34,35)/t18-,25?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Yes SH2 domain |
Bioorg Med Chem Lett 11: 2319-23 (2001)
BindingDB Entry DOI: 10.7270/Q2513XHJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM50103781

((4-{2-Acetylamino-2-[1-(3-carbamoyl-4-cyclohexylme...)Show SMILES CC(=O)NC(Cc1ccc(OCC(O)=O)c(c1)P(O)(O)=O)C(=O)NC(C)(C)c1ccc(OCC2CCCCC2)c(c1)C(N)=O Show InChI InChI=1S/C30H40N3O10P/c1-18(34)32-23(13-20-9-11-25(43-17-27(35)36)26(14-20)44(39,40)41)29(38)33-30(2,3)21-10-12-24(22(15-21)28(31)37)42-16-19-7-5-4-6-8-19/h9-12,14-15,19,23H,4-8,13,16-17H2,1-3H3,(H2,31,37)(H,32,34)(H,33,38)(H,35,36)(H2,39,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Zap70 protein kinase |
Bioorg Med Chem Lett 11: 2319-23 (2001)
BindingDB Entry DOI: 10.7270/Q2513XHJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM50103786

((4-{2-[1-(3-Carbamoyl-4-cyclohexylmethoxy-phenyl)-...)Show SMILES CC(C)(NC(=O)CCc1ccc(OCC(O)=O)c(c1)P(O)(O)=O)c1ccc(OCC2CCCCC2)c(c1)C(N)=O Show InChI InChI=1S/C28H37N2O9P/c1-28(2,20-10-12-22(21(15-20)27(29)34)38-16-19-6-4-3-5-7-19)30-25(31)13-9-18-8-11-23(39-17-26(32)33)24(14-18)40(35,36)37/h8,10-12,14-15,19H,3-7,9,13,16-17H2,1-2H3,(H2,29,34)(H,30,31)(H,32,33)(H2,35,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Zap70 protein kinase |
Bioorg Med Chem Lett 11: 2319-23 (2001)
BindingDB Entry DOI: 10.7270/Q2513XHJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM50103785

(CHEMBL263721 | {4-[2-Acetylamino-2-(3-carbamoyl-4-...)Show SMILES CC(=O)NC(Cc1ccc(OCC(O)=O)c(c1)P(O)(O)=O)C(=O)NCc1ccc(OCC2CCCCC2)c(c1)C(N)=O Show InChI InChI=1S/C28H36N3O10P/c1-17(32)31-22(12-19-7-10-24(41-16-26(33)34)25(13-19)42(37,38)39)28(36)30-14-20-8-9-23(21(11-20)27(29)35)40-15-18-5-3-2-4-6-18/h7-11,13,18,22H,2-6,12,14-16H2,1H3,(H2,29,35)(H,30,36)(H,31,32)(H,33,34)(H2,37,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Zap70 protein kinase |
Bioorg Med Chem Lett 11: 2319-23 (2001)
BindingDB Entry DOI: 10.7270/Q2513XHJ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50103783
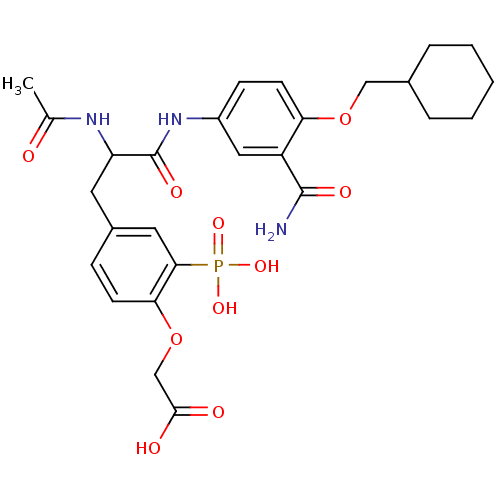
(CHEMBL308164 | {4-[2-Acetylamino-2-(3-carbamoyl-4-...)Show SMILES CC(=O)NC(Cc1ccc(OCC(O)=O)c(c1)P(O)(O)=O)C(=O)Nc1ccc(OCC2CCCCC2)c(c1)C(N)=O Show InChI InChI=1S/C27H34N3O10P/c1-16(31)29-21(11-18-7-9-23(40-15-25(32)33)24(12-18)41(36,37)38)27(35)30-19-8-10-22(20(13-19)26(28)34)39-14-17-5-3-2-4-6-17/h7-10,12-13,17,21H,2-6,11,14-15H2,1H3,(H2,28,34)(H,29,31)(H,30,35)(H,32,33)(H2,36,37,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Src SH2 domain |
Bioorg Med Chem Lett 11: 2319-23 (2001)
BindingDB Entry DOI: 10.7270/Q2513XHJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM50103779
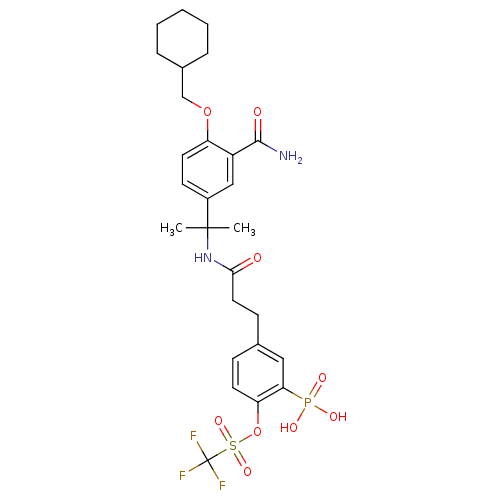
(CHEMBL430825 | Trifluoro-methanesulfonic acid 4-{2...)Show SMILES CC(C)(NC(=O)CCc1ccc(OS(=O)(=O)C(F)(F)F)c(c1)P(O)(O)=O)c1ccc(OCC2CCCCC2)c(c1)C(N)=O Show InChI InChI=1S/C27H34F3N2O9PS/c1-26(2,19-10-12-21(20(15-19)25(31)34)40-16-18-6-4-3-5-7-18)32-24(33)13-9-17-8-11-22(23(14-17)42(35,36)37)41-43(38,39)27(28,29)30/h8,10-12,14-15,18H,3-7,9,13,16H2,1-2H3,(H2,31,34)(H,32,33)(H2,35,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Zap70 protein kinase |
Bioorg Med Chem Lett 11: 2319-23 (2001)
BindingDB Entry DOI: 10.7270/Q2513XHJ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50103779

(CHEMBL430825 | Trifluoro-methanesulfonic acid 4-{2...)Show SMILES CC(C)(NC(=O)CCc1ccc(OS(=O)(=O)C(F)(F)F)c(c1)P(O)(O)=O)c1ccc(OCC2CCCCC2)c(c1)C(N)=O Show InChI InChI=1S/C27H34F3N2O9PS/c1-26(2,19-10-12-21(20(15-19)25(31)34)40-16-18-6-4-3-5-7-18)32-24(33)13-9-17-8-11-22(23(14-17)42(35,36)37)41-43(38,39)27(28,29)30/h8,10-12,14-15,18H,3-7,9,13,16H2,1-2H3,(H2,31,34)(H,32,33)(H2,35,36,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Src SH2 domain |
Bioorg Med Chem Lett 11: 2319-23 (2001)
BindingDB Entry DOI: 10.7270/Q2513XHJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM50103780

(CHEMBL308734 | [4-{2-Acetylamino-2-[(S)-1-(3-carba...)Show SMILES CCOP(O)(=O)c1cc(CC(NC(C)=O)C(=O)N[C@@H](C)c2ccc(OCC3CCCCC3)c(c2)C(N)=O)ccc1OCC(O)=O Show InChI InChI=1S/C31H42N3O10P/c1-4-44-45(40,41)28-15-22(10-12-27(28)43-18-29(36)37)14-25(34-20(3)35)31(39)33-19(2)23-11-13-26(24(16-23)30(32)38)42-17-21-8-6-5-7-9-21/h10-13,15-16,19,21,25H,4-9,14,17-18H2,1-3H3,(H2,32,38)(H,33,39)(H,34,35)(H,36,37)(H,40,41)/t19-,25?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Zap70 protein kinase |
Bioorg Med Chem Lett 11: 2319-23 (2001)
BindingDB Entry DOI: 10.7270/Q2513XHJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50103779

(CHEMBL430825 | Trifluoro-methanesulfonic acid 4-{2...)Show SMILES CC(C)(NC(=O)CCc1ccc(OS(=O)(=O)C(F)(F)F)c(c1)P(O)(O)=O)c1ccc(OCC2CCCCC2)c(c1)C(N)=O Show InChI InChI=1S/C27H34F3N2O9PS/c1-26(2,19-10-12-21(20(15-19)25(31)34)40-16-18-6-4-3-5-7-18)32-24(33)13-9-17-8-11-22(23(14-17)42(35,36)37)41-43(38,39)27(28,29)30/h8,10-12,14-15,18H,3-7,9,13,16H2,1-2H3,(H2,31,34)(H,32,33)(H2,35,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Yes SH2 domain |
Bioorg Med Chem Lett 11: 2319-23 (2001)
BindingDB Entry DOI: 10.7270/Q2513XHJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM50103778
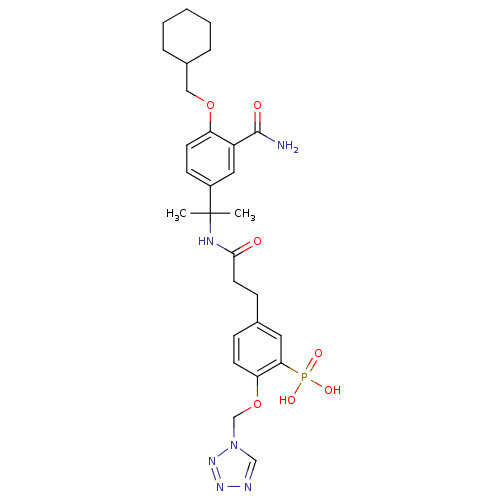
(CHEMBL311114 | [5-{2-[1-(3-Carbamoyl-4-cyclohexylm...)Show SMILES CC(C)(NC(=O)CCc1ccc(OCn2cnnn2)c(c1)P(O)(O)=O)c1ccc(OCC2CCCCC2)c(c1)C(N)=O Show InChI InChI=1S/C28H37N6O7P/c1-28(2,21-10-12-23(22(15-21)27(29)36)40-16-20-6-4-3-5-7-20)31-26(35)13-9-19-8-11-24(25(14-19)42(37,38)39)41-18-34-17-30-32-33-34/h8,10-12,14-15,17,20H,3-7,9,13,16,18H2,1-2H3,(H2,29,36)(H,31,35)(H2,37,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Zap70 protein kinase |
Bioorg Med Chem Lett 11: 2319-23 (2001)
BindingDB Entry DOI: 10.7270/Q2513XHJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50103778

(CHEMBL311114 | [5-{2-[1-(3-Carbamoyl-4-cyclohexylm...)Show SMILES CC(C)(NC(=O)CCc1ccc(OCn2cnnn2)c(c1)P(O)(O)=O)c1ccc(OCC2CCCCC2)c(c1)C(N)=O Show InChI InChI=1S/C28H37N6O7P/c1-28(2,21-10-12-23(22(15-21)27(29)36)40-16-20-6-4-3-5-7-20)31-26(35)13-9-19-8-11-24(25(14-19)42(37,38)39)41-18-34-17-30-32-33-34/h8,10-12,14-15,17,20H,3-7,9,13,16,18H2,1-2H3,(H2,29,36)(H,31,35)(H2,37,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Yes SH2 domain |
Bioorg Med Chem Lett 11: 2319-23 (2001)
BindingDB Entry DOI: 10.7270/Q2513XHJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM50103788
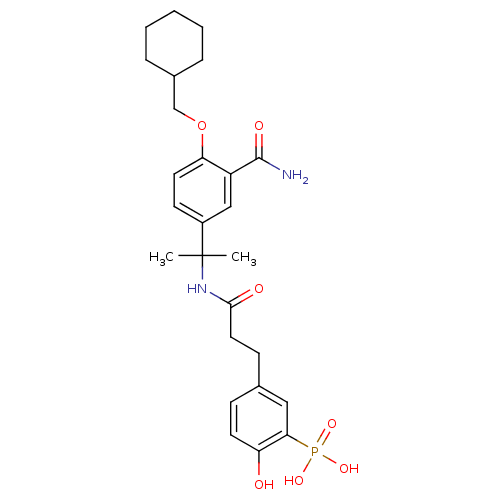
((5-{2-[1-(3-Carbamoyl-4-cyclohexylmethoxy-phenyl)-...)Show SMILES CC(C)(NC(=O)CCc1ccc(O)c(c1)P(O)(O)=O)c1ccc(OCC2CCCCC2)c(c1)C(N)=O Show InChI InChI=1S/C26H35N2O7P/c1-26(2,28-24(30)13-9-17-8-11-21(29)23(14-17)36(32,33)34)19-10-12-22(20(15-19)25(27)31)35-16-18-6-4-3-5-7-18/h8,10-12,14-15,18,29H,3-7,9,13,16H2,1-2H3,(H2,27,31)(H,28,30)(H2,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Zap70 protein kinase |
Bioorg Med Chem Lett 11: 2319-23 (2001)
BindingDB Entry DOI: 10.7270/Q2513XHJ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50103788

((5-{2-[1-(3-Carbamoyl-4-cyclohexylmethoxy-phenyl)-...)Show SMILES CC(C)(NC(=O)CCc1ccc(O)c(c1)P(O)(O)=O)c1ccc(OCC2CCCCC2)c(c1)C(N)=O Show InChI InChI=1S/C26H35N2O7P/c1-26(2,28-24(30)13-9-17-8-11-21(29)23(14-17)36(32,33)34)19-10-12-22(20(15-19)25(27)31)35-16-18-6-4-3-5-7-18/h8,10-12,14-15,18,29H,3-7,9,13,16H2,1-2H3,(H2,27,31)(H,28,30)(H2,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Src SH2 domain |
Bioorg Med Chem Lett 11: 2319-23 (2001)
BindingDB Entry DOI: 10.7270/Q2513XHJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data