

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50054502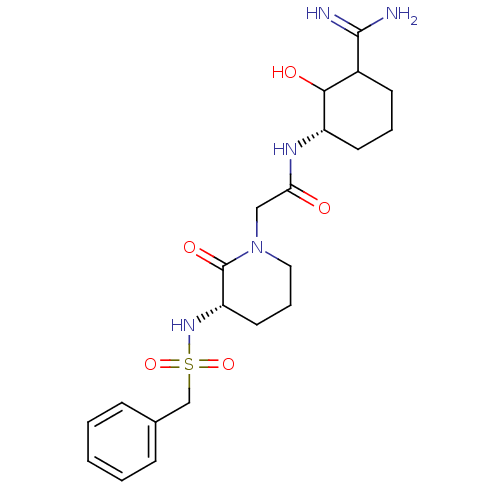 (CHEMBL140494 | N-((S)-3-Carbamimidoyl-2-hydroxy-cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description compound was tested in vitro for inhibition of serine protease thrombin(FIIa). | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076074 (1N-[1-amino(imino)methyl-6-hydroxy-(5S)-tetrahydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.505 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Concentration required for the in vitro inhibitory activity against human enzyme, thrombin cleavage of the chromogenic substrate | Bioorg Med Chem Lett 9: 895-900 (1999) BindingDB Entry DOI: 10.7270/Q2NS0T3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50054498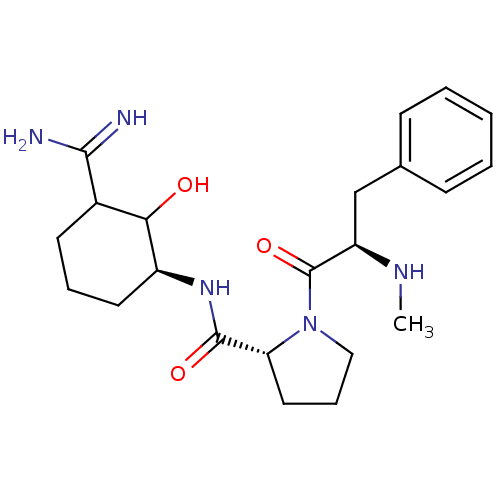 ((R)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of serine protease thrombin(FIIa). | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50054493 (CHEMBL343805 | N-((S)-3-Carbamimidoyl-2-hydroxy-cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of serine protease thrombin(FIIa). | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50054492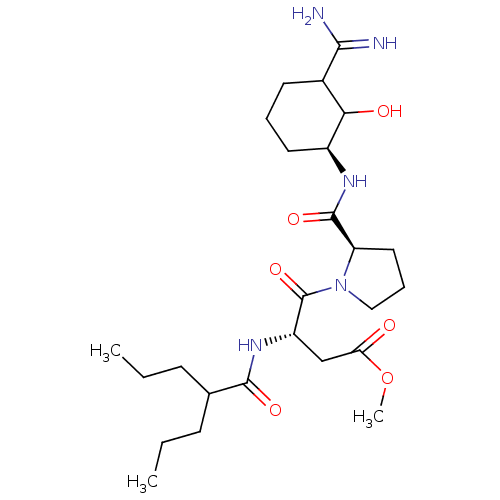 ((S)-4-[(R)-2-((S)-3-Carbamimidoyl-2-hydroxy-cycloh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of serine protease thrombin(FIIa). | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50054494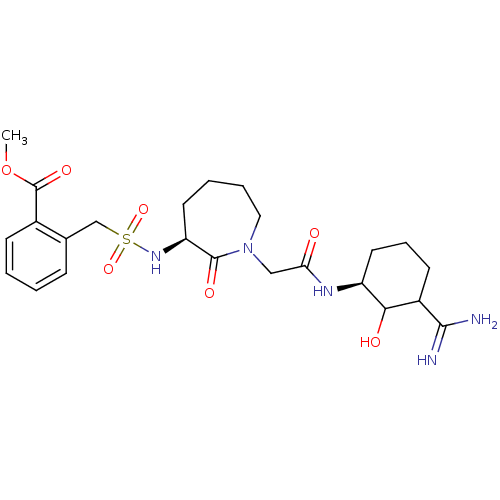 (2-({(S)-1-[((S)-3-Carbamimidoyl-2-hydroxy-cyclohex...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.12 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of serine protease thrombin(FIIa). | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50054492 ((S)-4-[(R)-2-((S)-3-Carbamimidoyl-2-hydroxy-cycloh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.36 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of serine protease Trypsin. | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50054498 ((R)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.52 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of serine protease Trypsin. | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076073 (1N-[1-amino(imino)methyl-6-hydroxy-(5S)-tetrahydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Concentration required for the in vitro inhibitory activity against human enzyme, thrombin cleavage of the chromogenic substrate | Bioorg Med Chem Lett 9: 895-900 (1999) BindingDB Entry DOI: 10.7270/Q2NS0T3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50054502 (CHEMBL140494 | N-((S)-3-Carbamimidoyl-2-hydroxy-cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of serine protease thrombin(FIIa). | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076070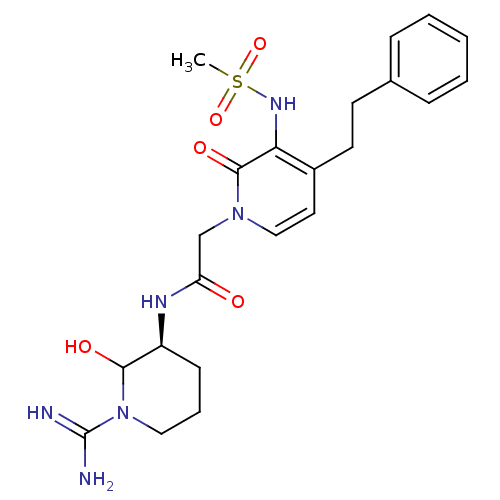 (1N-[1-amino(imino)methyl-6-hydroxy-(5S)-tetrahydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Concentration required for the in vitro inhibitory activity against human enzyme, thrombin cleavage of the chromogenic substrate | Bioorg Med Chem Lett 9: 895-900 (1999) BindingDB Entry DOI: 10.7270/Q2NS0T3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50054504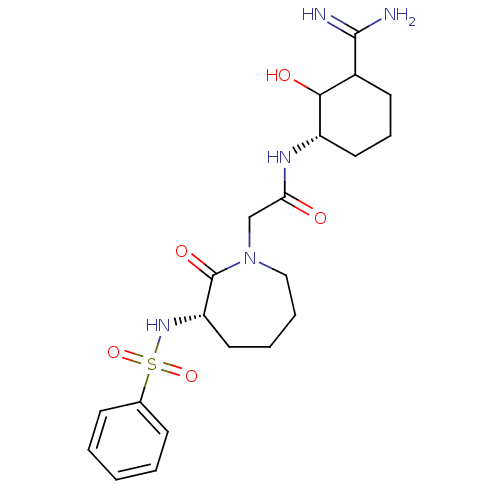 (2-((S)-3-Benzenesulfonylamino-2-oxo-azepan-1-yl)-N...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of serine protease thrombin(FIIa). | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50054504 (2-((S)-3-Benzenesulfonylamino-2-oxo-azepan-1-yl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of serine protease Trypsin. | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50054493 (CHEMBL343805 | N-((S)-3-Carbamimidoyl-2-hydroxy-cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of Coagulation factor X | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50076074 (1N-[1-amino(imino)methyl-6-hydroxy-(5S)-tetrahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Concentration required for the in vitro inhibitory activity against human enzyme, Factor Xa cleavage of the chromogenic substrate | Bioorg Med Chem Lett 9: 895-900 (1999) BindingDB Entry DOI: 10.7270/Q2NS0T3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50076074 (1N-[1-amino(imino)methyl-6-hydroxy-(5S)-tetrahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Concentration required for the in vitro inhibitory activity against human enzyme, trypsin cleavage of the chromogenic substrate | Bioorg Med Chem Lett 9: 895-900 (1999) BindingDB Entry DOI: 10.7270/Q2NS0T3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076069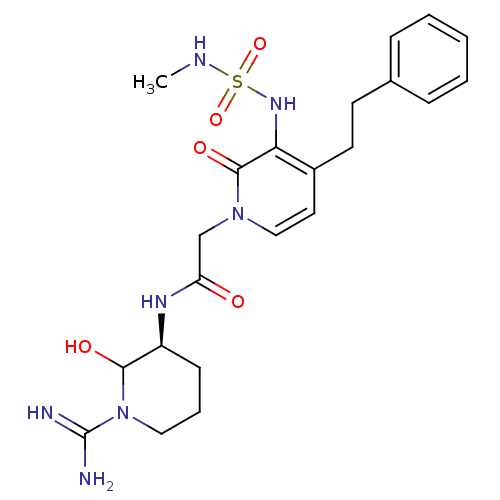 (1N-[1-amino(imino)methyl-6-hydroxy-(5S)-tetrahydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Concentration required for the in vitro inhibitory activity against human enzyme, thrombin cleavage of the chromogenic substrate | Bioorg Med Chem Lett 9: 895-900 (1999) BindingDB Entry DOI: 10.7270/Q2NS0T3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50054493 (CHEMBL343805 | N-((S)-3-Carbamimidoyl-2-hydroxy-cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 74.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of serine protease Trypsin. | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076068 (1N-[1-amino(imino)methyl-6-hydroxy-(5S)-tetrahydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Concentration required for the in vitro inhibitory activity against human enzyme, thrombin cleavage of the chromogenic substrate | Bioorg Med Chem Lett 9: 895-900 (1999) BindingDB Entry DOI: 10.7270/Q2NS0T3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50076069 (1N-[1-amino(imino)methyl-6-hydroxy-(5S)-tetrahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Concentration required for the in vitro inhibitory activity against human enzyme, trypsin cleavage of the chromogenic substrate | Bioorg Med Chem Lett 9: 895-900 (1999) BindingDB Entry DOI: 10.7270/Q2NS0T3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50054503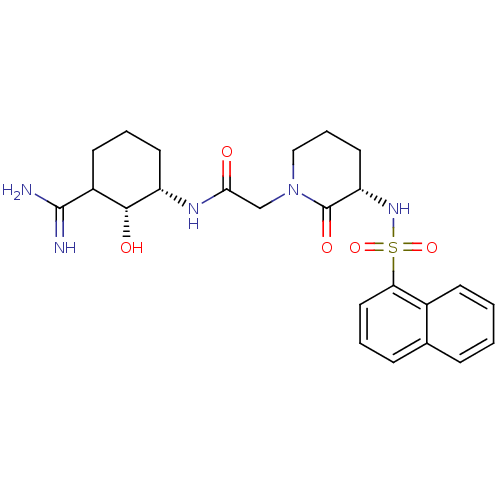 (CHEMBL263924 | N-((1S,2R)-3-Carbamimidoyl-2-hydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of serine protease thrombin(FIIa). | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50054505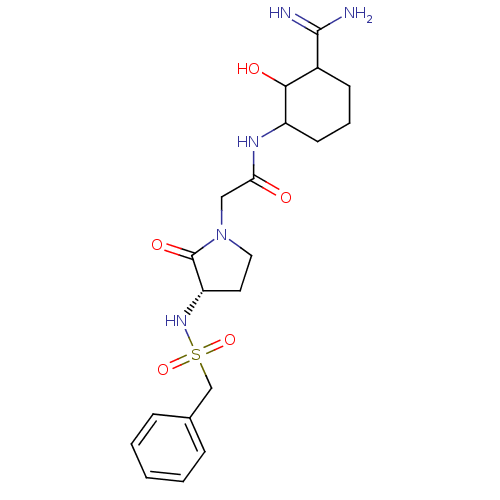 (CHEMBL421760 | N-(3-Carbamimidoyl-2-hydroxy-cycloh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description compound was tested in vitro for inhibition of serine protease thrombin(FIIa). | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50076070 (1N-[1-amino(imino)methyl-6-hydroxy-(5S)-tetrahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 135 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Concentration required for the in vitro inhibitory activity against human enzyme, trypsin cleavage of the chromogenic substrate | Bioorg Med Chem Lett 9: 895-900 (1999) BindingDB Entry DOI: 10.7270/Q2NS0T3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50054494 (2-({(S)-1-[((S)-3-Carbamimidoyl-2-hydroxy-cyclohex...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of serine protease Trypsin. | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50054495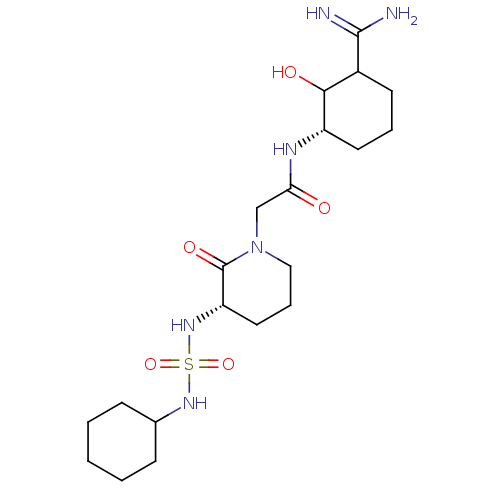 (CHEMBL422470 | Lactum Sulfonamide analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 147 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of serine protease Trypsin. | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50054489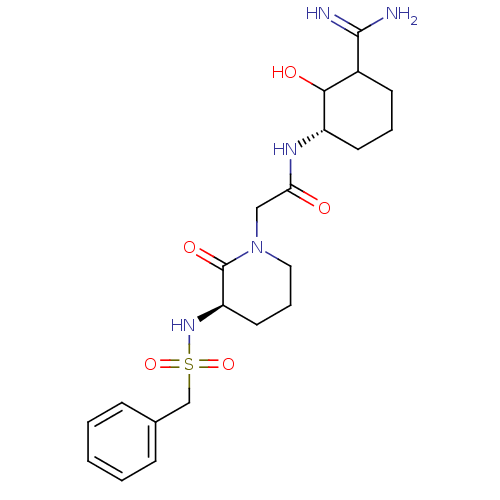 (CHEMBL139656 | N-((S)-3-Carbamimidoyl-2-hydroxy-cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 157 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of serine protease thrombin(FIIa). | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50054491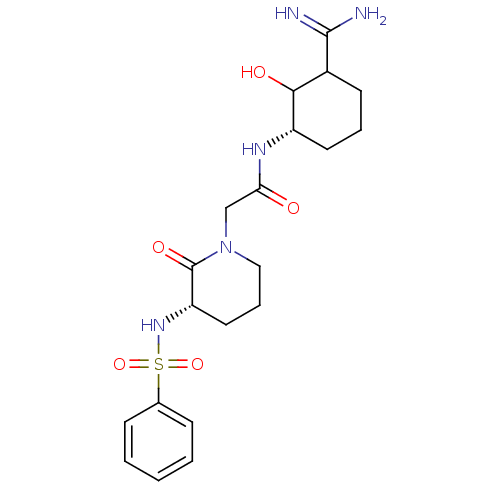 (2-((S)-3-Benzenesulfonylamino-2-oxo-piperidin-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 159 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of serine protease thrombin(FIIa). | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50076068 (1N-[1-amino(imino)methyl-6-hydroxy-(5S)-tetrahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 188 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Concentration required for the in vitro inhibitory activity against human enzyme, trypsin cleavage of the chromogenic substrate | Bioorg Med Chem Lett 9: 895-900 (1999) BindingDB Entry DOI: 10.7270/Q2NS0T3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076071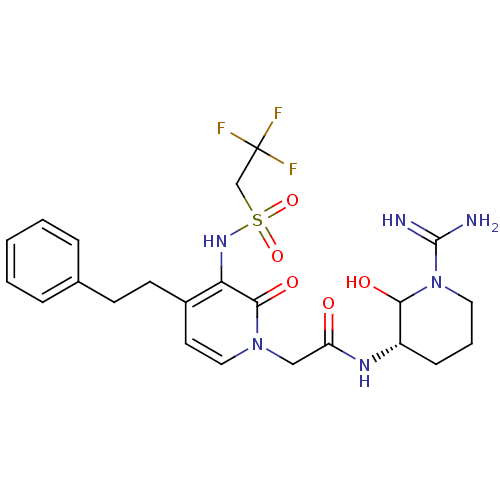 (1N-[1-amino(imino)methyl-6-hydroxy-(5S)-tetrahydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 194 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Concentration required for the in vitro inhibitory activity against human enzyme, thrombin cleavage of the chromogenic substrate | Bioorg Med Chem Lett 9: 895-900 (1999) BindingDB Entry DOI: 10.7270/Q2NS0T3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076066 (1N-[1-amino(imino)methyl-6-hydroxy-(5S)-tetrahydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 203 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Concentration required for the in vitro inhibitory activity against human enzyme, thrombin cleavage of the chromogenic substrate | Bioorg Med Chem Lett 9: 895-900 (1999) BindingDB Entry DOI: 10.7270/Q2NS0T3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50054495 (CHEMBL422470 | Lactum Sulfonamide analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 235 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of serine protease thrombin(FIIa). | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50076069 (1N-[1-amino(imino)methyl-6-hydroxy-(5S)-tetrahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Concentration required for the in vitro inhibitory activity against human enzyme, Plasmin cleavage of the chromogenic substrate | Bioorg Med Chem Lett 9: 895-900 (1999) BindingDB Entry DOI: 10.7270/Q2NS0T3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076067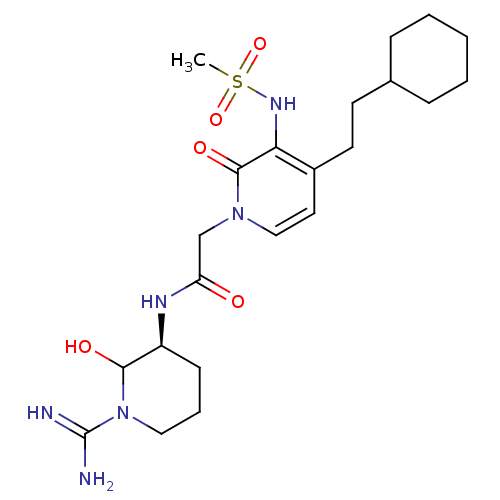 (1N-[1-amino(imino)methyl-6-hydroxy-(5S)-tetrahydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 256 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Concentration required for the in vitro inhibitory activity against human enzyme, thrombin cleavage of the chromogenic substrate | Bioorg Med Chem Lett 9: 895-900 (1999) BindingDB Entry DOI: 10.7270/Q2NS0T3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50076067 (1N-[1-amino(imino)methyl-6-hydroxy-(5S)-tetrahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 257 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Concentration required for the in vitro inhibitory activity against human enzyme, trypsin cleavage of the chromogenic substrate | Bioorg Med Chem Lett 9: 895-900 (1999) BindingDB Entry DOI: 10.7270/Q2NS0T3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50054499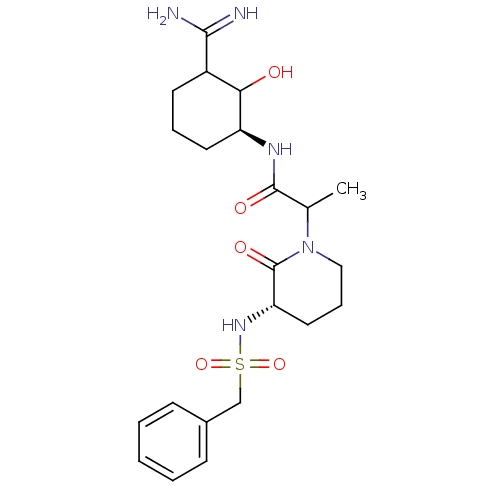 (CHEMBL334454 | N-((S)-3-Carbamimidoyl-2-hydroxy-cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 286 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of serine protease thrombin(FIIa). | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50054492 ((S)-4-[(R)-2-((S)-3-Carbamimidoyl-2-hydroxy-cycloh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of Coagulation factor X | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anionic trypsin (Bos taurus) | BDBM50054499 (CHEMBL334454 | N-((S)-3-Carbamimidoyl-2-hydroxy-cy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 293 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description compound was tested in vitro for inhibition of serine protease Trypsin. | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50054490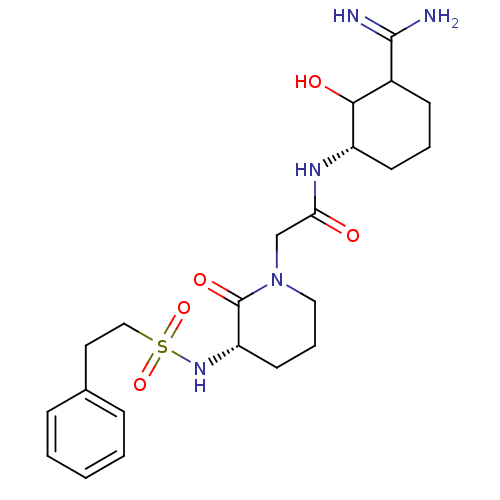 (CHEMBL138243 | N-((S)-3-Carbamimidoyl-2-hydroxy-cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of serine protease thrombin(FIIa). | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50054489 (CHEMBL139656 | N-((S)-3-Carbamimidoyl-2-hydroxy-cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 467 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of serine protease Trypsin. | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50054494 (2-({(S)-1-[((S)-3-Carbamimidoyl-2-hydroxy-cyclohex...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 471 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of Coagulation factor X | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076072 (1N-[1-amino(imino)methyl-6-hydroxy-(5S)-tetrahydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 472 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Concentration required for the in vitro inhibitory activity against human enzyme, thrombin cleavage of the chromogenic substrate | Bioorg Med Chem Lett 9: 895-900 (1999) BindingDB Entry DOI: 10.7270/Q2NS0T3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50054504 (2-((S)-3-Benzenesulfonylamino-2-oxo-azepan-1-yl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 479 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of Coagulation factor X | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50076066 (1N-[1-amino(imino)methyl-6-hydroxy-(5S)-tetrahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 479 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Concentration required for the in vitro inhibitory activity against human enzyme, trypsin cleavage of the chromogenic substrate | Bioorg Med Chem Lett 9: 895-900 (1999) BindingDB Entry DOI: 10.7270/Q2NS0T3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anionic trypsin (Bos taurus) | BDBM50054505 (CHEMBL421760 | N-(3-Carbamimidoyl-2-hydroxy-cycloh...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 538 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description compound was tested in vitro for inhibition of serine protease Trypsin. | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50054501 (2-[(S)-3-(Butane-1-sulfonylamino)-2-oxo-piperidin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 709 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of serine protease thrombin(FIIa). | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50054491 (2-((S)-3-Benzenesulfonylamino-2-oxo-piperidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of serine protease Trypsin. | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50076073 (1N-[1-amino(imino)methyl-6-hydroxy-(5S)-tetrahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 791 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Concentration required for the in vitro inhibitory activity against human enzyme, trypsin cleavage of the chromogenic substrate | Bioorg Med Chem Lett 9: 895-900 (1999) BindingDB Entry DOI: 10.7270/Q2NS0T3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50054502 (CHEMBL140494 | N-((S)-3-Carbamimidoyl-2-hydroxy-cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 791 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of serine protease Trypsin. | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anionic trypsin (Bos taurus) | BDBM50054503 (CHEMBL263924 | N-((1S,2R)-3-Carbamimidoyl-2-hydrox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description compound was tested in vitro for inhibition of serine protease Trypsin. | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50054491 (2-((S)-3-Benzenesulfonylamino-2-oxo-piperidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of Coagulation factor X | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 86 total ) | Next | Last >> |