

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50240845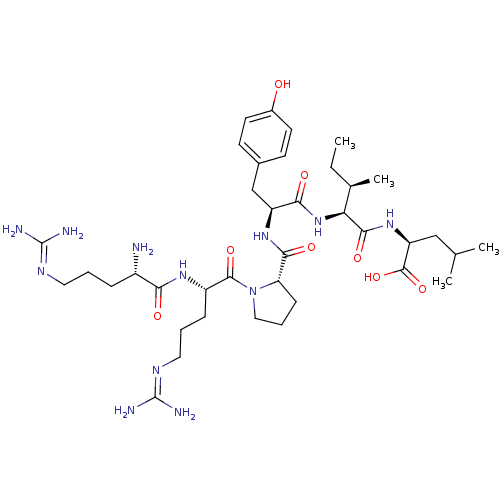 ((S)-2-{(2S,3R)-2-[(S)-2-({(S)-1-[(S)-2-((S)-2-Amin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Binding affinity to human NTR1 | J Med Chem 52: 1803-13 (2009) Article DOI: 10.1021/jm801072v BindingDB Entry DOI: 10.7270/Q2VD6Z9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50257185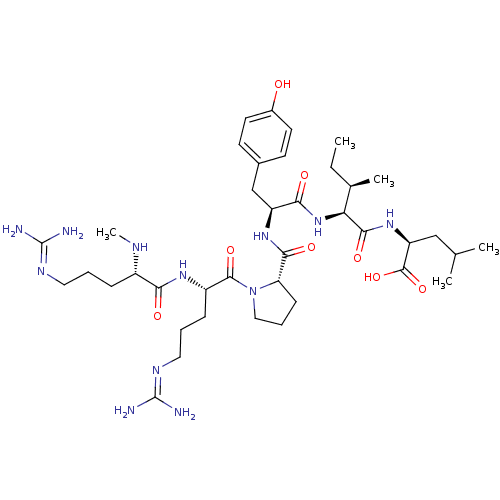 ((S)-2-((2S,3R)-2-((S)-2-((S)-1-((S)-5-guanidino-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Displacement of [125I]I-Tyr(3)NT from human NTR1 | J Med Chem 52: 1803-13 (2009) Article DOI: 10.1021/jm801072v BindingDB Entry DOI: 10.7270/Q2VD6Z9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50257186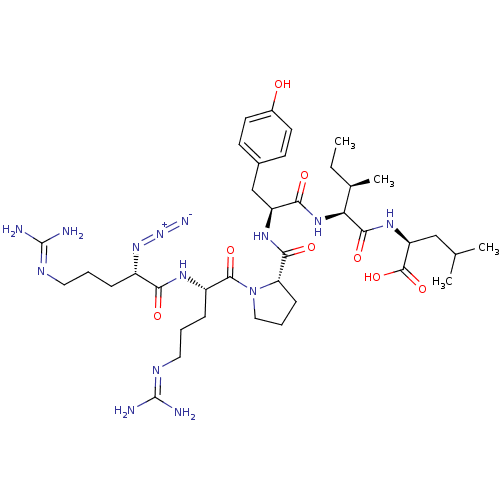 ((S)-2-((2S,3R)-2-((S)-2-((S)-1-((S)-2-((S)-2-azido...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Displacement of [125I]I-Tyr(3)NT from human NTR1 | J Med Chem 52: 1803-13 (2009) Article DOI: 10.1021/jm801072v BindingDB Entry DOI: 10.7270/Q2VD6Z9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50240845 ((S)-2-{(2S,3R)-2-[(S)-2-({(S)-1-[(S)-2-((S)-2-Amin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.05 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Displacement of [125I]I-Tyr(3)NT from human NTR1 | J Med Chem 52: 1803-13 (2009) Article DOI: 10.1021/jm801072v BindingDB Entry DOI: 10.7270/Q2VD6Z9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50130880 (CHEMBL407196 | NT(1-13) | neurotensin | pGlu-Leu-T...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Displacement of [125I]I-Tyr(3)NT from human NTR1 | J Med Chem 52: 1803-13 (2009) Article DOI: 10.1021/jm801072v BindingDB Entry DOI: 10.7270/Q2VD6Z9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50240844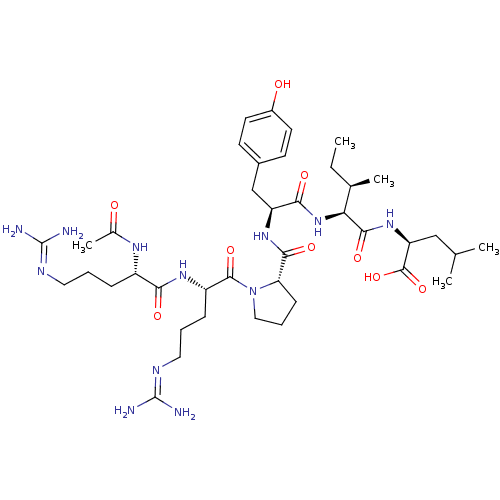 ((S)-2-((2S,3R)-2-((S)-2-((S)-1-((S)-2-((S)-2-aceta...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Displacement of [125I]I-Tyr(3)NT from human NTR1 | J Med Chem 52: 1803-13 (2009) Article DOI: 10.1021/jm801072v BindingDB Entry DOI: 10.7270/Q2VD6Z9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50257188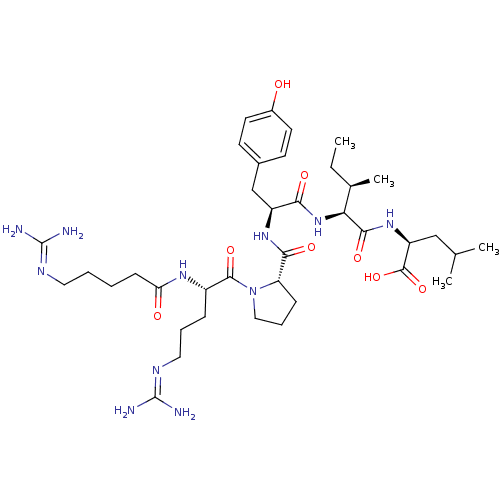 ((S)-2-((2S,3R)-2-((S)-2-((S)-1-((S)-5-guanidino-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Displacement of [125I]I-Tyr(3)NT from human NTR1 | J Med Chem 52: 1803-13 (2009) Article DOI: 10.1021/jm801072v BindingDB Entry DOI: 10.7270/Q2VD6Z9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50300041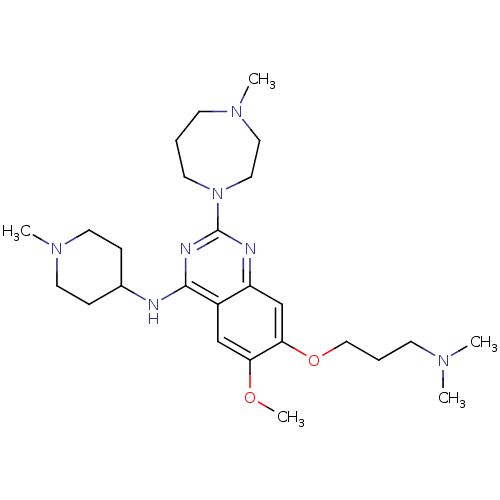 (7-(3-(dimethylamino)propoxy)-6-methoxy-2-(4-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to human recombinant G9a catalytic domain amino acid 913 to 1193 expressed in Escherichia coli BL21 (DE3) by isothermal titration ca... | Eur J Med Chem 56: 179-194 (2012) Article DOI: 10.1016/j.ejmech.2012.08.010 BindingDB Entry DOI: 10.7270/Q2TQ62NX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50257194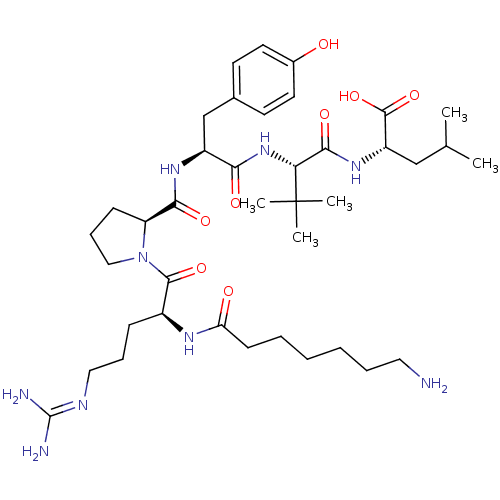 ((S)-2-((S)-2-((S)-2-((S)-1-((S)-2-(7-aminoheptanam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Displacement of [125I]I-Tyr(3)NT from human NTR1 | J Med Chem 52: 1803-13 (2009) Article DOI: 10.1021/jm801072v BindingDB Entry DOI: 10.7270/Q2VD6Z9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50353128 (CHEMBL1231795) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of G9a by fluorescence polarization assay in presence of fluorescein-labeled H3 peptide | Eur J Med Chem 56: 179-194 (2012) Article DOI: 10.1016/j.ejmech.2012.08.010 BindingDB Entry DOI: 10.7270/Q2TQ62NX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50257192 ((S)-2-((S)-2-((S)-2-((S)-1-((S)-2-((S)-7-amino-3-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Binding affinity to human NTR1 | J Med Chem 52: 1803-13 (2009) Article DOI: 10.1021/jm801072v BindingDB Entry DOI: 10.7270/Q2VD6Z9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50257187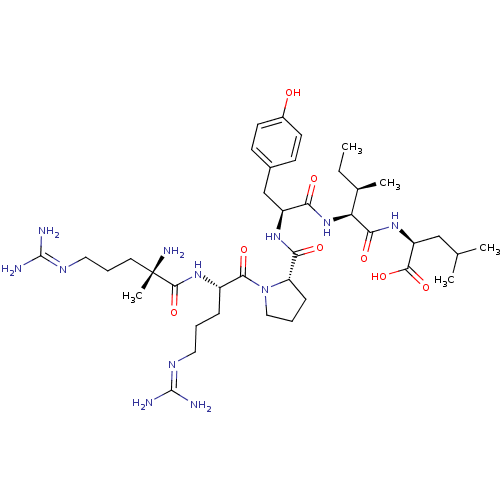 ((S)-2-((2S,3R)-2-((S)-2-((S)-1-((S)-2-((S)-2-amino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Displacement of [125I]I-Tyr(3)NT from human NTR1 | J Med Chem 52: 1803-13 (2009) Article DOI: 10.1021/jm801072v BindingDB Entry DOI: 10.7270/Q2VD6Z9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50257192 ((S)-2-((S)-2-((S)-2-((S)-1-((S)-2-((S)-7-amino-3-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Displacement of [125I]I-Tyr(3)NT from human NTR1 | J Med Chem 52: 1803-13 (2009) Article DOI: 10.1021/jm801072v BindingDB Entry DOI: 10.7270/Q2VD6Z9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50257189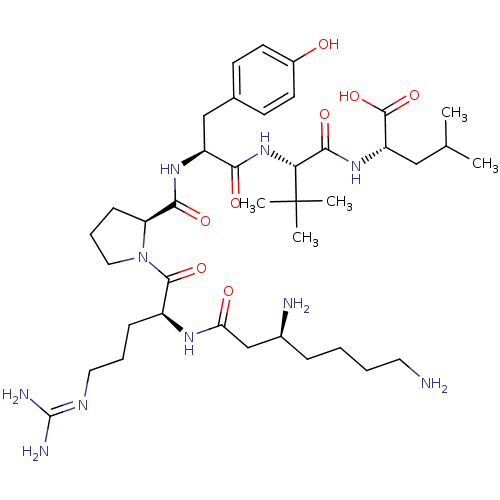 ((S)-2-((S)-2-((S)-2-((S)-1-((S)-2-((S)-3,7-diamino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.07 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Displacement of [125I]I-Tyr(3)NT from human NTR1 | J Med Chem 52: 1803-13 (2009) Article DOI: 10.1021/jm801072v BindingDB Entry DOI: 10.7270/Q2VD6Z9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50257193 ((S)-2-((S)-2-((S)-2-((S)-1-((S)-2-((S)-3,7-diamino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Binding affinity to human NTR1 | J Med Chem 52: 1803-13 (2009) Article DOI: 10.1021/jm801072v BindingDB Entry DOI: 10.7270/Q2VD6Z9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50257193 ((S)-2-((S)-2-((S)-2-((S)-1-((S)-2-((S)-3,7-diamino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Displacement of [125I]I-Tyr(3)NT from human NTR1 | J Med Chem 52: 1803-13 (2009) Article DOI: 10.1021/jm801072v BindingDB Entry DOI: 10.7270/Q2VD6Z9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50257191 ((S)-2-((S)-2-((S)-2-((S)-1-((S)-2-((S)-7-amino-3-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Displacement of [125I]I-Tyr(3)NT from human NTR1 | J Med Chem 52: 1803-13 (2009) Article DOI: 10.1021/jm801072v BindingDB Entry DOI: 10.7270/Q2VD6Z9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50240339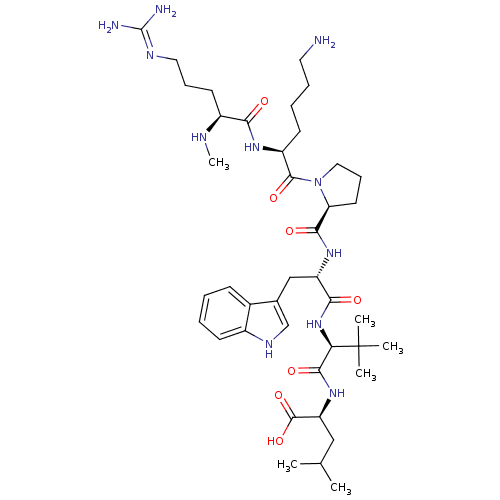 ((S)-2-((S)-2-((S)-2-((S)-1-((S)-6-amino-2-((S)-5-g...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Binding affinity to human NTR1 | J Med Chem 52: 1803-13 (2009) Article DOI: 10.1021/jm801072v BindingDB Entry DOI: 10.7270/Q2VD6Z9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50257197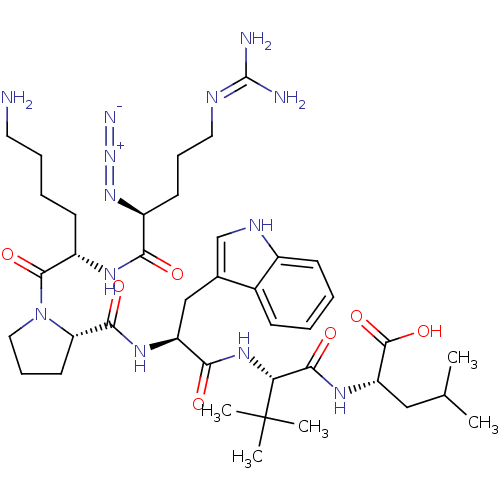 ((S)-2-((S)-2-((S)-2-((S)-1-((S)-6-amino-2-((S)-2-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Displacement of [125I]I-Tyr(3)NT from human NTR1 | J Med Chem 52: 1803-13 (2009) Article DOI: 10.1021/jm801072v BindingDB Entry DOI: 10.7270/Q2VD6Z9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50257190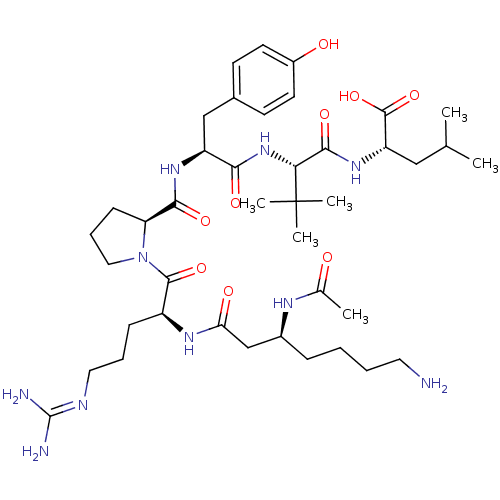 ((S)-2-((S)-2-((S)-2-((S)-1-((S)-2-((S)-3-acetamido...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 136 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Displacement of [125I]I-Tyr(3)NT from human NTR1 | J Med Chem 52: 1803-13 (2009) Article DOI: 10.1021/jm801072v BindingDB Entry DOI: 10.7270/Q2VD6Z9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50240339 ((S)-2-((S)-2-((S)-2-((S)-1-((S)-6-amino-2-((S)-5-g...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Displacement of [125I]I-Tyr(3)NT from human NTR1 | J Med Chem 52: 1803-13 (2009) Article DOI: 10.1021/jm801072v BindingDB Entry DOI: 10.7270/Q2VD6Z9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50396023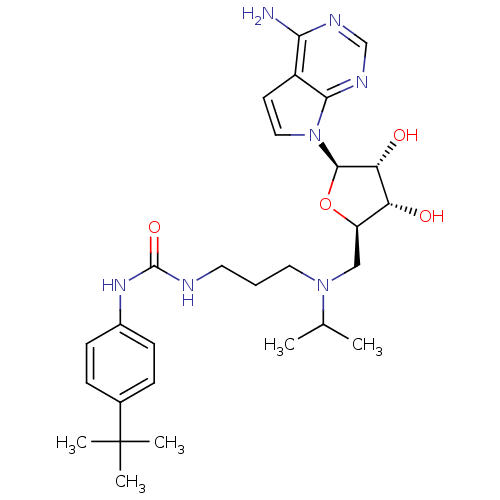 (CHEMBL2169919) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human recombinant DOT1L using [3H]-SAM as substrate after 30 mins | Eur J Med Chem 56: 179-194 (2012) Article DOI: 10.1016/j.ejmech.2012.08.010 BindingDB Entry DOI: 10.7270/Q2TQ62NX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50396022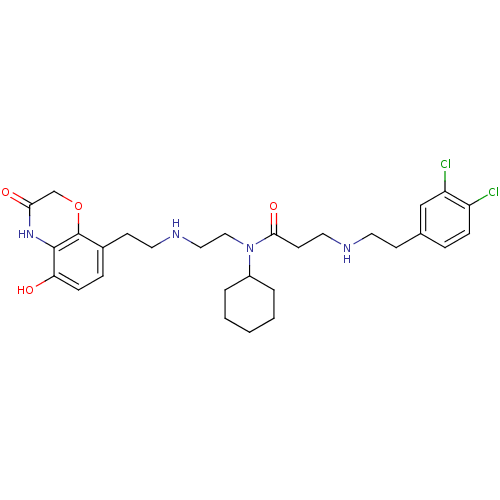 (CHEMBL2169920) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive binding affinity to full length human SMYD2 amino acid 1 to 433 expressed in Escherichia coli BL21 (DE3) after 90 mins by radioactive fil... | Eur J Med Chem 56: 179-194 (2012) Article DOI: 10.1016/j.ejmech.2012.08.010 BindingDB Entry DOI: 10.7270/Q2TQ62NX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50257195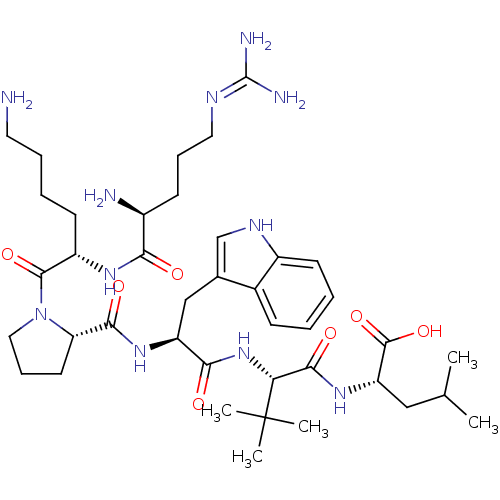 ((S)-2-((S)-2-((S)-2-((S)-1-((S)-6-amino-2-((S)-2-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 303 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Displacement of [125I]I-Tyr(3)NT from human NTR1 | J Med Chem 52: 1803-13 (2009) Article DOI: 10.1021/jm801072v BindingDB Entry DOI: 10.7270/Q2VD6Z9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50396014 (CHEMBL2169888) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of LSD1 | Eur J Med Chem 56: 179-194 (2012) Article DOI: 10.1016/j.ejmech.2012.08.010 BindingDB Entry DOI: 10.7270/Q2TQ62NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50257199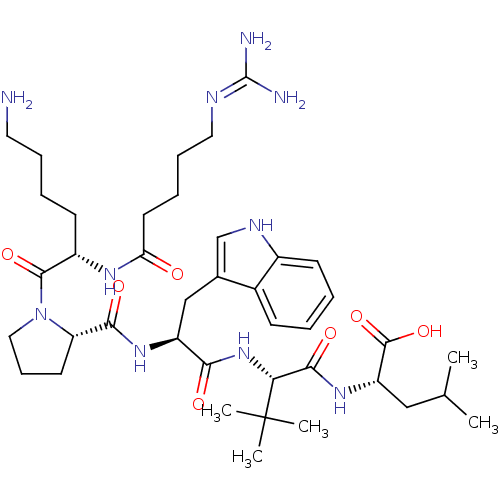 ((S)-2-((S)-2-((S)-2-((S)-1-((S)-6-amino-2-(5-guani...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Displacement of [125I]I-Tyr(3)NT from human NTR1 | J Med Chem 52: 1803-13 (2009) Article DOI: 10.1021/jm801072v BindingDB Entry DOI: 10.7270/Q2VD6Z9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50257196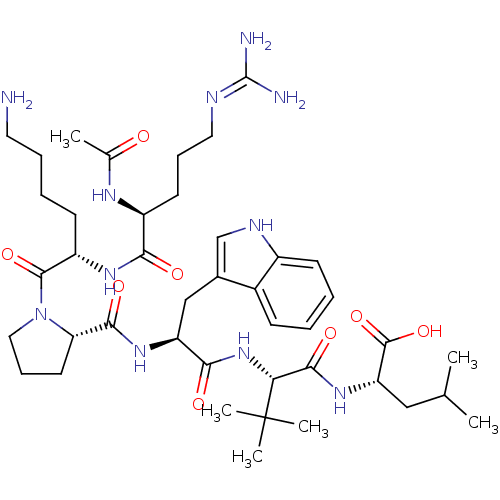 ((S)-2-((S)-2-((S)-2-((S)-1-((S)-2-((S)-2-acetamido...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 647 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Displacement of [125I]I-Tyr(3)NT from human NTR1 | J Med Chem 52: 1803-13 (2009) Article DOI: 10.1021/jm801072v BindingDB Entry DOI: 10.7270/Q2VD6Z9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50396015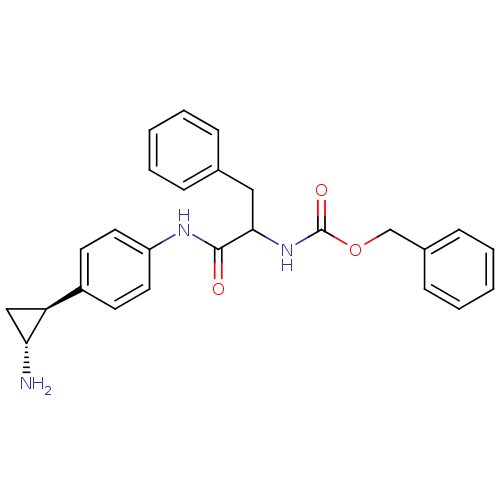 (CHEMBL2169921) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human LSD1 using histone H3 peptide as substrate | Eur J Med Chem 56: 179-194 (2012) Article DOI: 10.1016/j.ejmech.2012.08.010 BindingDB Entry DOI: 10.7270/Q2TQ62NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein REV1 (Homo sapiens) | BDBM50463119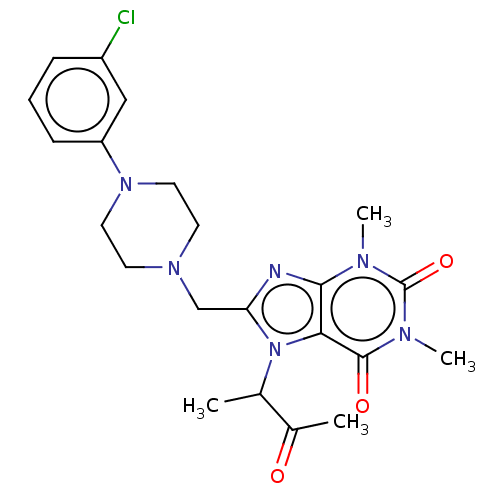 (CHEMBL4240676) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Displacement of FAM-labelled polkappa-RIR peptide from recombinant human C-terminal Rev1 (1158 to 1251 residues) expressed in Escherichia coli BL21(D... | Bioorg Med Chem 26: 4301-4309 (2018) Article DOI: 10.1016/j.bmc.2018.07.029 BindingDB Entry DOI: 10.7270/Q2K93B5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50257198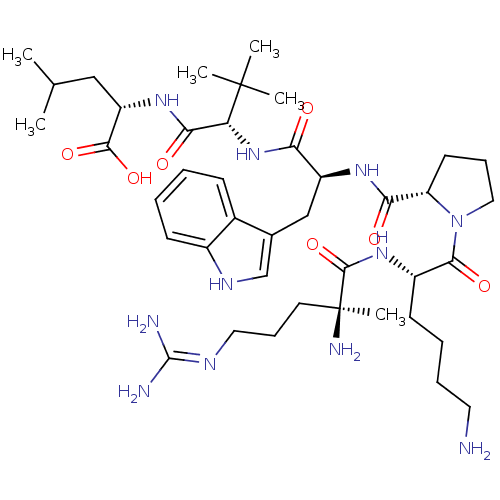 ((S)-2-((S)-2-((S)-2-((S)-1-((S)-6-amino-2-((S)-2-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Displacement of [125I]I-Tyr(3)NT from human NTR1 | J Med Chem 52: 1803-13 (2009) Article DOI: 10.1021/jm801072v BindingDB Entry DOI: 10.7270/Q2VD6Z9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50346874 (CHEMBL1797649) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 catalytic domain amino acid 178 to 831 expressed in Sf9 cells infected with baculovirus using diMeK4H3-21 as substrate | Eur J Med Chem 56: 179-194 (2012) Article DOI: 10.1016/j.ejmech.2012.08.010 BindingDB Entry DOI: 10.7270/Q2TQ62NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein REV1 (Homo sapiens) | BDBM50463115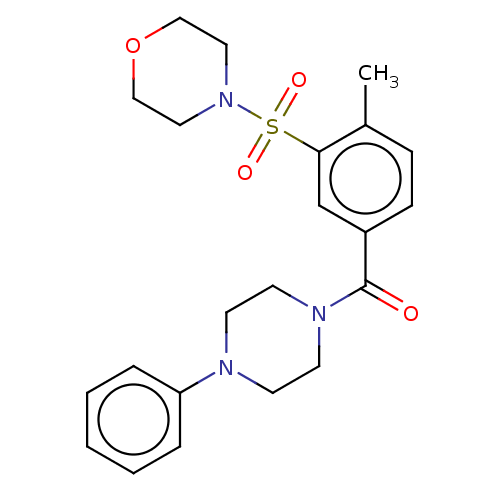 (CHEMBL1717725) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Displacement of FAM-labelled polkappa-RIR peptide from recombinant human C-terminal Rev1 (1158 to 1251 residues) expressed in Escherichia coli BL21(D... | Bioorg Med Chem 26: 4301-4309 (2018) Article DOI: 10.1016/j.bmc.2018.07.029 BindingDB Entry DOI: 10.7270/Q2K93B5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50346862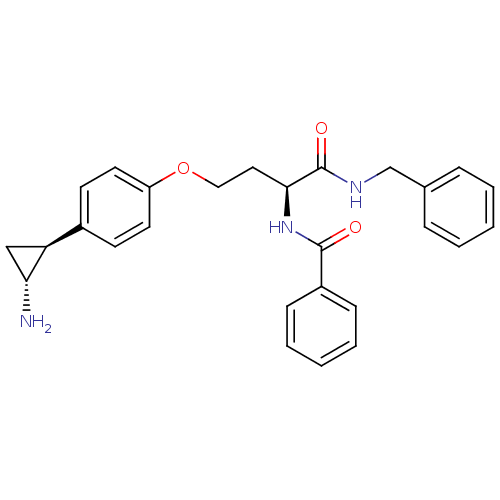 (CHEMBL1215658) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of N terminal hexahistidine-tag recombinant human LSD1 expressed in Escherichia coli BL21 (DE3) using histone H3 peptide as substrate prei... | Eur J Med Chem 56: 179-194 (2012) Article DOI: 10.1016/j.ejmech.2012.08.010 BindingDB Entry DOI: 10.7270/Q2TQ62NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein REV1 (Homo sapiens) | BDBM50463120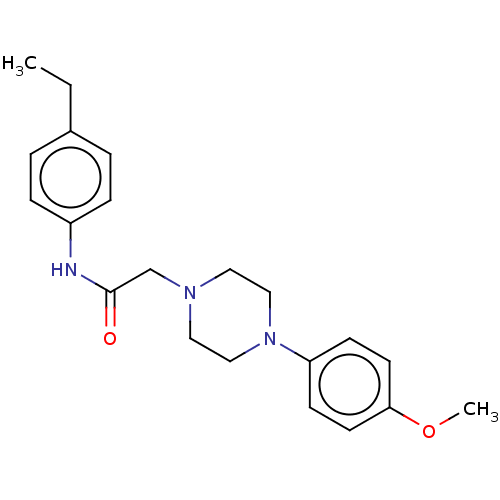 (CHEMBL1721787) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Displacement of FAM-labelled polkappa-RIR peptide from recombinant human C-terminal Rev1 (1158 to 1251 residues) expressed in Escherichia coli BL21(D... | Bioorg Med Chem 26: 4301-4309 (2018) Article DOI: 10.1016/j.bmc.2018.07.029 BindingDB Entry DOI: 10.7270/Q2K93B5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein REV1 (Homo sapiens) | BDBM50463107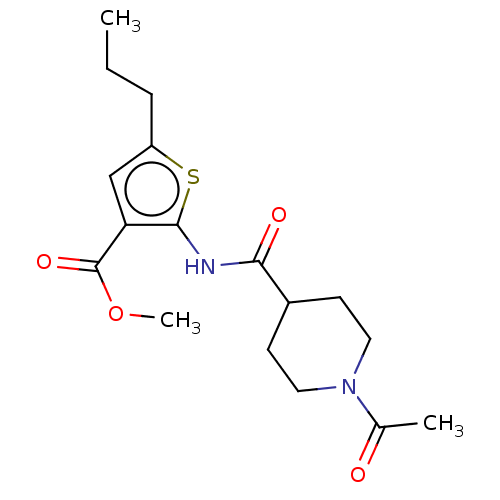 (CHEMBL4245882) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Displacement of FAM-labelled polkappa-RIR peptide from recombinant human C-terminal Rev1 (1158 to 1251 residues) expressed in Escherichia coli BL21(D... | Bioorg Med Chem 26: 4301-4309 (2018) Article DOI: 10.1016/j.bmc.2018.07.029 BindingDB Entry DOI: 10.7270/Q2K93B5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein REV1 (Homo sapiens) | BDBM50463117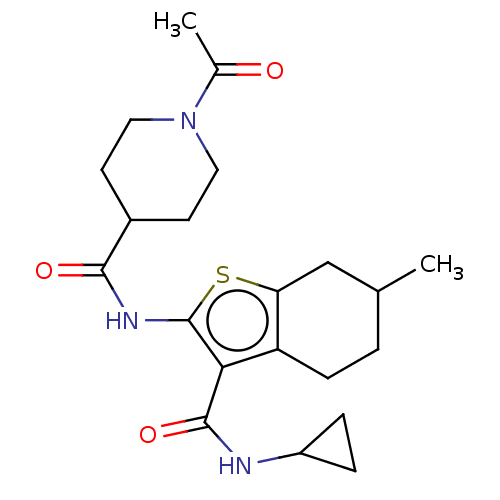 (CHEMBL4249624) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Displacement of FAM-labelled polkappa-RIR peptide from recombinant human C-terminal Rev1 (1158 to 1251 residues) expressed in Escherichia coli BL21(D... | Bioorg Med Chem 26: 4301-4309 (2018) Article DOI: 10.1016/j.bmc.2018.07.029 BindingDB Entry DOI: 10.7270/Q2K93B5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein REV1 (Homo sapiens) | BDBM50463116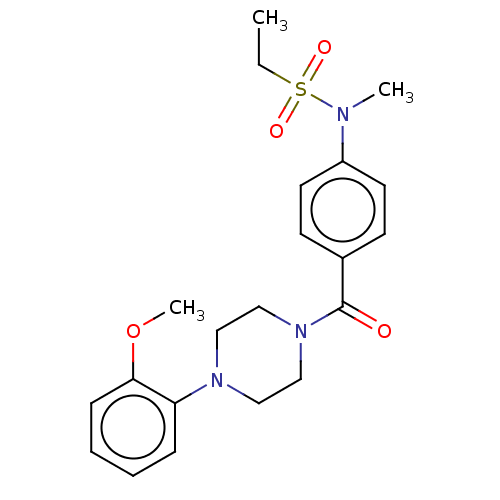 (CHEMBL4237457) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Displacement of FAM-labelled polkappa-RIR peptide from recombinant human C-terminal Rev1 (1158 to 1251 residues) expressed in Escherichia coli BL21(D... | Bioorg Med Chem 26: 4301-4309 (2018) Article DOI: 10.1016/j.bmc.2018.07.029 BindingDB Entry DOI: 10.7270/Q2K93B5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein REV1 (Homo sapiens) | BDBM50463122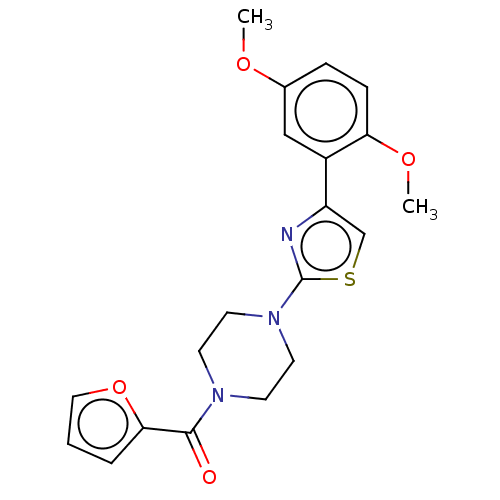 (CHEMBL4249812) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Displacement of FAM-labelled polkappa-RIR peptide from recombinant human C-terminal Rev1 (1158 to 1251 residues) expressed in Escherichia coli BL21(D... | Bioorg Med Chem 26: 4301-4309 (2018) Article DOI: 10.1016/j.bmc.2018.07.029 BindingDB Entry DOI: 10.7270/Q2K93B5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein REV1 (Homo sapiens) | BDBM50463118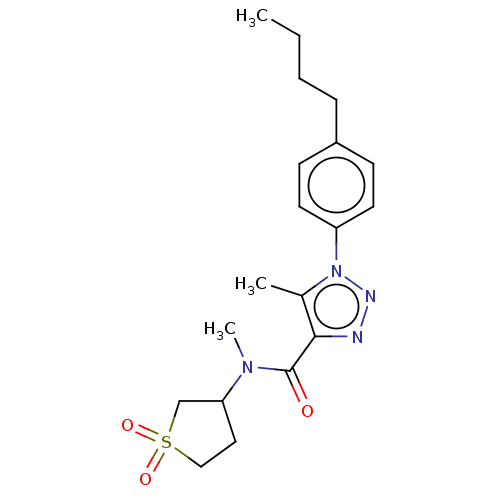 (CHEMBL4246571) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Displacement of FAM-labelled polkappa-RIR peptide from recombinant human C-terminal Rev1 (1158 to 1251 residues) expressed in Escherichia coli BL21(D... | Bioorg Med Chem 26: 4301-4309 (2018) Article DOI: 10.1016/j.bmc.2018.07.029 BindingDB Entry DOI: 10.7270/Q2K93B5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50346534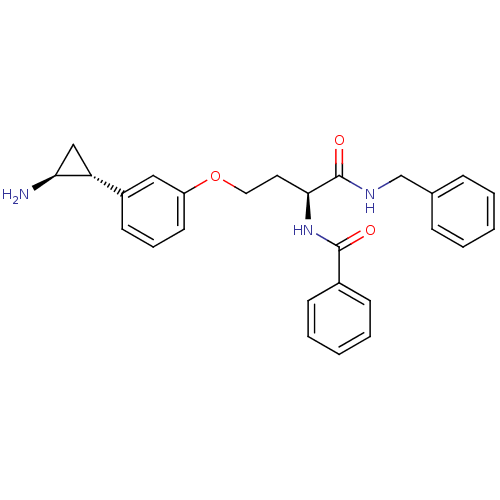 (CHEMBL1797705) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of N terminal hexahistidine-tag recombinant human LSD1 expressed in Escherichia coli BL21 (DE3) using histone H3 peptide as substrate prei... | Eur J Med Chem 56: 179-194 (2012) Article DOI: 10.1016/j.ejmech.2012.08.010 BindingDB Entry DOI: 10.7270/Q2TQ62NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein REV1 (Homo sapiens) | BDBM50463106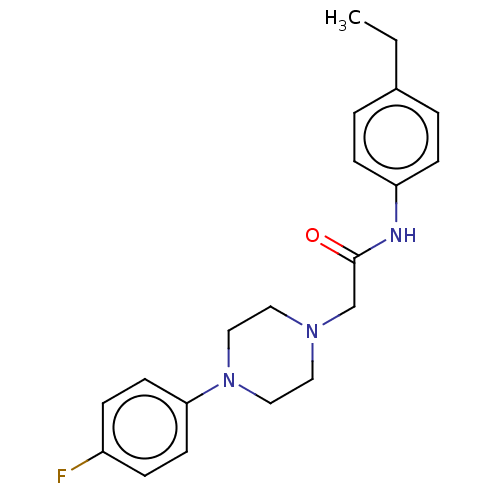 (CHEMBL4238011) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Displacement of FAM-labelled polkappa-RIR peptide from recombinant human C-terminal Rev1 (1158 to 1251 residues) expressed in Escherichia coli BL21(D... | Bioorg Med Chem 26: 4301-4309 (2018) Article DOI: 10.1016/j.bmc.2018.07.029 BindingDB Entry DOI: 10.7270/Q2K93B5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein REV1 (Homo sapiens) | BDBM50463105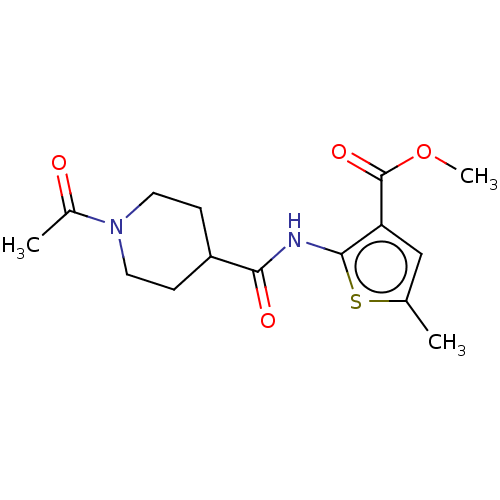 (CHEMBL4244254) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Displacement of FAM-labelled polkappa-RIR peptide from recombinant human C-terminal Rev1 (1158 to 1251 residues) expressed in Escherichia coli BL21(D... | Bioorg Med Chem 26: 4301-4309 (2018) Article DOI: 10.1016/j.bmc.2018.07.029 BindingDB Entry DOI: 10.7270/Q2K93B5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50346865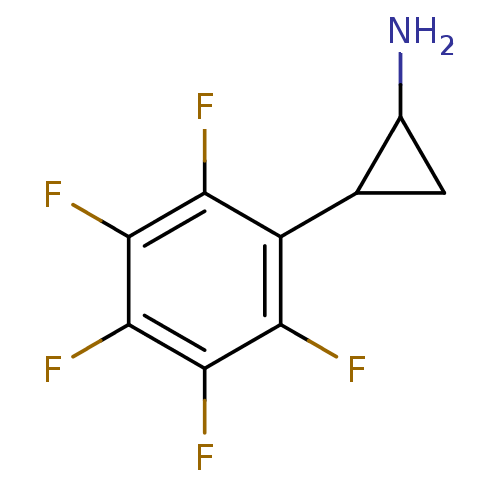 (2-PFPA | CHEMBL1797642) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of LSD1 | Eur J Med Chem 56: 179-194 (2012) Article DOI: 10.1016/j.ejmech.2012.08.010 BindingDB Entry DOI: 10.7270/Q2TQ62NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein REV1 (Homo sapiens) | BDBM50463108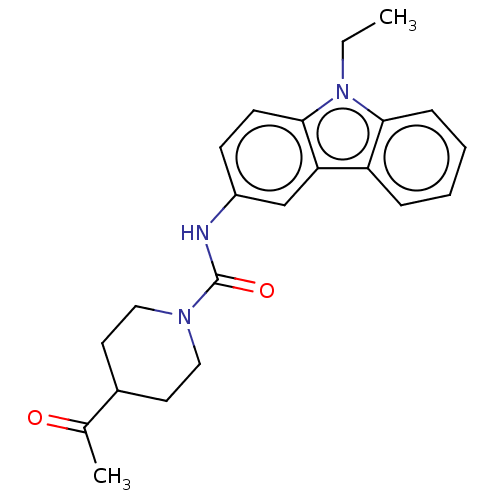 (CHEMBL4244874) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Displacement of FAM-labelled polkappa-RIR peptide from recombinant human C-terminal Rev1 (1158 to 1251 residues) expressed in Escherichia coli BL21(D... | Bioorg Med Chem 26: 4301-4309 (2018) Article DOI: 10.1016/j.bmc.2018.07.029 BindingDB Entry DOI: 10.7270/Q2K93B5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein REV1 (Homo sapiens) | BDBM50463113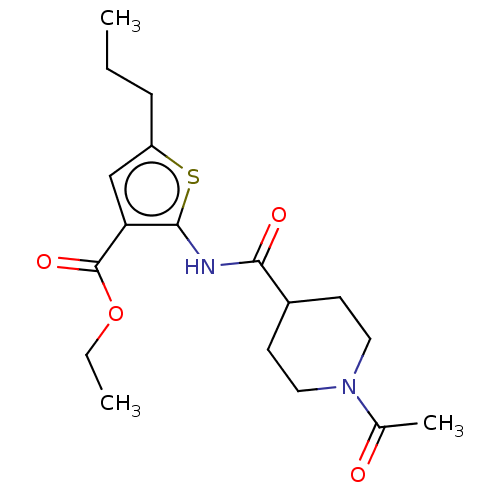 (CHEMBL4238016) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Displacement of FAM-labelled polkappa-RIR peptide from recombinant human C-terminal Rev1 (1158 to 1251 residues) expressed in Escherichia coli BL21(D... | Bioorg Med Chem 26: 4301-4309 (2018) Article DOI: 10.1016/j.bmc.2018.07.029 BindingDB Entry DOI: 10.7270/Q2K93B5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein REV1 (Homo sapiens) | BDBM50463114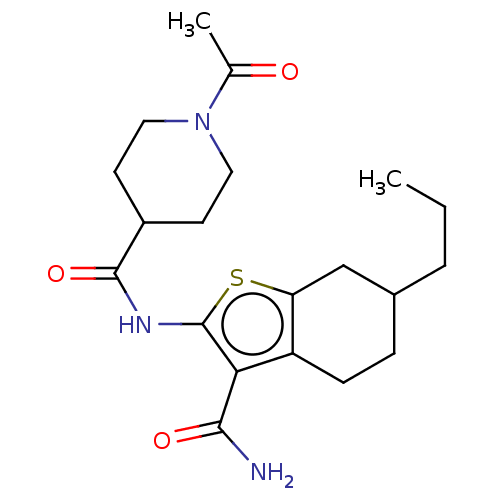 (CHEMBL4241371) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Displacement of FAM-labelled polkappa-RIR peptide from recombinant human C-terminal Rev1 (1158 to 1251 residues) expressed in Escherichia coli BL21(D... | Bioorg Med Chem 26: 4301-4309 (2018) Article DOI: 10.1016/j.bmc.2018.07.029 BindingDB Entry DOI: 10.7270/Q2K93B5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein REV1 (Homo sapiens) | BDBM50463103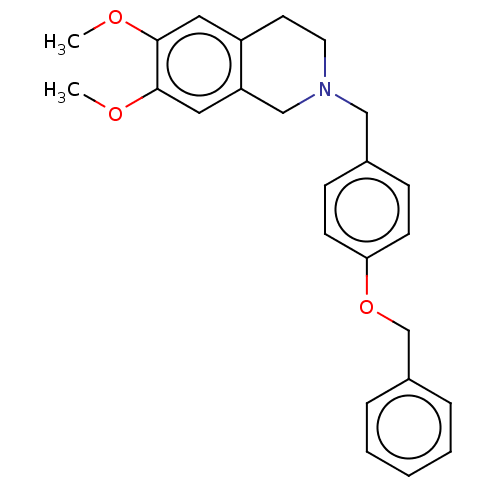 (CHEMBL4237396) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Displacement of FAM-labelled polkappa-RIR peptide from recombinant human C-terminal Rev1 (1158 to 1251 residues) expressed in Escherichia coli BL21(D... | Bioorg Med Chem 26: 4301-4309 (2018) Article DOI: 10.1016/j.bmc.2018.07.029 BindingDB Entry DOI: 10.7270/Q2K93B5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein REV1 (Homo sapiens) | BDBM50463123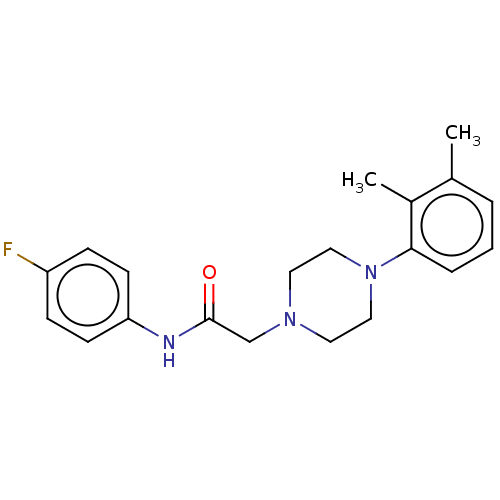 (CHEMBL4239688) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Displacement of FAM-labelled polkappa-RIR peptide from recombinant human C-terminal Rev1 (1158 to 1251 residues) expressed in Escherichia coli BL21(D... | Bioorg Med Chem 26: 4301-4309 (2018) Article DOI: 10.1016/j.bmc.2018.07.029 BindingDB Entry DOI: 10.7270/Q2K93B5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein REV1 (Homo sapiens) | BDBM50463111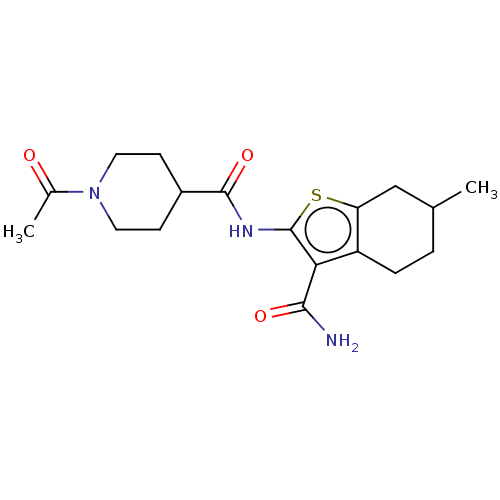 (CHEMBL4239342) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Displacement of FAM-labelled polkappa-RIR peptide from recombinant human C-terminal Rev1 (1158 to 1251 residues) expressed in Escherichia coli BL21(D... | Bioorg Med Chem 26: 4301-4309 (2018) Article DOI: 10.1016/j.bmc.2018.07.029 BindingDB Entry DOI: 10.7270/Q2K93B5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein REV1 (Homo sapiens) | BDBM50463109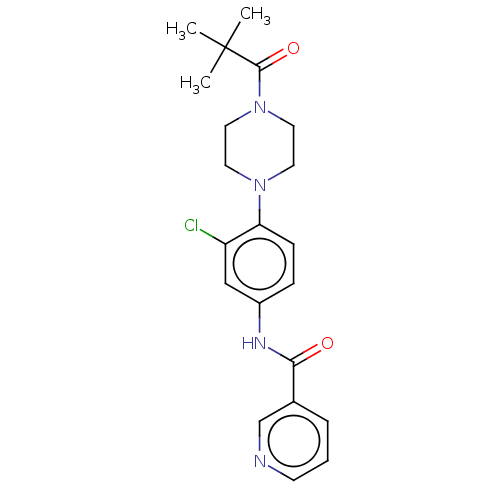 (CHEMBL4241418) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Displacement of FAM-labelled polkappa-RIR peptide from recombinant human C-terminal Rev1 (1158 to 1251 residues) expressed in Escherichia coli BL21(D... | Bioorg Med Chem 26: 4301-4309 (2018) Article DOI: 10.1016/j.bmc.2018.07.029 BindingDB Entry DOI: 10.7270/Q2K93B5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 440 total ) | Next | Last >> |