 Found 47 hits with Last Name = 'haile' and Initial = 'wh'
Found 47 hits with Last Name = 'haile' and Initial = 'wh' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Folylpolyglutamate synthase, mitochondrial
(Homo sapiens (Human)) | BDBM50051747

(5-Amino-2-{4-[(2-amino-4-hydroxy-5,6,7,8-tetrahydr...)Show SMILES NCCCC(NC(=O)c1ccc(NCC2CNc3nc(N)[nH]c(=O)c3N2)cc1)C(O)=O Show InChI InChI=1S/C19H26N8O4/c20-7-1-2-13(18(30)31)25-16(28)10-3-5-11(6-4-10)22-8-12-9-23-15-14(24-12)17(29)27-19(21)26-15/h3-6,12-13,22,24H,1-2,7-9,20H2,(H,25,28)(H,30,31)(H4,21,23,26,27,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibitory activity against mammalian Folyl-polyglutamate synthase |
J Med Chem 39: 2536-40 (1996)
Article DOI: 10.1021/jm960046w
BindingDB Entry DOI: 10.7270/Q2000158 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibitory concentration against dihydrofolate reductase (DHFR) enzyme isolated from CCRF-CEM human leukemia cells. |
J Med Chem 39: 2536-40 (1996)
Article DOI: 10.1021/jm960046w
BindingDB Entry DOI: 10.7270/Q2000158 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049156
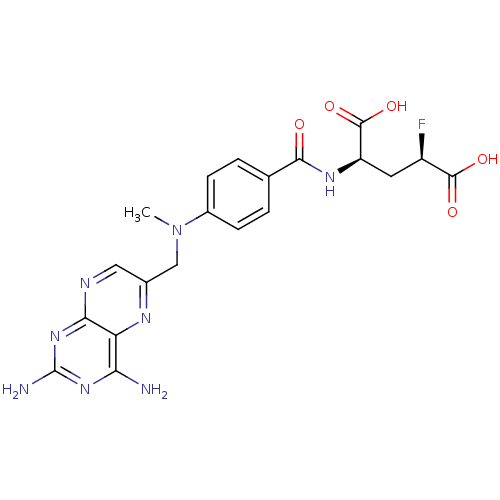
((2R,4R)-2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-me...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@H](C[C@@H](F)C(O)=O)C(O)=O Show InChI InChI=1S/C20H21FN8O5/c1-29(8-10-7-24-16-14(25-10)15(22)27-20(23)28-16)11-4-2-9(3-5-11)17(30)26-13(19(33)34)6-12(21)18(31)32/h2-5,7,12-13H,6,8H2,1H3,(H,26,30)(H,31,32)(H,33,34)(H4,22,23,24,27,28)/t12-,13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibitory activity against Dihydrofolate reductase (DHFR) isolated from CCRF-CEM human leukemia cells in experiment 1 |
J Med Chem 39: 56-65 (1996)
Article DOI: 10.1021/jm950515e
BindingDB Entry DOI: 10.7270/Q2Q81DR3 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50011886
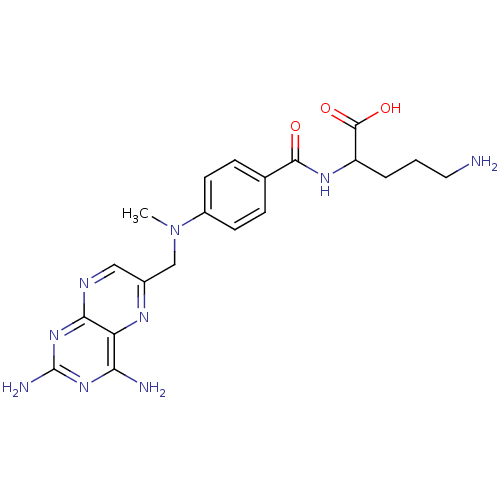
(5-Amino-2-{4-[(2,4-diamino-pteridin-6-ylmethyl)-me...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)NC(CCCN)C(O)=O Show InChI InChI=1S/C20H25N9O3/c1-29(10-12-9-24-17-15(25-12)16(22)27-20(23)28-17)13-6-4-11(5-7-13)18(30)26-14(19(31)32)3-2-8-21/h4-7,9,14H,2-3,8,10,21H2,1H3,(H,26,30)(H,31,32)(H4,22,23,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibitory concentration against dihydrofolate reductase enzyme (DHFR) enzyme isolated from CCRF-CEM human leukemia cells. value mentioned is from li... |
J Med Chem 39: 2536-40 (1996)
Article DOI: 10.1021/jm960046w
BindingDB Entry DOI: 10.7270/Q2000158 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1.18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibitory activity against Dihydrofolate reductase (DHFR) isolated from CCRF-CEM human leukemia cells in experiment 1 |
J Med Chem 39: 56-65 (1996)
Article DOI: 10.1021/jm950515e
BindingDB Entry DOI: 10.7270/Q2Q81DR3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50051746
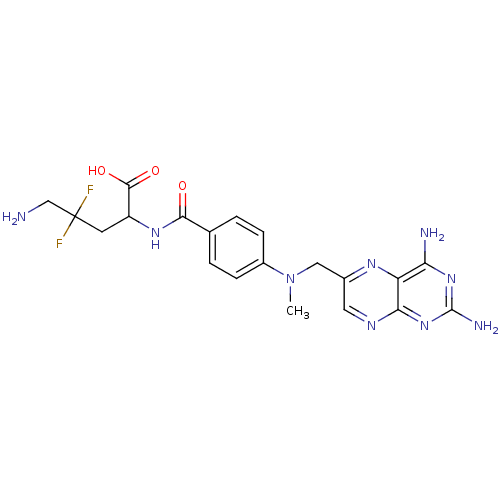
(5-Amino-2-{4-[(2,4-diamino-pteridin-6-ylmethyl)-me...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)NC(CC(F)(F)CN)C(O)=O Show InChI InChI=1S/C20H23F2N9O3/c1-31(8-11-7-26-16-14(27-11)15(24)29-19(25)30-16)12-4-2-10(3-5-12)17(32)28-13(18(33)34)6-20(21,22)9-23/h2-5,7,13H,6,8-9,23H2,1H3,(H,28,32)(H,33,34)(H4,24,25,26,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibitory concentration against dihydrofolate reductase enzyme (DHFR) enzyme isolated from CCRF-CEM human leukemia cells. |
J Med Chem 39: 2536-40 (1996)
Article DOI: 10.1021/jm960046w
BindingDB Entry DOI: 10.7270/Q2000158 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049154

(2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-ami...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)NC(C(O)=O)C(F)(F)CC(O)=O Show InChI InChI=1S/C20H20F2N8O5/c1-30(8-10-7-25-16-13(26-10)15(23)28-19(24)29-16)11-4-2-9(3-5-11)17(33)27-14(18(34)35)20(21,22)6-12(31)32/h2-5,7,14H,6,8H2,1H3,(H,27,33)(H,31,32)(H,34,35)(H4,23,24,25,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibitory activity against Dihydrofolate reductase (DHFR) isolated from CCRF-CEM human leukemia cells in experiment 2 |
J Med Chem 39: 56-65 (1996)
Article DOI: 10.1021/jm950515e
BindingDB Entry DOI: 10.7270/Q2Q81DR3 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibitory activity against Dihydrofolate reductase (DHFR) isolated from CCRF-CEM human leukemia cells in experiment 2 |
J Med Chem 39: 56-65 (1996)
Article DOI: 10.1021/jm950515e
BindingDB Entry DOI: 10.7270/Q2Q81DR3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049155
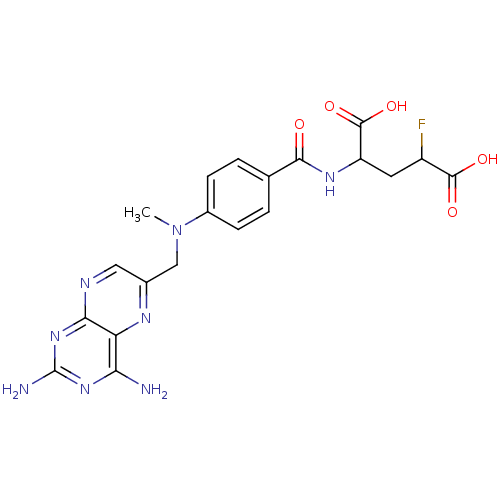
(2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-ami...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)NC(CC(F)C(O)=O)C(O)=O Show InChI InChI=1S/C20H21FN8O5/c1-29(8-10-7-24-16-14(25-10)15(22)27-20(23)28-16)11-4-2-9(3-5-11)17(30)26-13(19(33)34)6-12(21)18(31)32/h2-5,7,12-13H,6,8H2,1H3,(H,26,30)(H,31,32)(H,33,34)(H4,22,23,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibitory activity against Dihydrofolate reductase (DHFR) isolated from CCRF-CEM human leukemia cells in experiment 1 |
J Med Chem 39: 56-65 (1996)
Article DOI: 10.1021/jm950515e
BindingDB Entry DOI: 10.7270/Q2Q81DR3 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibitory activity against Dihydrofolate reductase (DHFR) isolated from CCRF-CEM human leukemia cells in experiment 2 |
J Med Chem 39: 56-65 (1996)
Article DOI: 10.1021/jm950515e
BindingDB Entry DOI: 10.7270/Q2Q81DR3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50011886

(5-Amino-2-{4-[(2,4-diamino-pteridin-6-ylmethyl)-me...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)NC(CCCN)C(O)=O Show InChI InChI=1S/C20H25N9O3/c1-29(10-12-9-24-17-15(25-12)16(22)27-20(23)28-17)13-6-4-11(5-7-13)18(30)26-14(19(31)32)3-2-8-21/h4-7,9,14H,2-3,8,10,21H2,1H3,(H,26,30)(H,31,32)(H4,22,23,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibitory concentration against dihydrofolate reductase enzyme (DHFR) enzyme isolated from CCRF-CEM human leukemia cells. |
J Med Chem 39: 2536-40 (1996)
Article DOI: 10.1021/jm960046w
BindingDB Entry DOI: 10.7270/Q2000158 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated as inhibitor of Escherichia coli Dihydrofolate reductase |
J Med Chem 46: 591-600 (2003)
Article DOI: 10.1021/jm0203534
BindingDB Entry DOI: 10.7270/Q2JQ11RG |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM18069

(5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...)Show InChI InChI=1S/C14H18N4O3/c1-19-10-5-8(6-11(20-2)12(10)21-3)4-9-7-17-14(16)18-13(9)15/h5-7H,4H2,1-3H3,(H4,15,16,17,18) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated as inhibitor of Escherichia coli Dihydrofolate reductase |
J Med Chem 46: 591-600 (2003)
Article DOI: 10.1021/jm0203534
BindingDB Entry DOI: 10.7270/Q2JQ11RG |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated as inhibitor of human Dihydrofolate reductase |
J Med Chem 46: 591-600 (2003)
Article DOI: 10.1021/jm0203534
BindingDB Entry DOI: 10.7270/Q2JQ11RG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Lactobacillus casei) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated as inhibitor of Lactobacillus casei Dihydrofolate reductase |
J Med Chem 46: 591-600 (2003)
Article DOI: 10.1021/jm0203534
BindingDB Entry DOI: 10.7270/Q2JQ11RG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Lactobacillus casei) | BDBM50092651
((S)-2-{4-[2-(2-Amino-4-methyl-7H-pyrrolo[2,3-d]pyr...)Show SMILES Cc1nc(N)nc2[nH]cc(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c12 Show InChI InChI=1S/C21H23N5O5/c1-11-17-14(10-23-18(17)26-21(22)24-11)7-4-12-2-5-13(6-3-12)19(29)25-15(20(30)31)8-9-16(27)28/h2-3,5-6,10,15H,4,7-9H2,1H3,(H,25,29)(H,27,28)(H,30,31)(H3,22,23,24,26)/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated as inhibitor of Lactobacillus casei Dihydrofolate reductase |
J Med Chem 46: 591-600 (2003)
Article DOI: 10.1021/jm0203534
BindingDB Entry DOI: 10.7270/Q2JQ11RG |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18771

((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...)Show SMILES Nc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C24H23N5O6/c1-2-11-29(13-14-3-8-18-17(12-14)22(33)28-24(25)27-18)16-6-4-15(5-7-16)21(32)26-19(23(34)35)9-10-20(30)31/h1,3-8,12,19H,9-11,13H2,(H,26,32)(H,30,31)(H,34,35)(H3,25,27,28,33)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated as inhibitor of Lactobacillus casei thymidylate synthase |
J Med Chem 46: 591-600 (2003)
Article DOI: 10.1021/jm0203534
BindingDB Entry DOI: 10.7270/Q2JQ11RG |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18771

((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...)Show SMILES Nc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C24H23N5O6/c1-2-11-29(13-14-3-8-18-17(12-14)22(33)28-24(25)27-18)16-6-4-15(5-7-16)21(32)26-19(23(34)35)9-10-20(30)31/h1,3-8,12,19H,9-11,13H2,(H,26,32)(H,30,31)(H,34,35)(H3,25,27,28,33)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated as inhibitor of Escherichia coli thymidylate synthase |
J Med Chem 46: 591-600 (2003)
Article DOI: 10.1021/jm0203534
BindingDB Entry DOI: 10.7270/Q2JQ11RG |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18771

((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...)Show SMILES Nc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C24H23N5O6/c1-2-11-29(13-14-3-8-18-17(12-14)22(33)28-24(25)27-18)16-6-4-15(5-7-16)21(32)26-19(23(34)35)9-10-20(30)31/h1,3-8,12,19H,9-11,13H2,(H,26,32)(H,30,31)(H,34,35)(H3,25,27,28,33)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated as inhibitor of human thymidylate synthase |
J Med Chem 46: 591-600 (2003)
Article DOI: 10.1021/jm0203534
BindingDB Entry DOI: 10.7270/Q2JQ11RG |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Lactobacillus casei) | BDBM18069

(5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...)Show InChI InChI=1S/C14H18N4O3/c1-19-10-5-8(6-11(20-2)12(10)21-3)4-9-7-17-14(16)18-13(9)15/h5-7H,4H2,1-3H3,(H4,15,16,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated as inhibitor of Lactobacillus casei Dihydrofolate reductase |
J Med Chem 46: 591-600 (2003)
Article DOI: 10.1021/jm0203534
BindingDB Entry DOI: 10.7270/Q2JQ11RG |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50123630
((S)-2-{4-[2-(2-Amino-4-ethyl-7H-pyrrolo[2,3-d]pyri...)Show SMILES CCc1nc(N)nc2[nH]cc(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c12 Show InChI InChI=1S/C22H25N5O5/c1-2-15-18-14(11-24-19(18)27-22(23)26-15)8-5-12-3-6-13(7-4-12)20(30)25-16(21(31)32)9-10-17(28)29/h3-4,6-7,11,16H,2,5,8-10H2,1H3,(H,25,30)(H,28,29)(H,31,32)(H3,23,24,26,27)/t16-/m0/s1 | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated as inhibitor of Escherichia coli Dihydrofolate reductase |
J Med Chem 46: 591-600 (2003)
Article DOI: 10.1021/jm0203534
BindingDB Entry DOI: 10.7270/Q2JQ11RG |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50123630

((S)-2-{4-[2-(2-Amino-4-ethyl-7H-pyrrolo[2,3-d]pyri...)Show SMILES CCc1nc(N)nc2[nH]cc(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c12 Show InChI InChI=1S/C22H25N5O5/c1-2-15-18-14(11-24-19(18)27-22(23)26-15)8-5-12-3-6-13(7-4-12)20(30)25-16(21(31)32)9-10-17(28)29/h3-4,6-7,11,16H,2,5,8-10H2,1H3,(H,25,30)(H,28,29)(H,31,32)(H3,23,24,26,27)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated as inhibitor of human Dihydrofolate reductase |
J Med Chem 46: 591-600 (2003)
Article DOI: 10.1021/jm0203534
BindingDB Entry DOI: 10.7270/Q2JQ11RG |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50092651

((S)-2-{4-[2-(2-Amino-4-methyl-7H-pyrrolo[2,3-d]pyr...)Show SMILES Cc1nc(N)nc2[nH]cc(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c12 Show InChI InChI=1S/C21H23N5O5/c1-11-17-14(10-23-18(17)26-21(22)24-11)7-4-12-2-5-13(6-3-12)19(29)25-15(20(30)31)8-9-16(27)28/h2-3,5-6,10,15H,4,7-9H2,1H3,(H,25,29)(H,27,28)(H,30,31)(H3,22,23,24,26)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated as inhibitor of human thymidylate synthase |
J Med Chem 46: 591-600 (2003)
Article DOI: 10.1021/jm0203534
BindingDB Entry DOI: 10.7270/Q2JQ11RG |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18795
((2S)-2-[(5-{methyl[(2-methyl-4-oxo-1,4-dihydroquin...)Show SMILES CN(Cc1ccc2nc(C)[nH]c(=O)c2c1)c1ccc(s1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C21H22N4O6S/c1-11-22-14-4-3-12(9-13(14)19(28)23-11)10-25(2)17-7-6-16(32-17)20(29)24-15(21(30)31)5-8-18(26)27/h3-4,6-7,9,15H,5,8,10H2,1-2H3,(H,24,29)(H,26,27)(H,30,31)(H,22,23,28)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated as inhibitor of human thymidylate synthase |
J Med Chem 46: 591-600 (2003)
Article DOI: 10.1021/jm0203534
BindingDB Entry DOI: 10.7270/Q2JQ11RG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50092651

((S)-2-{4-[2-(2-Amino-4-methyl-7H-pyrrolo[2,3-d]pyr...)Show SMILES Cc1nc(N)nc2[nH]cc(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c12 Show InChI InChI=1S/C21H23N5O5/c1-11-17-14(10-23-18(17)26-21(22)24-11)7-4-12-2-5-13(6-3-12)19(29)25-15(20(30)31)8-9-16(27)28/h2-3,5-6,10,15H,4,7-9H2,1H3,(H,25,29)(H,27,28)(H,30,31)(H3,22,23,24,26)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated as inhibitor of human Dihydrofolate reductase |
J Med Chem 46: 591-600 (2003)
Article DOI: 10.1021/jm0203534
BindingDB Entry DOI: 10.7270/Q2JQ11RG |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18796
((2S)-2-{[4-(2-{2-amino-4-oxo-1H,4H,7H-pyrrolo[2,3-...)Show SMILES Nc1nc2[nH]cc(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c2c(=O)[nH]1 |r| Show InChI InChI=1S/C20H21N5O6/c21-20-24-16-15(18(29)25-20)12(9-22-16)6-3-10-1-4-11(5-2-10)17(28)23-13(19(30)31)7-8-14(26)27/h1-2,4-5,9,13H,3,6-8H2,(H,23,28)(H,26,27)(H,30,31)(H4,21,22,24,25,29)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated as inhibitor of human Dihydrofolate reductase |
J Med Chem 46: 591-600 (2003)
Article DOI: 10.1021/jm0203534
BindingDB Entry DOI: 10.7270/Q2JQ11RG |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50092651

((S)-2-{4-[2-(2-Amino-4-methyl-7H-pyrrolo[2,3-d]pyr...)Show SMILES Cc1nc(N)nc2[nH]cc(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c12 Show InChI InChI=1S/C21H23N5O5/c1-11-17-14(10-23-18(17)26-21(22)24-11)7-4-12-2-5-13(6-3-12)19(29)25-15(20(30)31)8-9-16(27)28/h2-3,5-6,10,15H,4,7-9H2,1H3,(H,25,29)(H,27,28)(H,30,31)(H3,22,23,24,26)/t15-/m0/s1 | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated as inhibitor of Escherichia coli Dihydrofolate reductase |
J Med Chem 46: 591-600 (2003)
Article DOI: 10.1021/jm0203534
BindingDB Entry DOI: 10.7270/Q2JQ11RG |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50092651

((S)-2-{4-[2-(2-Amino-4-methyl-7H-pyrrolo[2,3-d]pyr...)Show SMILES Cc1nc(N)nc2[nH]cc(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c12 Show InChI InChI=1S/C21H23N5O5/c1-11-17-14(10-23-18(17)26-21(22)24-11)7-4-12-2-5-13(6-3-12)19(29)25-15(20(30)31)8-9-16(27)28/h2-3,5-6,10,15H,4,7-9H2,1H3,(H,25,29)(H,27,28)(H,30,31)(H3,22,23,24,26)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated as inhibitor of Escherichia coli thymidylate synthase |
J Med Chem 46: 591-600 (2003)
Article DOI: 10.1021/jm0203534
BindingDB Entry DOI: 10.7270/Q2JQ11RG |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18069

(5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...)Show InChI InChI=1S/C14H18N4O3/c1-19-10-5-8(6-11(20-2)12(10)21-3)4-9-7-17-14(16)18-13(9)15/h5-7H,4H2,1-3H3,(H4,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated as inhibitor of human Dihydrofolate reductase |
J Med Chem 46: 591-600 (2003)
Article DOI: 10.1021/jm0203534
BindingDB Entry DOI: 10.7270/Q2JQ11RG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18795

((2S)-2-[(5-{methyl[(2-methyl-4-oxo-1,4-dihydroquin...)Show SMILES CN(Cc1ccc2nc(C)[nH]c(=O)c2c1)c1ccc(s1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C21H22N4O6S/c1-11-22-14-4-3-12(9-13(14)19(28)23-11)10-25(2)17-7-6-16(32-17)20(29)24-15(21(30)31)5-8-18(26)27/h3-4,6-7,9,15H,5,8,10H2,1-2H3,(H,24,29)(H,26,27)(H,30,31)(H,22,23,28)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated as inhibitor of Lactobacillus casei thymidylate synthase |
J Med Chem 46: 591-600 (2003)
Article DOI: 10.1021/jm0203534
BindingDB Entry DOI: 10.7270/Q2JQ11RG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50092651

((S)-2-{4-[2-(2-Amino-4-methyl-7H-pyrrolo[2,3-d]pyr...)Show SMILES Cc1nc(N)nc2[nH]cc(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c12 Show InChI InChI=1S/C21H23N5O5/c1-11-17-14(10-23-18(17)26-21(22)24-11)7-4-12-2-5-13(6-3-12)19(29)25-15(20(30)31)8-9-16(27)28/h2-3,5-6,10,15H,4,7-9H2,1H3,(H,25,29)(H,27,28)(H,30,31)(H3,22,23,24,26)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated as inhibitor of Lactobacillus casei thymidylate synthase |
J Med Chem 46: 591-600 (2003)
Article DOI: 10.1021/jm0203534
BindingDB Entry DOI: 10.7270/Q2JQ11RG |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50123629

((S)-2-{4-[2-(2-Amino-4-ethyl-6-methyl-7H-pyrrolo[2...)Show SMILES CCc1nc(N)nc2[nH]c(C)c(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c12 Show InChI InChI=1S/C23H27N5O5/c1-3-16-19-15(12(2)25-20(19)28-23(24)27-16)9-6-13-4-7-14(8-5-13)21(31)26-17(22(32)33)10-11-18(29)30/h4-5,7-8,17H,3,6,9-11H2,1-2H3,(H,26,31)(H,29,30)(H,32,33)(H3,24,25,27,28)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated as inhibitor of human thymidylate synthase |
J Med Chem 46: 591-600 (2003)
Article DOI: 10.1021/jm0203534
BindingDB Entry DOI: 10.7270/Q2JQ11RG |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18795

((2S)-2-[(5-{methyl[(2-methyl-4-oxo-1,4-dihydroquin...)Show SMILES CN(Cc1ccc2nc(C)[nH]c(=O)c2c1)c1ccc(s1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C21H22N4O6S/c1-11-22-14-4-3-12(9-13(14)19(28)23-11)10-25(2)17-7-6-16(32-17)20(29)24-15(21(30)31)5-8-18(26)27/h3-4,6-7,9,15H,5,8,10H2,1-2H3,(H,24,29)(H,26,27)(H,30,31)(H,22,23,28)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated as inhibitor of Escherichia coli thymidylate synthase |
J Med Chem 46: 591-600 (2003)
Article DOI: 10.1021/jm0203534
BindingDB Entry DOI: 10.7270/Q2JQ11RG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18796

((2S)-2-{[4-(2-{2-amino-4-oxo-1H,4H,7H-pyrrolo[2,3-...)Show SMILES Nc1nc2[nH]cc(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c2c(=O)[nH]1 |r| Show InChI InChI=1S/C20H21N5O6/c21-20-24-16-15(18(29)25-20)12(9-22-16)6-3-10-1-4-11(5-2-10)17(28)23-13(19(30)31)7-8-14(26)27/h1-2,4-5,9,13H,3,6-8H2,(H,23,28)(H,26,27)(H,30,31)(H4,21,22,24,25,29)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated as inhibitor of human thymidylate synthase |
J Med Chem 46: 591-600 (2003)
Article DOI: 10.1021/jm0203534
BindingDB Entry DOI: 10.7270/Q2JQ11RG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50123629

((S)-2-{4-[2-(2-Amino-4-ethyl-6-methyl-7H-pyrrolo[2...)Show SMILES CCc1nc(N)nc2[nH]c(C)c(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c12 Show InChI InChI=1S/C23H27N5O5/c1-3-16-19-15(12(2)25-20(19)28-23(24)27-16)9-6-13-4-7-14(8-5-13)21(31)26-17(22(32)33)10-11-18(29)30/h4-5,7-8,17H,3,6,9-11H2,1-2H3,(H,26,31)(H,29,30)(H,32,33)(H3,24,25,27,28)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated as inhibitor of Escherichia coli thymidylate synthase |
J Med Chem 46: 591-600 (2003)
Article DOI: 10.1021/jm0203534
BindingDB Entry DOI: 10.7270/Q2JQ11RG |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18796

((2S)-2-{[4-(2-{2-amino-4-oxo-1H,4H,7H-pyrrolo[2,3-...)Show SMILES Nc1nc2[nH]cc(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c2c(=O)[nH]1 |r| Show InChI InChI=1S/C20H21N5O6/c21-20-24-16-15(18(29)25-20)12(9-22-16)6-3-10-1-4-11(5-2-10)17(28)23-13(19(30)31)7-8-14(26)27/h1-2,4-5,9,13H,3,6-8H2,(H,23,28)(H,26,27)(H,30,31)(H4,21,22,24,25,29)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated as inhibitor of Lactobacillus casei thymidylate synthase |
J Med Chem 46: 591-600 (2003)
Article DOI: 10.1021/jm0203534
BindingDB Entry DOI: 10.7270/Q2JQ11RG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Escherichia coli) | BDBM50123629

((S)-2-{4-[2-(2-Amino-4-ethyl-6-methyl-7H-pyrrolo[2...)Show SMILES CCc1nc(N)nc2[nH]c(C)c(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c12 Show InChI InChI=1S/C23H27N5O5/c1-3-16-19-15(12(2)25-20(19)28-23(24)27-16)9-6-13-4-7-14(8-5-13)21(31)26-17(22(32)33)10-11-18(29)30/h4-5,7-8,17H,3,6,9-11H2,1-2H3,(H,26,31)(H,29,30)(H,32,33)(H3,24,25,27,28)/t17-/m0/s1 | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated as inhibitor of Escherichia coli Dihydrofolate reductase |
J Med Chem 46: 591-600 (2003)
Article DOI: 10.1021/jm0203534
BindingDB Entry DOI: 10.7270/Q2JQ11RG |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50123629

((S)-2-{4-[2-(2-Amino-4-ethyl-6-methyl-7H-pyrrolo[2...)Show SMILES CCc1nc(N)nc2[nH]c(C)c(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c12 Show InChI InChI=1S/C23H27N5O5/c1-3-16-19-15(12(2)25-20(19)28-23(24)27-16)9-6-13-4-7-14(8-5-13)21(31)26-17(22(32)33)10-11-18(29)30/h4-5,7-8,17H,3,6,9-11H2,1-2H3,(H,26,31)(H,29,30)(H,32,33)(H3,24,25,27,28)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated as inhibitor of human Dihydrofolate reductase |
J Med Chem 46: 591-600 (2003)
Article DOI: 10.1021/jm0203534
BindingDB Entry DOI: 10.7270/Q2JQ11RG |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Homo sapiens (Human)) | BDBM50011886

(5-Amino-2-{4-[(2,4-diamino-pteridin-6-ylmethyl)-me...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)NC(CCCN)C(O)=O Show InChI InChI=1S/C20H25N9O3/c1-29(10-12-9-24-17-15(25-12)16(22)27-20(23)28-17)13-6-4-11(5-7-13)18(30)26-14(19(31)32)3-2-8-21/h4-7,9,14H,2-3,8,10,21H2,1H3,(H,26,30)(H,31,32)(H4,22,23,24,27,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Folyl-polyglutamate synthase isolated from CCRF-CEM human leukemia cells. |
J Med Chem 39: 2536-40 (1996)
Article DOI: 10.1021/jm960046w
BindingDB Entry DOI: 10.7270/Q2000158 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Lactobacillus casei) | BDBM50123629

((S)-2-{4-[2-(2-Amino-4-ethyl-6-methyl-7H-pyrrolo[2...)Show SMILES CCc1nc(N)nc2[nH]c(C)c(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c12 Show InChI InChI=1S/C23H27N5O5/c1-3-16-19-15(12(2)25-20(19)28-23(24)27-16)9-6-13-4-7-14(8-5-13)21(31)26-17(22(32)33)10-11-18(29)30/h4-5,7-8,17H,3,6,9-11H2,1-2H3,(H,26,31)(H,29,30)(H,32,33)(H3,24,25,27,28)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated as inhibitor of Lactobacillus casei Dihydrofolate reductase |
J Med Chem 46: 591-600 (2003)
Article DOI: 10.1021/jm0203534
BindingDB Entry DOI: 10.7270/Q2JQ11RG |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50123630

((S)-2-{4-[2-(2-Amino-4-ethyl-7H-pyrrolo[2,3-d]pyri...)Show SMILES CCc1nc(N)nc2[nH]cc(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c12 Show InChI InChI=1S/C22H25N5O5/c1-2-15-18-14(11-24-19(18)27-22(23)26-15)8-5-12-3-6-13(7-4-12)20(30)25-16(21(31)32)9-10-17(28)29/h3-4,6-7,11,16H,2,5,8-10H2,1H3,(H,25,30)(H,28,29)(H,31,32)(H3,23,24,26,27)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated as inhibitor of Escherichia coli thymidylate synthase |
J Med Chem 46: 591-600 (2003)
Article DOI: 10.1021/jm0203534
BindingDB Entry DOI: 10.7270/Q2JQ11RG |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50123630

((S)-2-{4-[2-(2-Amino-4-ethyl-7H-pyrrolo[2,3-d]pyri...)Show SMILES CCc1nc(N)nc2[nH]cc(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c12 Show InChI InChI=1S/C22H25N5O5/c1-2-15-18-14(11-24-19(18)27-22(23)26-15)8-5-12-3-6-13(7-4-12)20(30)25-16(21(31)32)9-10-17(28)29/h3-4,6-7,11,16H,2,5,8-10H2,1H3,(H,25,30)(H,28,29)(H,31,32)(H3,23,24,26,27)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated as inhibitor of human thymidylate synthase |
J Med Chem 46: 591-600 (2003)
Article DOI: 10.1021/jm0203534
BindingDB Entry DOI: 10.7270/Q2JQ11RG |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50123629

((S)-2-{4-[2-(2-Amino-4-ethyl-6-methyl-7H-pyrrolo[2...)Show SMILES CCc1nc(N)nc2[nH]c(C)c(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c12 Show InChI InChI=1S/C23H27N5O5/c1-3-16-19-15(12(2)25-20(19)28-23(24)27-16)9-6-13-4-7-14(8-5-13)21(31)26-17(22(32)33)10-11-18(29)30/h4-5,7-8,17H,3,6,9-11H2,1-2H3,(H,26,31)(H,29,30)(H,32,33)(H3,24,25,27,28)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated as inhibitor of Lactobacillus casei thymidylate synthase |
J Med Chem 46: 591-600 (2003)
Article DOI: 10.1021/jm0203534
BindingDB Entry DOI: 10.7270/Q2JQ11RG |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18796

((2S)-2-{[4-(2-{2-amino-4-oxo-1H,4H,7H-pyrrolo[2,3-...)Show SMILES Nc1nc2[nH]cc(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c2c(=O)[nH]1 |r| Show InChI InChI=1S/C20H21N5O6/c21-20-24-16-15(18(29)25-20)12(9-22-16)6-3-10-1-4-11(5-2-10)17(28)23-13(19(30)31)7-8-14(26)27/h1-2,4-5,9,13H,3,6-8H2,(H,23,28)(H,26,27)(H,30,31)(H4,21,22,24,25,29)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated as inhibitor of Escherichia coli thymidylate synthase |
J Med Chem 46: 591-600 (2003)
Article DOI: 10.1021/jm0203534
BindingDB Entry DOI: 10.7270/Q2JQ11RG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Lactobacillus casei) | BDBM18796

((2S)-2-{[4-(2-{2-amino-4-oxo-1H,4H,7H-pyrrolo[2,3-...)Show SMILES Nc1nc2[nH]cc(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c2c(=O)[nH]1 |r| Show InChI InChI=1S/C20H21N5O6/c21-20-24-16-15(18(29)25-20)12(9-22-16)6-3-10-1-4-11(5-2-10)17(28)23-13(19(30)31)7-8-14(26)27/h1-2,4-5,9,13H,3,6-8H2,(H,23,28)(H,26,27)(H,30,31)(H4,21,22,24,25,29)/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated as inhibitor of Lactobacillus casei Dihydrofolate reductase |
J Med Chem 46: 591-600 (2003)
Article DOI: 10.1021/jm0203534
BindingDB Entry DOI: 10.7270/Q2JQ11RG |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM18796

((2S)-2-{[4-(2-{2-amino-4-oxo-1H,4H,7H-pyrrolo[2,3-...)Show SMILES Nc1nc2[nH]cc(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c2c(=O)[nH]1 |r| Show InChI InChI=1S/C20H21N5O6/c21-20-24-16-15(18(29)25-20)12(9-22-16)6-3-10-1-4-11(5-2-10)17(28)23-13(19(30)31)7-8-14(26)27/h1-2,4-5,9,13H,3,6-8H2,(H,23,28)(H,26,27)(H,30,31)(H4,21,22,24,25,29)/t13-/m0/s1 | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated as inhibitor of Escherichia coli Dihydrofolate reductase |
J Med Chem 46: 591-600 (2003)
Article DOI: 10.1021/jm0203534
BindingDB Entry DOI: 10.7270/Q2JQ11RG |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Homo sapiens (Human)) | BDBM50051746

(5-Amino-2-{4-[(2,4-diamino-pteridin-6-ylmethyl)-me...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)NC(CC(F)(F)CN)C(O)=O Show InChI InChI=1S/C20H23F2N9O3/c1-31(8-11-7-26-16-14(27-11)15(24)29-19(25)30-16)12-4-2-10(3-5-12)17(32)28-13(18(33)34)6-20(21,22)9-23/h2-5,7,13H,6,8-9,23H2,1H3,(H,28,32)(H,33,34)(H4,24,25,26,29,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Folyl-polyglutamate synthase isolated from CCRF-CEM human leukemia cells. |
J Med Chem 39: 2536-40 (1996)
Article DOI: 10.1021/jm960046w
BindingDB Entry DOI: 10.7270/Q2000158 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data