 Found 1683 hits with Last Name = 'hajdu' and Initial = 'r'
Found 1683 hits with Last Name = 'hajdu' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50021345
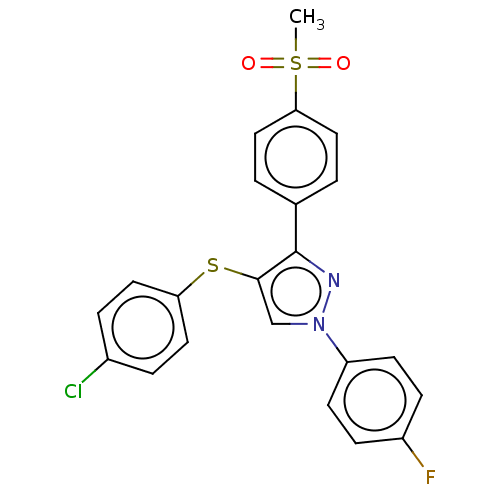
(CHEMBL3287928)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1nn(cc1Sc1ccc(Cl)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C22H16ClFN2O2S2/c1-30(27,28)20-12-2-15(3-13-20)22-21(29-19-10-4-16(23)5-11-19)14-26(25-22)18-8-6-17(24)7-9-18/h2-14H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 637 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG |
ACS Med Chem Lett 5: 717-21 (2014)
Article DOI: 10.1021/ml5001239
BindingDB Entry DOI: 10.7270/Q2JQ12KN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50021331
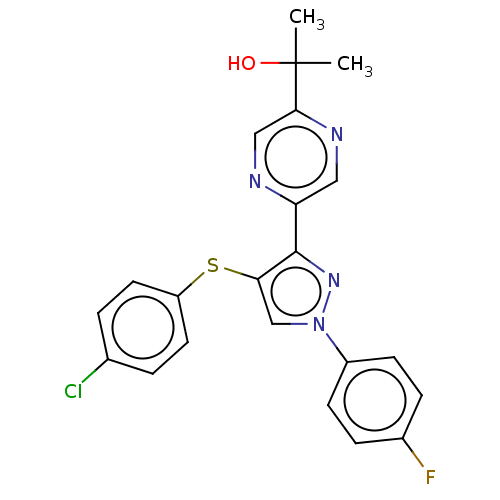
(CHEMBL3287930)Show SMILES CC(C)(O)c1cnc(cn1)-c1nn(cc1Sc1ccc(Cl)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C22H18ClFN4OS/c1-22(2,29)20-12-25-18(11-26-20)21-19(30-17-9-3-14(23)4-10-17)13-28(27-21)16-7-5-15(24)6-8-16/h3-13,29H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG |
ACS Med Chem Lett 5: 717-21 (2014)
Article DOI: 10.1021/ml5001239
BindingDB Entry DOI: 10.7270/Q2JQ12KN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50350538
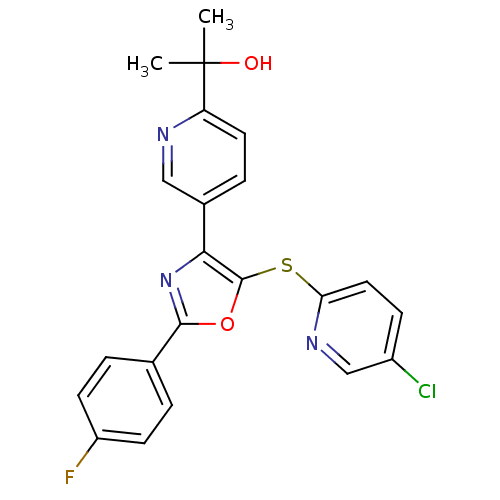
(CHEMBL1812717)Show SMILES CC(C)(O)c1ccc(cn1)-c1nc(oc1Sc1ccc(Cl)cn1)-c1ccc(F)cc1 Show InChI InChI=1S/C22H17ClFN3O2S/c1-22(2,28)17-9-5-14(11-25-17)19-21(30-18-10-6-15(23)12-26-18)29-20(27-19)13-3-7-16(24)8-4-13/h3-12,28H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG |
ACS Med Chem Lett 5: 717-21 (2014)
Article DOI: 10.1021/ml5001239
BindingDB Entry DOI: 10.7270/Q2JQ12KN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50021346
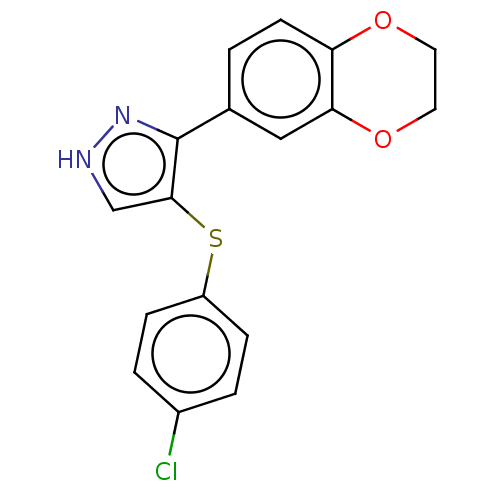
(CHEMBL3287926)Show InChI InChI=1S/C17H13ClN2O2S/c18-12-2-4-13(5-3-12)23-16-10-19-20-17(16)11-1-6-14-15(9-11)22-8-7-21-14/h1-6,9-10H,7-8H2,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG |
ACS Med Chem Lett 5: 717-21 (2014)
Article DOI: 10.1021/ml5001239
BindingDB Entry DOI: 10.7270/Q2JQ12KN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50021329
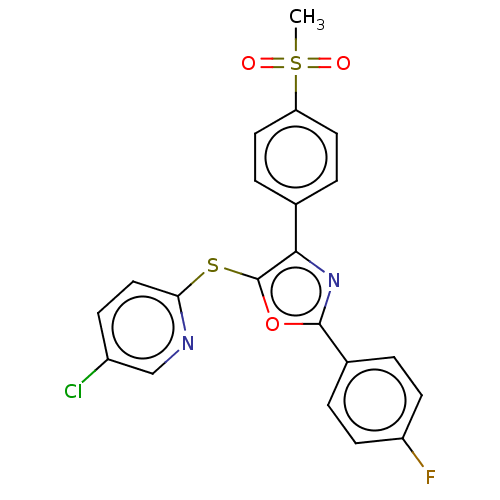
(CHEMBL3287932)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1nc(oc1Sc1ccc(Cl)cn1)-c1ccc(F)cc1 Show InChI InChI=1S/C21H14ClFN2O3S2/c1-30(26,27)17-9-4-13(5-10-17)19-21(29-18-11-6-15(22)12-24-18)28-20(25-19)14-2-7-16(23)8-3-14/h2-12H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG |
ACS Med Chem Lett 5: 717-21 (2014)
Article DOI: 10.1021/ml5001239
BindingDB Entry DOI: 10.7270/Q2JQ12KN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50021344
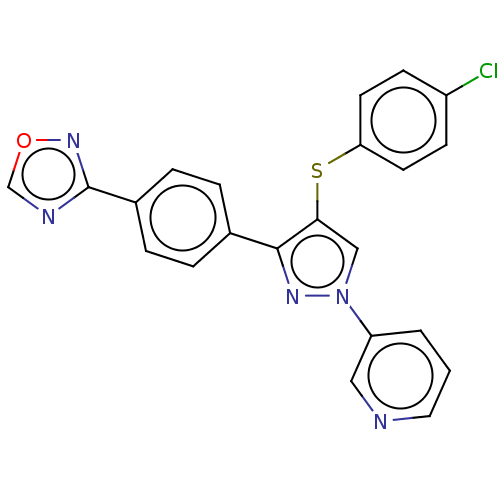
(CHEMBL3287929)Show SMILES Clc1ccc(Sc2cn(nc2-c2ccc(cc2)-c2ncon2)-c2cccnc2)cc1 Show InChI InChI=1S/C22H14ClN5OS/c23-17-7-9-19(10-8-17)30-20-13-28(18-2-1-11-24-12-18)26-21(20)15-3-5-16(6-4-15)22-25-14-29-27-22/h1-14H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG |
ACS Med Chem Lett 5: 717-21 (2014)
Article DOI: 10.1021/ml5001239
BindingDB Entry DOI: 10.7270/Q2JQ12KN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50021334
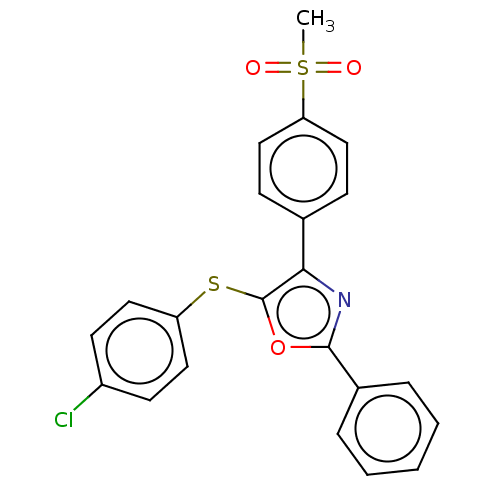
(CHEMBL3287931)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1nc(oc1Sc1ccc(Cl)cc1)-c1ccccc1 Show InChI InChI=1S/C22H16ClNO3S2/c1-29(25,26)19-13-7-15(8-14-19)20-22(28-18-11-9-17(23)10-12-18)27-21(24-20)16-5-3-2-4-6-16/h2-14H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG |
ACS Med Chem Lett 5: 717-21 (2014)
Article DOI: 10.1021/ml5001239
BindingDB Entry DOI: 10.7270/Q2JQ12KN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50021330
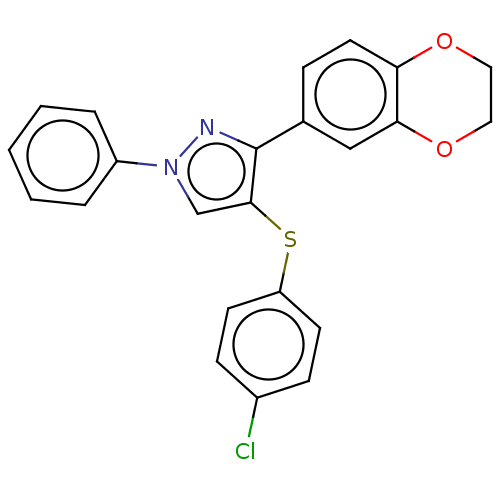
(CHEMBL3287927)Show SMILES Clc1ccc(Sc2cn(nc2-c2ccc3OCCOc3c2)-c2ccccc2)cc1 Show InChI InChI=1S/C23H17ClN2O2S/c24-17-7-9-19(10-8-17)29-22-15-26(18-4-2-1-3-5-18)25-23(22)16-6-11-20-21(14-16)28-13-12-27-20/h1-11,14-15H,12-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG |
ACS Med Chem Lett 5: 717-21 (2014)
Article DOI: 10.1021/ml5001239
BindingDB Entry DOI: 10.7270/Q2JQ12KN |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50158348

((2S,3R,4E)-2-amino-3-hydroxyoctadec-4-en-1-yl dihy...)Show SMILES CCCCCCCCCCCCC\C=C\[C@@H](O)[C@@H](N)COP(O)(O)=O |r| Show InChI InChI=1S/C18H38NO5P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18(20)17(19)16-24-25(21,22)23/h14-15,17-18,20H,2-13,16,19H2,1H3,(H2,21,22,23)/b15-14+/t17-,18+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 3 expressed on CHO cell membranes |
Bioorg Med Chem Lett 14: 3351-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.106
BindingDB Entry DOI: 10.7270/Q2QN667K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50147703
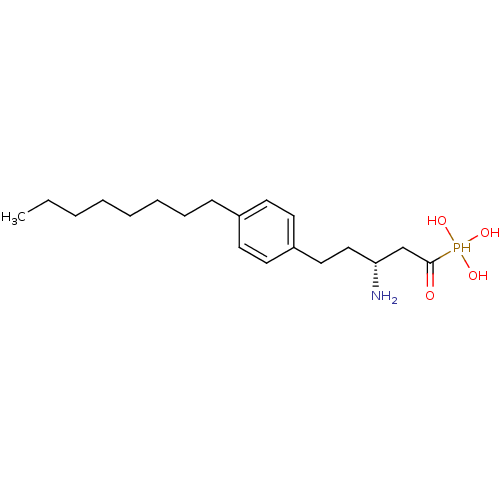
(CHEMBL115554 | CHEMBL115713 | [(1S,3R)-3-Amino-1-h...)Show SMILES CCCCCCCCc1ccc(CC[C@@H](N)CC(=O)P(O)(O)O)cc1 Show InChI InChI=1S/C19H34NO4P/c1-2-3-4-5-6-7-8-16-9-11-17(12-10-16)13-14-18(20)15-19(21)25(22,23)24/h9-12,18,22-25H,2-8,13-15,20H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 1 expressed on CHO cell membranes |
Bioorg Med Chem Lett 14: 3351-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.106
BindingDB Entry DOI: 10.7270/Q2QN667K |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50147703

(CHEMBL115554 | CHEMBL115713 | [(1S,3R)-3-Amino-1-h...)Show SMILES CCCCCCCCc1ccc(CC[C@@H](N)CC(=O)P(O)(O)O)cc1 Show InChI InChI=1S/C19H34NO4P/c1-2-3-4-5-6-7-8-16-9-11-17(12-10-16)13-14-18(20)15-19(21)25(22,23)24/h9-12,18,22-25H,2-8,13-15,20H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human Sphingosine 1-phosphate receptor 1 expressed in CHO cells was determined by using [33P]-S1P as radioligand |
Bioorg Med Chem Lett 14: 3495-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.069
BindingDB Entry DOI: 10.7270/Q2SF2WRM |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50147713
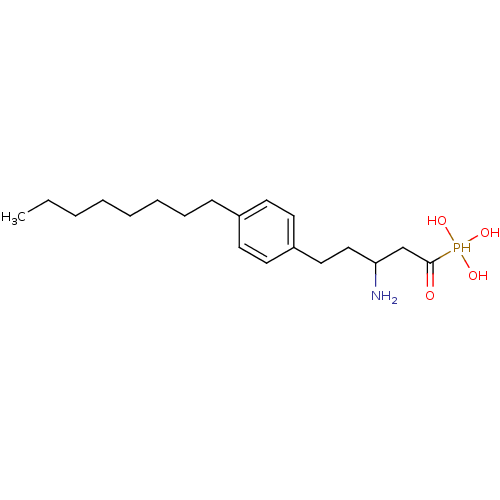
(CHEMBL113344 | [3-Amino-1-hydroxy-5-(4-octyl-pheny...)Show InChI InChI=1S/C19H34NO4P/c1-2-3-4-5-6-7-8-16-9-11-17(12-10-16)13-14-18(20)15-19(21)25(22,23)24/h9-12,18,22-25H,2-8,13-15,20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 1 expressed on CHO cell membranes |
Bioorg Med Chem Lett 14: 3351-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.106
BindingDB Entry DOI: 10.7270/Q2QN667K |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50152322

(CHEMBL184879 | {2-[5-(4-Nonyl-phenyl)-pyrrolidin-2...)Show InChI InChI=1S/C21H36NO3P/c1-2-3-4-5-6-7-8-9-18-10-12-19(13-11-18)21-15-14-20(22-21)16-17-26(23,24)25/h10-13,20-22H,2-9,14-17H2,1H3,(H2,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 1 expressed on CHO cell membranes |
Bioorg Med Chem Lett 14: 4861-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.049
BindingDB Entry DOI: 10.7270/Q2BK1D38 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50152322

(CHEMBL184879 | {2-[5-(4-Nonyl-phenyl)-pyrrolidin-2...)Show InChI InChI=1S/C21H36NO3P/c1-2-3-4-5-6-7-8-9-18-10-12-19(13-11-18)21-15-14-20(22-21)16-17-26(23,24)25/h10-13,20-22H,2-9,14-17H2,1H3,(H2,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 1 expressed on CHO cell membranes |
Bioorg Med Chem Lett 14: 4861-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.049
BindingDB Entry DOI: 10.7270/Q2BK1D38 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50158348

((2S,3R,4E)-2-amino-3-hydroxyoctadec-4-en-1-yl dihy...)Show SMILES CCCCCCCCCCCCC\C=C\[C@@H](O)[C@@H](N)COP(O)(O)=O |r| Show InChI InChI=1S/C18H38NO5P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18(20)17(19)16-24-25(21,22)23/h14-15,17-18,20H,2-13,16,19H2,1H3,(H2,21,22,23)/b15-14+/t17-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 1 expressed on CHO cell membranes |
Bioorg Med Chem Lett 14: 3351-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.106
BindingDB Entry DOI: 10.7270/Q2QN667K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50152328
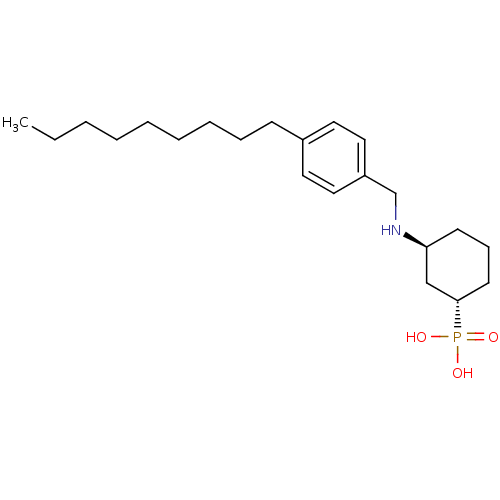
(CHEMBL181597 | [(1S,3S)-3-(4-Nonyl-benzylamino)-cy...)Show SMILES CCCCCCCCCc1ccc(CN[C@H]2CCC[C@@H](C2)P(O)(O)=O)cc1 Show InChI InChI=1S/C22H38NO3P/c1-2-3-4-5-6-7-8-10-19-13-15-20(16-14-19)18-23-21-11-9-12-22(17-21)27(24,25)26/h13-16,21-23H,2-12,17-18H2,1H3,(H2,24,25,26)/t21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 1 expressed on CHO cell membranes |
Bioorg Med Chem Lett 14: 4861-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.049
BindingDB Entry DOI: 10.7270/Q2BK1D38 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50148394
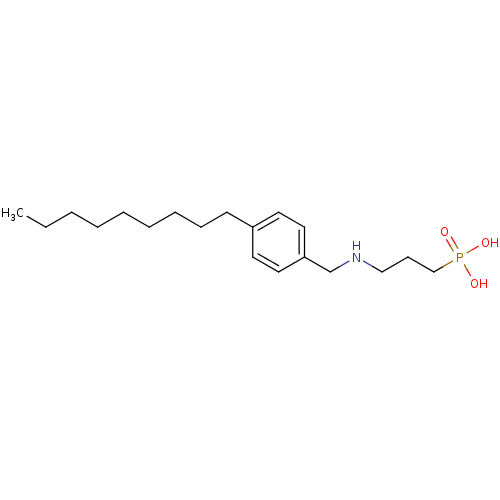
(CHEMBL118860 | [3-(4-Nonyl-benzylamino)-propyl]-ph...)Show InChI InChI=1S/C19H34NO3P/c1-2-3-4-5-6-7-8-10-18-11-13-19(14-12-18)17-20-15-9-16-24(21,22)23/h11-14,20H,2-10,15-17H2,1H3,(H2,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human Sphingosine 1-phosphate receptor 1 expressed in CHO cells was determined by using [33P]-S1P as radioligand |
Bioorg Med Chem Lett 14: 3495-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.069
BindingDB Entry DOI: 10.7270/Q2SF2WRM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092190
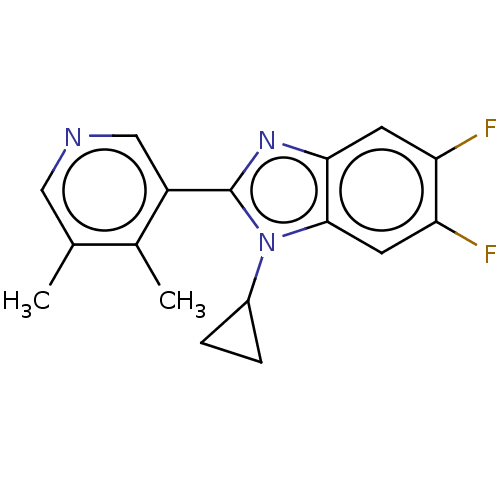
(CHEMBL3582482)Show InChI InChI=1S/C17H15F2N3/c1-9-7-20-8-12(10(9)2)17-21-15-5-13(18)14(19)6-16(15)22(17)11-3-4-11/h5-8,11H,3-4H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50148394

(CHEMBL118860 | [3-(4-Nonyl-benzylamino)-propyl]-ph...)Show InChI InChI=1S/C19H34NO3P/c1-2-3-4-5-6-7-8-10-18-11-13-19(14-12-18)17-20-15-9-16-24(21,22)23/h11-14,20H,2-10,15-17H2,1H3,(H2,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human sphingosine 1-phosphate receptor 1 expressed in CHO cells was determined by using [33P]-S1P as radioligand |
Bioorg Med Chem Lett 14: 3501-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.070
BindingDB Entry DOI: 10.7270/Q2W66K76 |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50385806
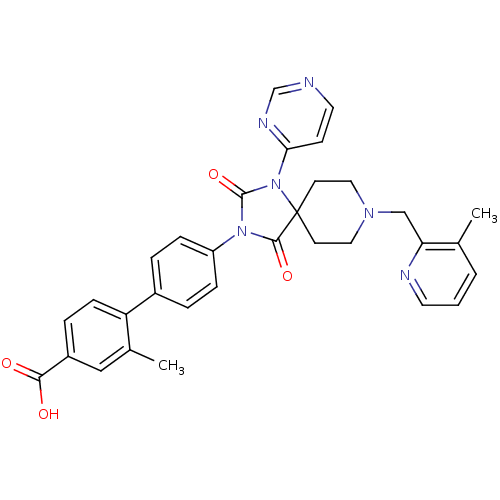
(CHEMBL2041193)Show SMILES Cc1cccnc1CN1CCC2(CC1)N(C(=O)N(C2=O)c1ccc(cc1)-c1ccc(cc1C)C(O)=O)c1ccncn1 Show InChI InChI=1S/C32H30N6O4/c1-21-4-3-14-34-27(21)19-36-16-12-32(13-17-36)30(41)37(31(42)38(32)28-11-15-33-20-35-28)25-8-5-23(6-9-25)26-10-7-24(29(39)40)18-22(26)2/h3-11,14-15,18,20H,12-13,16-17,19H2,1-2H3,(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM50385814
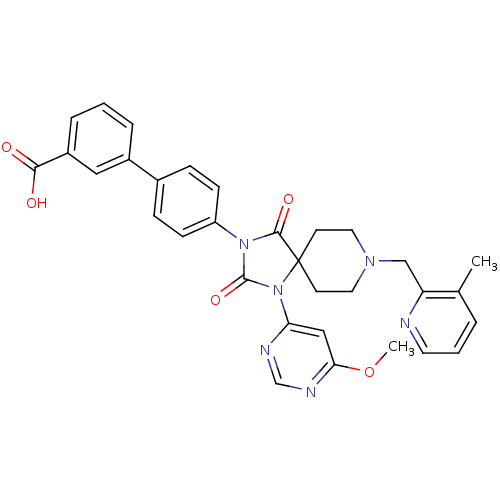
(CHEMBL2043169)Show SMILES COc1cc(ncn1)N1C(=O)N(C(=O)C11CCN(Cc2ncccc2C)CC1)c1ccc(cc1)-c1cccc(c1)C(O)=O Show InChI InChI=1S/C32H30N6O5/c1-21-5-4-14-33-26(21)19-36-15-12-32(13-16-36)30(41)37(31(42)38(32)27-18-28(43-2)35-20-34-27)25-10-8-22(9-11-25)23-6-3-7-24(17-23)29(39)40/h3-11,14,17-18,20H,12-13,15-16,19H2,1-2H3,(H,39,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PHD1 |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50148394

(CHEMBL118860 | [3-(4-Nonyl-benzylamino)-propyl]-ph...)Show InChI InChI=1S/C19H34NO3P/c1-2-3-4-5-6-7-8-10-18-11-13-19(14-12-18)17-20-15-9-16-24(21,22)23/h11-14,20H,2-10,15-17H2,1H3,(H2,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human sphingosine 1-phosphate receptor 2 expressed in CHO cells was determined by using [33P]-S1P as radioligand |
Bioorg Med Chem Lett 14: 3495-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.069
BindingDB Entry DOI: 10.7270/Q2SF2WRM |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50385819
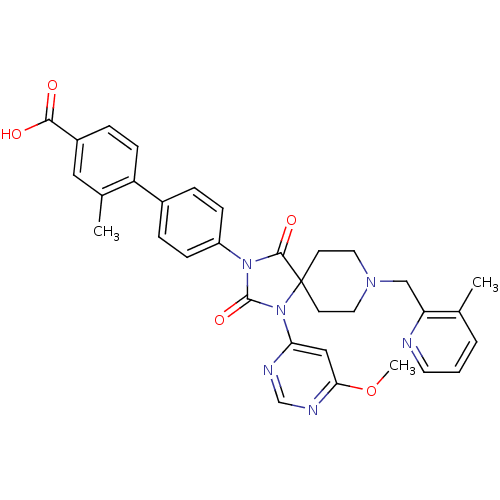
(CHEMBL2043325)Show SMILES COc1cc(ncn1)N1C(=O)N(C(=O)C11CCN(Cc2ncccc2C)CC1)c1ccc(cc1)-c1ccc(cc1C)C(O)=O Show InChI InChI=1S/C33H32N6O5/c1-21-5-4-14-34-27(21)19-37-15-12-33(13-16-37)31(42)38(32(43)39(33)28-18-29(44-3)36-20-35-28)25-9-6-23(7-10-25)26-11-8-24(30(40)41)17-22(26)2/h4-11,14,17-18,20H,12-13,15-16,19H2,1-3H3,(H,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50385782
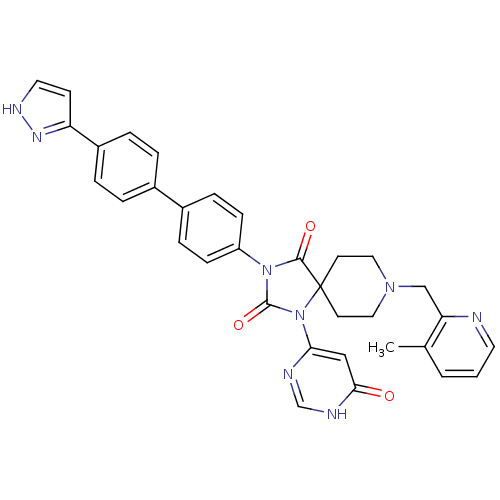
(CHEMBL2041169)Show SMILES Cc1cccnc1CN1CCC2(CC1)N(C(=O)N(C2=O)c1ccc(cc1)-c1ccc(cc1)-c1cc[nH]n1)c1cc(=O)[nH]cn1 Show InChI InChI=1S/C33H30N8O3/c1-22-3-2-15-34-28(22)20-39-17-13-33(14-18-39)31(43)40(32(44)41(33)29-19-30(42)36-21-35-29)26-10-8-24(9-11-26)23-4-6-25(7-5-23)27-12-16-37-38-27/h2-12,15-16,19,21H,13-14,17-18,20H2,1H3,(H,37,38)(H,35,36,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50385788
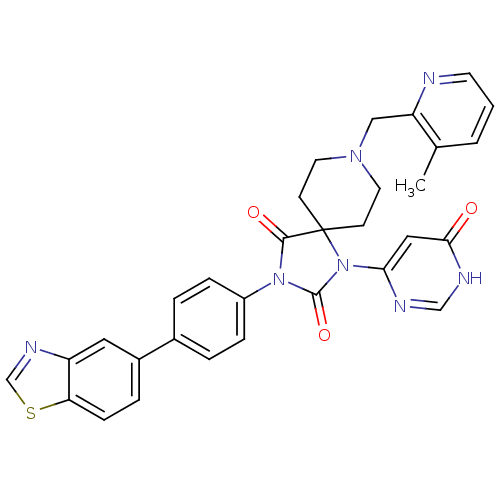
(CHEMBL2041175)Show SMILES Cc1cccnc1CN1CCC2(CC1)N(C(=O)N(C2=O)c1ccc(cc1)-c1ccc2scnc2c1)c1cc(=O)[nH]cn1 Show InChI InChI=1S/C31H27N7O3S/c1-20-3-2-12-32-25(20)17-36-13-10-31(11-14-36)29(40)37(30(41)38(31)27-16-28(39)34-18-33-27)23-7-4-21(5-8-23)22-6-9-26-24(15-22)35-19-42-26/h2-9,12,15-16,18-19H,10-11,13-14,17H2,1H3,(H,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50385796
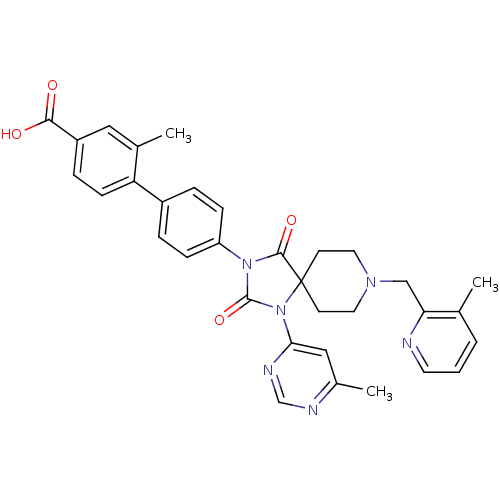
(CHEMBL2041182)Show SMILES Cc1cc(ncn1)N1C(=O)N(C(=O)C11CCN(Cc2ncccc2C)CC1)c1ccc(cc1)-c1ccc(cc1C)C(O)=O Show InChI InChI=1S/C33H32N6O4/c1-21-5-4-14-34-28(21)19-37-15-12-33(13-16-37)31(42)38(32(43)39(33)29-18-23(3)35-20-36-29)26-9-6-24(7-10-26)27-11-8-25(30(40)41)17-22(27)2/h4-11,14,17-18,20H,12-13,15-16,19H2,1-3H3,(H,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50385830
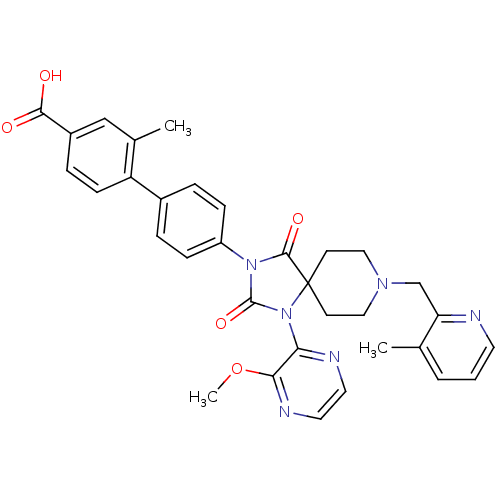
(CHEMBL2041185)Show SMILES COc1nccnc1N1C(=O)N(C(=O)C11CCN(Cc2ncccc2C)CC1)c1ccc(cc1)-c1ccc(cc1C)C(O)=O Show InChI InChI=1S/C33H32N6O5/c1-21-5-4-14-34-27(21)20-37-17-12-33(13-18-37)31(42)38(32(43)39(33)28-29(44-3)36-16-15-35-28)25-9-6-23(7-10-25)26-11-8-24(30(40)41)19-22(26)2/h4-11,14-16,19H,12-13,17-18,20H2,1-3H3,(H,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50385799
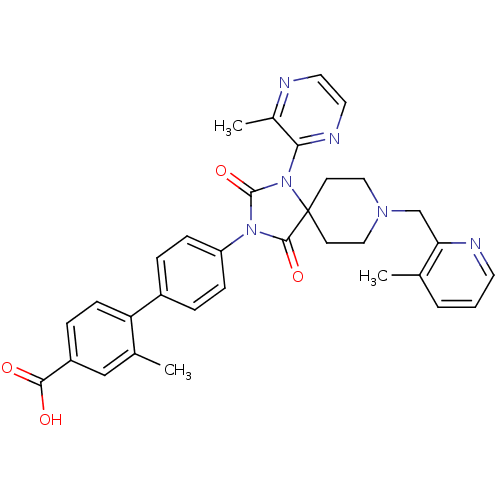
(CHEMBL2041186)Show SMILES Cc1cccnc1CN1CCC2(CC1)N(C(=O)N(C2=O)c1ccc(cc1)-c1ccc(cc1C)C(O)=O)c1nccnc1C Show InChI InChI=1S/C33H32N6O4/c1-21-5-4-14-35-28(21)20-37-17-12-33(13-18-37)31(42)38(32(43)39(33)29-23(3)34-15-16-36-29)26-9-6-24(7-10-26)27-11-8-25(30(40)41)19-22(27)2/h4-11,14-16,19H,12-13,17-18,20H2,1-3H3,(H,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50385803
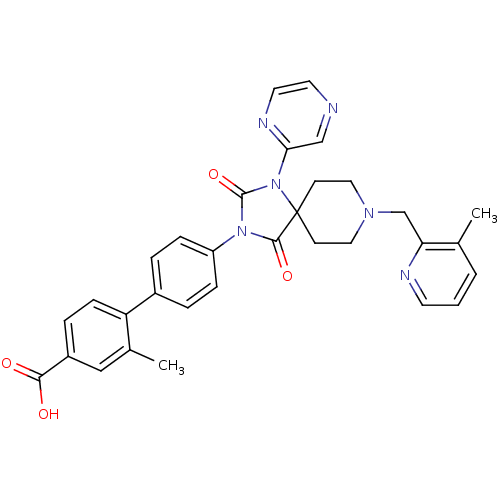
(CHEMBL2041190)Show SMILES Cc1cccnc1CN1CCC2(CC1)N(C(=O)N(C2=O)c1ccc(cc1)-c1ccc(cc1C)C(O)=O)c1cnccn1 Show InChI InChI=1S/C32H30N6O4/c1-21-4-3-13-34-27(21)20-36-16-11-32(12-17-36)30(41)37(31(42)38(32)28-19-33-14-15-35-28)25-8-5-23(6-9-25)26-10-7-24(29(39)40)18-22(26)2/h3-10,13-15,18-19H,11-12,16-17,20H2,1-2H3,(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50385805
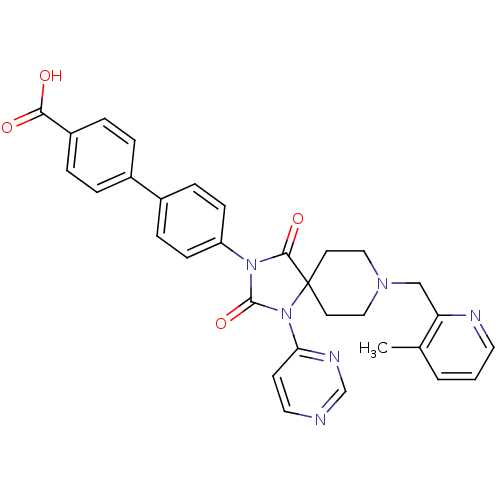
(CHEMBL2041192)Show SMILES Cc1cccnc1CN1CCC2(CC1)N(C(=O)N(C2=O)c1ccc(cc1)-c1ccc(cc1)C(O)=O)c1ccncn1 Show InChI InChI=1S/C31H28N6O4/c1-21-3-2-15-33-26(21)19-35-17-13-31(14-18-35)29(40)36(30(41)37(31)27-12-16-32-20-34-27)25-10-8-23(9-11-25)22-4-6-24(7-5-22)28(38)39/h2-12,15-16,20H,13-14,17-19H2,1H3,(H,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM50385788

(CHEMBL2041175)Show SMILES Cc1cccnc1CN1CCC2(CC1)N(C(=O)N(C2=O)c1ccc(cc1)-c1ccc2scnc2c1)c1cc(=O)[nH]cn1 Show InChI InChI=1S/C31H27N7O3S/c1-20-3-2-12-32-25(20)17-36-13-10-31(11-14-36)29(40)37(30(41)38(31)27-16-28(39)34-18-33-27)23-7-4-21(5-8-23)22-6-9-26-24(15-22)35-19-42-26/h2-9,12,15-16,18-19H,10-11,13-14,17H2,1H3,(H,33,34,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PHD1 |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50385798
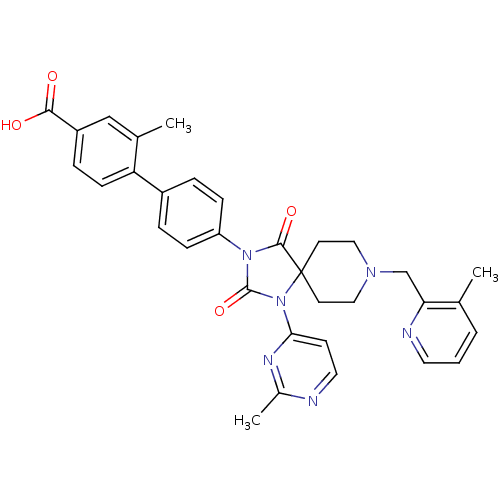
(CHEMBL2041184)Show SMILES Cc1nccc(n1)N1C(=O)N(C(=O)C11CCN(Cc2ncccc2C)CC1)c1ccc(cc1)-c1ccc(cc1C)C(O)=O Show InChI InChI=1S/C33H32N6O4/c1-21-5-4-15-35-28(21)20-37-17-13-33(14-18-37)31(42)38(32(43)39(33)29-12-16-34-23(3)36-29)26-9-6-24(7-10-26)27-11-8-25(30(40)41)19-22(27)2/h4-12,15-16,19H,13-14,17-18,20H2,1-3H3,(H,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM50385819

(CHEMBL2043325)Show SMILES COc1cc(ncn1)N1C(=O)N(C(=O)C11CCN(Cc2ncccc2C)CC1)c1ccc(cc1)-c1ccc(cc1C)C(O)=O Show InChI InChI=1S/C33H32N6O5/c1-21-5-4-14-34-27(21)19-37-15-12-33(13-16-37)31(42)38(32(43)39(33)28-18-29(44-3)36-20-35-28)25-9-6-23(7-10-25)26-11-8-24(30(40)41)17-22(26)2/h4-11,14,17-18,20H,12-13,15-16,19H2,1-3H3,(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PHD1 |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM50385782

(CHEMBL2041169)Show SMILES Cc1cccnc1CN1CCC2(CC1)N(C(=O)N(C2=O)c1ccc(cc1)-c1ccc(cc1)-c1cc[nH]n1)c1cc(=O)[nH]cn1 Show InChI InChI=1S/C33H30N8O3/c1-22-3-2-15-34-28(22)20-39-17-13-33(14-18-39)31(43)40(32(44)41(33)29-19-30(42)36-21-35-29)26-10-8-24(9-11-26)23-4-6-25(7-5-23)27-12-16-37-38-27/h2-12,15-16,19,21H,13-14,17-18,20H2,1H3,(H,37,38)(H,35,36,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PHD1 |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM50385796

(CHEMBL2041182)Show SMILES Cc1cc(ncn1)N1C(=O)N(C(=O)C11CCN(Cc2ncccc2C)CC1)c1ccc(cc1)-c1ccc(cc1C)C(O)=O Show InChI InChI=1S/C33H32N6O4/c1-21-5-4-14-34-28(21)19-37-15-12-33(13-16-37)31(42)38(32(43)39(33)29-18-23(3)35-20-36-29)26-9-6-24(7-10-26)27-11-8-25(30(40)41)17-22(27)2/h4-11,14,17-18,20H,12-13,15-16,19H2,1-3H3,(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PHD1 |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM50385830

(CHEMBL2041185)Show SMILES COc1nccnc1N1C(=O)N(C(=O)C11CCN(Cc2ncccc2C)CC1)c1ccc(cc1)-c1ccc(cc1C)C(O)=O Show InChI InChI=1S/C33H32N6O5/c1-21-5-4-14-34-27(21)20-37-17-12-33(13-18-37)31(42)38(32(43)39(33)28-29(44-3)36-16-15-35-28)25-9-6-23(7-10-25)26-11-8-24(30(40)41)19-22(26)2/h4-11,14-16,19H,12-13,17-18,20H2,1-3H3,(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PHD1 |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM50385803

(CHEMBL2041190)Show SMILES Cc1cccnc1CN1CCC2(CC1)N(C(=O)N(C2=O)c1ccc(cc1)-c1ccc(cc1C)C(O)=O)c1cnccn1 Show InChI InChI=1S/C32H30N6O4/c1-21-4-3-13-34-27(21)20-36-16-11-32(12-17-36)30(41)37(31(42)38(32)28-19-33-14-15-35-28)25-8-5-23(6-9-25)26-10-7-24(29(39)40)18-22(26)2/h3-10,13-15,18-19H,11-12,16-17,20H2,1-2H3,(H,39,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PHD1 |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM50385806

(CHEMBL2041193)Show SMILES Cc1cccnc1CN1CCC2(CC1)N(C(=O)N(C2=O)c1ccc(cc1)-c1ccc(cc1C)C(O)=O)c1ccncn1 Show InChI InChI=1S/C32H30N6O4/c1-21-4-3-14-34-27(21)19-36-16-12-32(13-17-36)30(41)37(31(42)38(32)28-11-15-33-20-35-28)25-8-5-23(6-9-25)26-10-7-24(29(39)40)18-22(26)2/h3-11,14-15,18,20H,12-13,16-17,19H2,1-2H3,(H,39,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PHD1 |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 2
(Homo sapiens (Human)) | BDBM50158348

((2S,3R,4E)-2-amino-3-hydroxyoctadec-4-en-1-yl dihy...)Show SMILES CCCCCCCCCCCCC\C=C\[C@@H](O)[C@@H](N)COP(O)(O)=O |r| Show InChI InChI=1S/C18H38NO5P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18(20)17(19)16-24-25(21,22)23/h14-15,17-18,20H,2-13,16,19H2,1H3,(H2,21,22,23)/b15-14+/t17-,18+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 5 expressed on CHO cell membranes |
Bioorg Med Chem Lett 14: 3351-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.106
BindingDB Entry DOI: 10.7270/Q2QN667K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50158348

((2S,3R,4E)-2-amino-3-hydroxyoctadec-4-en-1-yl dihy...)Show SMILES CCCCCCCCCCCCC\C=C\[C@@H](O)[C@@H](N)COP(O)(O)=O |r| Show InChI InChI=1S/C18H38NO5P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18(20)17(19)16-24-25(21,22)23/h14-15,17-18,20H,2-13,16,19H2,1H3,(H2,21,22,23)/b15-14+/t17-,18+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine 1 phosphate from human S1P3 receptor expressed in CHO cells |
J Med Chem 47: 6662-5 (2004)
Article DOI: 10.1021/jm0492507
BindingDB Entry DOI: 10.7270/Q2TQ611F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50152337
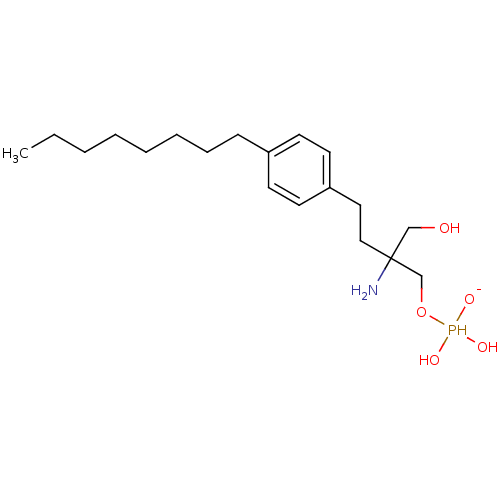
(2-amino-2-({[dihydroxy(oxido)--phosphanyl]oxy}meth...)Show InChI InChI=1S/C19H35NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21-23,26H,2-8,13-16,20H2,1H3/q-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 1 expressed on CHO cell membranes |
Bioorg Med Chem Lett 14: 4861-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.049
BindingDB Entry DOI: 10.7270/Q2BK1D38 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM23163
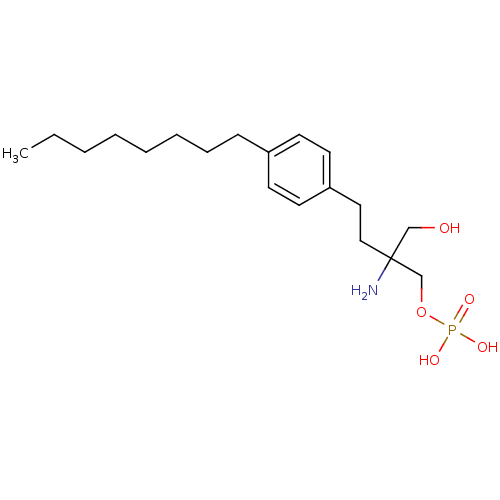
(CHEMBL114606 | FTY720-phosphate, rac-2 | {2-amino-...)Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 1 expressed on CHO cell membranes |
Bioorg Med Chem Lett 14: 3351-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.106
BindingDB Entry DOI: 10.7270/Q2QN667K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM23163

(CHEMBL114606 | FTY720-phosphate, rac-2 | {2-amino-...)Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine 1 phosphate from human S1P1 receptor expressed in CHO cells |
J Med Chem 47: 6662-5 (2004)
Article DOI: 10.1021/jm0492507
BindingDB Entry DOI: 10.7270/Q2TQ611F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50148399

(CHEMBL117130 | Phosphoric acid mono-[1-amino-1-hyd...)Show InChI InChI=1S/C18H32NO5P/c1-2-3-4-5-6-7-8-16-9-11-17(12-10-16)13-14-18(19,15-20)24-25(21,22)23/h9-12,20H,2-8,13-15,19H2,1H3,(H2,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human Sphingosine 1-phosphate receptor 1 expressed in CHO cells was determined by using [33P]-S1P as radioligand |
Bioorg Med Chem Lett 14: 3495-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.069
BindingDB Entry DOI: 10.7270/Q2SF2WRM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092142

(CHEMBL3582477)Show SMILES CC(C)(O)c1cncc(c1)-c1nc2cc(F)c(F)cc2n1C1CC1 Show InChI InChI=1S/C11H17NO8/c1-4(14)12-8-5(15)2-7(11(18)19)20-10(8)9(17)6(16)3-13/h2,5-6,8-10,13,15-17H,3H2,1H3,(H,12,14)(H,18,19)/t5-,6-,8+,9-,10+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50148421
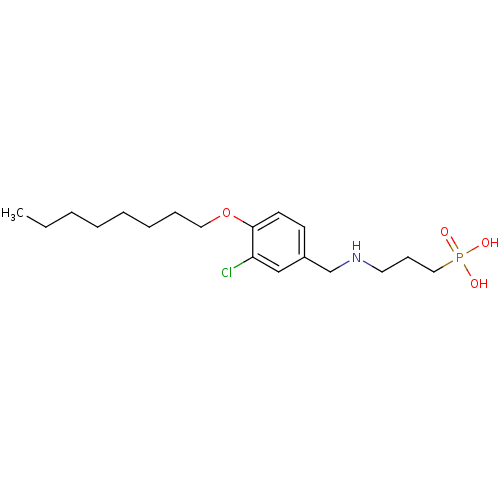
(CHEMBL119413 | [3-(3-Chloro-4-octyloxy-benzylamino...)Show InChI InChI=1S/C18H31ClNO4P/c1-2-3-4-5-6-7-12-24-18-10-9-16(14-17(18)19)15-20-11-8-13-25(21,22)23/h9-10,14,20H,2-8,11-13,15H2,1H3,(H2,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human sphingosine 1-phosphate receptor 1 expressed in CHO cells was determined by using [33P]-S1P as radioligand |
Bioorg Med Chem Lett 14: 3501-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.070
BindingDB Entry DOI: 10.7270/Q2W66K76 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50148433
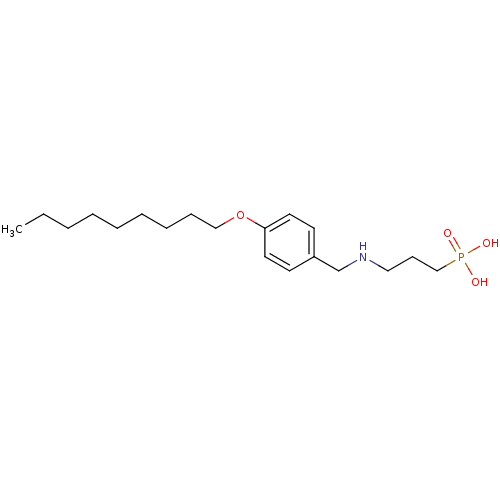
(CHEMBL333335 | [3-(4-Nonyloxy-benzylamino)-propyl]...)Show InChI InChI=1S/C19H34NO4P/c1-2-3-4-5-6-7-8-15-24-19-12-10-18(11-13-19)17-20-14-9-16-25(21,22)23/h10-13,20H,2-9,14-17H2,1H3,(H2,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human sphingosine 1-phosphate receptor 1 expressed in CHO cells was determined by using [33P]-S1P as radioligand |
Bioorg Med Chem Lett 14: 3501-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.070
BindingDB Entry DOI: 10.7270/Q2W66K76 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM22218

(1,2,4-oxadiazole based compound, 35 | 1-[(4-{5-[4-...)Show SMILES OC(=O)C1CN(Cc2ccc(cc2)-c2noc(n2)-c2ccc(CCC(F)(F)F)cc2)C1 Show InChI InChI=1S/C22H20F3N3O3/c23-22(24,25)10-9-14-1-7-17(8-2-14)20-26-19(27-31-20)16-5-3-15(4-6-16)11-28-12-18(13-28)21(29)30/h1-8,18H,9-13H2,(H,29,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Research Laboratories
| Assay Description
The assay measures the displacement of [33P]-labeled sphingosine-1-phosphate (S1P) by test compounds from human S1P receptors expressed on CHO cell m... |
J Med Chem 48: 6169-73 (2005)
Article DOI: 10.1021/jm0503244
BindingDB Entry DOI: 10.7270/Q26971W9 |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM50385813
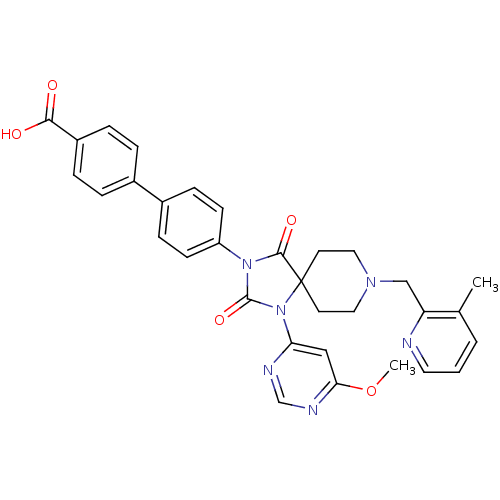
(CHEMBL2043168)Show SMILES COc1cc(ncn1)N1C(=O)N(C(=O)C11CCN(Cc2ncccc2C)CC1)c1ccc(cc1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C32H30N6O5/c1-21-4-3-15-33-26(21)19-36-16-13-32(14-17-36)30(41)37(31(42)38(32)27-18-28(43-2)35-20-34-27)25-11-9-23(10-12-25)22-5-7-24(8-6-22)29(39)40/h3-12,15,18,20H,13-14,16-17,19H2,1-2H3,(H,39,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PHD1 |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM50385805

(CHEMBL2041192)Show SMILES Cc1cccnc1CN1CCC2(CC1)N(C(=O)N(C2=O)c1ccc(cc1)-c1ccc(cc1)C(O)=O)c1ccncn1 Show InChI InChI=1S/C31H28N6O4/c1-21-3-2-15-33-26(21)19-35-17-13-31(14-18-35)29(40)36(30(41)37(31)27-12-16-32-20-34-27)25-10-8-23(9-11-25)22-4-6-24(7-5-22)28(38)39/h2-12,15-16,20H,13-14,17-19H2,1H3,(H,38,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PHD1 |
J Med Chem 55: 2945-59 (2012)
Article DOI: 10.1021/jm201542d
BindingDB Entry DOI: 10.7270/Q27945QW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data