 Found 18708 hits with Last Name = 'ham' and Initial = 'j'
Found 18708 hits with Last Name = 'ham' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Oxidized purine nucleoside triphosphate hydrolase
(Homo sapiens (Human)) | BDBM50511450
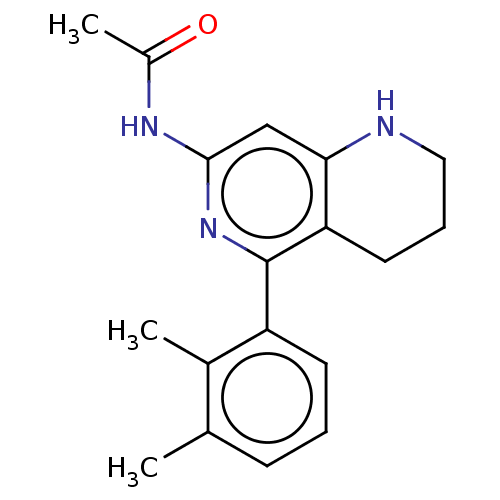
(CHEMBL4436749)Show InChI InChI=1S/C18H21N3O/c1-11-6-4-7-14(12(11)2)18-15-8-5-9-19-16(15)10-17(21-18)20-13(3)22/h4,6-7,10,19H,5,8-9H2,1-3H3,(H,20,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for... |
ACS Med Chem Lett 11: 358-364 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00420
BindingDB Entry DOI: 10.7270/Q2PV6PPZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxidized purine nucleoside triphosphate hydrolase
(Homo sapiens (Human)) | BDBM50511450

(CHEMBL4436749)Show InChI InChI=1S/C18H21N3O/c1-11-6-4-7-14(12(11)2)18-15-8-5-9-19-16(15)10-17(21-18)20-13(3)22/h4,6-7,10,19H,5,8-9H2,1-3H3,(H,20,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for... |
ACS Med Chem Lett 11: 358-364 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00420
BindingDB Entry DOI: 10.7270/Q2PV6PPZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50475328

(CHEMBL414782)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(F)cc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](=O)-[#7](-[#7])-[#7])-[#6](=O)-[#7]-[#7] Show InChI InChI=1S/C82H140FN35O21/c1-43(104-62(123)41-103-79(139)65(45(3)120)116-76(136)57(37-47-24-26-48(83)27-25-47)106-63(124)40-101-61(122)39-102-68(128)49(87)36-46-16-5-4-6-17-46)66(126)107-53(21-13-33-98-80(89)90)70(130)111-52(20-9-12-32-86)74(134)115-59(42-119)77(137)105-44(2)67(127)108-54(22-14-34-99-81(91)92)71(131)109-50(18-7-10-30-84)69(129)112-55(23-15-35-100-82(93)94)72(132)110-51(19-8-11-31-85)73(133)114-58(38-60(88)121)75(135)113-56(78(138)117-95)28-29-64(125)118(96)97/h4-6,16-17,24-27,43-45,49-59,65,119-120H,7-15,18-23,28-42,84-87,95-97H2,1-3H3,(H2,88,121)(H,101,122)(H,102,128)(H,103,139)(H,104,123)(H,105,137)(H,106,124)(H,107,126)(H,108,127)(H,109,131)(H,110,132)(H,111,130)(H,112,129)(H,113,135)(H,114,133)(H,115,134)(H,116,136)(H,117,138)(H4,89,90,98)(H4,91,92,99)(H4,93,94,100)/t43-,44-,45+,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,65-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Receptor binding affinity for recombinant human N/OFQ peptide receptor (NOP) expressed in chinese hamster ovary cells |
J Med Chem 48: 1421-7 (2005)
Article DOI: 10.1021/jm040106v
BindingDB Entry DOI: 10.7270/Q2J38WBW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50475327
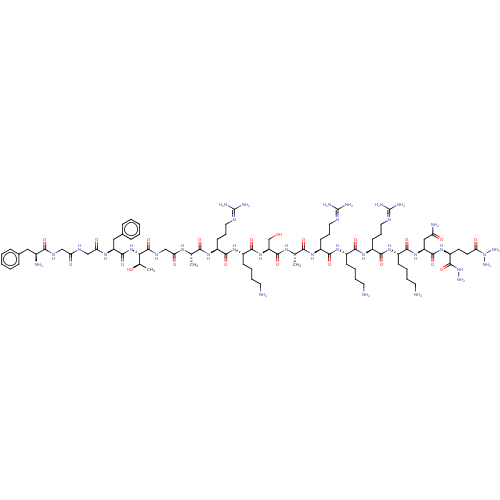
(CHEMBL410653)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](=O)-[#7](-[#7])-[#7])-[#6](=O)-[#7]-[#7] Show InChI InChI=1S/C82H141N35O21/c1-44(103-62(122)42-102-79(138)65(46(3)119)115-76(135)57(38-48-21-8-5-9-22-48)105-63(123)41-100-61(121)40-101-68(127)49(86)37-47-19-6-4-7-20-47)66(125)106-53(26-16-34-97-80(88)89)70(129)110-52(25-12-15-33-85)74(133)114-59(43-118)77(136)104-45(2)67(126)107-54(27-17-35-98-81(90)91)71(130)108-50(23-10-13-31-83)69(128)111-55(28-18-36-99-82(92)93)72(131)109-51(24-11-14-32-84)73(132)113-58(39-60(87)120)75(134)112-56(78(137)116-94)29-30-64(124)117(95)96/h4-9,19-22,44-46,49-59,65,118-119H,10-18,23-43,83-86,94-96H2,1-3H3,(H2,87,120)(H,100,121)(H,101,127)(H,102,138)(H,103,122)(H,104,136)(H,105,123)(H,106,125)(H,107,126)(H,108,130)(H,109,131)(H,110,129)(H,111,128)(H,112,134)(H,113,132)(H,114,133)(H,115,135)(H,116,137)(H4,88,89,97)(H4,90,91,98)(H4,92,93,99)/t44-,45-,46+,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,65-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Receptor binding affinity for recombinant human N/OFQ peptide receptor (NOP) expressed in chinese hamster ovary cells |
J Med Chem 48: 1421-7 (2005)
Article DOI: 10.1021/jm040106v
BindingDB Entry DOI: 10.7270/Q2J38WBW |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 642-9 (2000)
Article DOI: 10.1124/mol.57.3.642
BindingDB Entry DOI: 10.7270/Q21C1VF8 |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 642-9 (2000)
Article DOI: 10.1124/mol.57.3.642
BindingDB Entry DOI: 10.7270/Q21C1VF8 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50049750

((S)-3-(azetidin-2-ylmethoxy)pyridine | 3-((S)-1-Az...)Show InChI InChI=1S/C9H12N2O/c1-2-9(6-10-4-1)12-7-8-3-5-11-8/h1-2,4,6,8,11H,3,5,7H2/t8-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 642-9 (2000)
Article DOI: 10.1124/mol.57.3.642
BindingDB Entry DOI: 10.7270/Q21C1VF8 |
More data for this
Ligand-Target Pair | |
Son of sevenless homolog 1 [564-1049]
() | BDBM609452

(US11702418, Example 10-11)Show SMILES C[C@@H](Nc1nnc(C)c2ccc(cc12)N1CCn2c(C)nc(C)c2C1)c1cccc(c1C)C(F)(F)F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2C251J6 |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50049750

((S)-3-(azetidin-2-ylmethoxy)pyridine | 3-((S)-1-Az...)Show InChI InChI=1S/C9H12N2O/c1-2-9(6-10-4-1)12-7-8-3-5-11-8/h1-2,4,6,8,11H,3,5,7H2/t8-/m0/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 642-9 (2000)
Article DOI: 10.1124/mol.57.3.642
BindingDB Entry DOI: 10.7270/Q21C1VF8 |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50066789
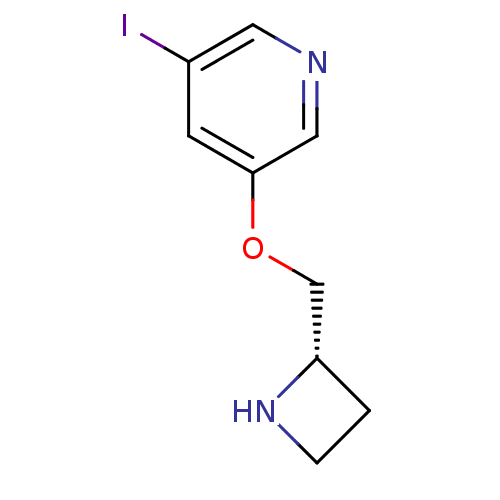
(3-((S)-1-Azetidin-2-ylmethoxy)-5-iodo-pyridine | A...)Show InChI InChI=1S/C9H11IN2O/c10-7-3-9(5-11-4-7)13-6-8-1-2-12-8/h3-5,8,12H,1-2,6H2/t8-/m0/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 642-9 (2000)
Article DOI: 10.1124/mol.57.3.642
BindingDB Entry DOI: 10.7270/Q21C1VF8 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50365814
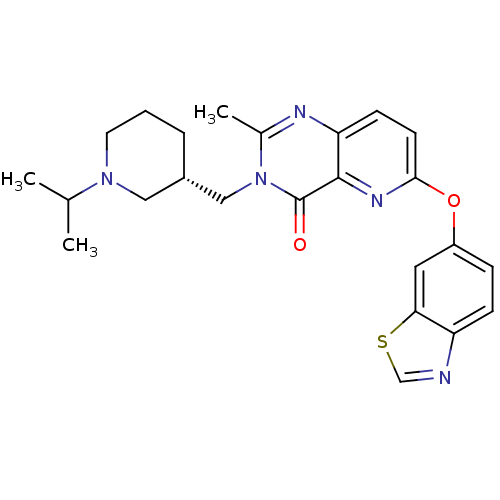
(CHEMBL1956993)Show SMILES CC(C)N1CCC[C@H](Cn2c(C)nc3ccc(Oc4ccc5ncsc5c4)nc3c2=O)C1 |r| Show InChI InChI=1S/C24H27N5O2S/c1-15(2)28-10-4-5-17(12-28)13-29-16(3)26-20-8-9-22(27-23(20)24(29)30)31-18-6-7-19-21(11-18)32-14-25-19/h6-9,11,14-15,17H,4-5,10,12-13H2,1-3H3/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Prosidion Ltd
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Ghrelin from human GHSR membranes overexpressing GSH-R1a by scintillation counting |
Bioorg Med Chem Lett 22: 2271-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.078
BindingDB Entry DOI: 10.7270/Q20G3KMP |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50365815
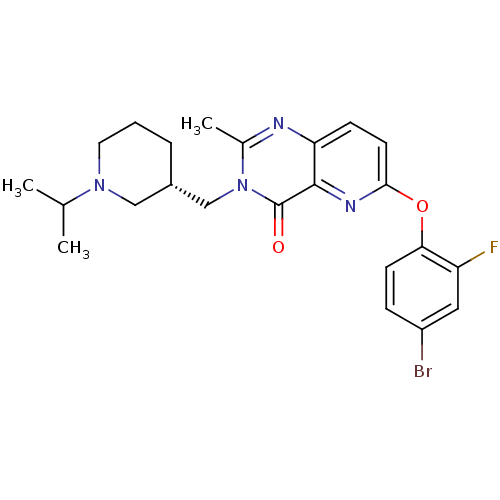
(CHEMBL1956994)Show SMILES CC(C)N1CCC[C@H](Cn2c(C)nc3ccc(Oc4ccc(Br)cc4F)nc3c2=O)C1 |r| Show InChI InChI=1S/C23H26BrFN4O2/c1-14(2)28-10-4-5-16(12-28)13-29-15(3)26-19-7-9-21(27-22(19)23(29)30)31-20-8-6-17(24)11-18(20)25/h6-9,11,14,16H,4-5,10,12-13H2,1-3H3/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Prosidion Ltd
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Ghrelin from human GHSR membranes overexpressing GSH-R1a by scintillation counting |
Bioorg Med Chem Lett 22: 2271-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.078
BindingDB Entry DOI: 10.7270/Q20G3KMP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50231448

(CHEMBL253022 | N-(6-(2-(4-(benzo[d]isothiazol-3-yl...)Show SMILES CCC(=O)NC1CCc2ccc(CCN3CCN(CC3)c3nsc4ccccc34)cc12 |w:5.4| Show InChI InChI=1S/C25H30N4OS/c1-2-24(30)26-22-10-9-19-8-7-18(17-21(19)22)11-12-28-13-15-29(16-14-28)25-20-5-3-4-6-23(20)31-27-25/h3-8,17,22H,2,9-16H2,1H3,(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in Swiss 3T3 cells |
Bioorg Med Chem Lett 18: 489-93 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.106
BindingDB Entry DOI: 10.7270/Q2611023 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50231459

(CHEMBL252818 | N-(6-(2-(4-(benzo[d]isothiazol-3-yl...)Show SMILES CC(=O)NC1CCc2ccc(CCN3CCN(CC3)c3nsc4ccccc34)cc12 |w:4.3| Show InChI InChI=1S/C24H28N4OS/c1-17(29)25-22-9-8-19-7-6-18(16-21(19)22)10-11-27-12-14-28(15-13-27)24-20-4-2-3-5-23(20)30-26-24/h2-7,16,22H,8-15H2,1H3,(H,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in Swiss 3T3 cells |
Bioorg Med Chem Lett 18: 489-93 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.106
BindingDB Entry DOI: 10.7270/Q2611023 |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM484497
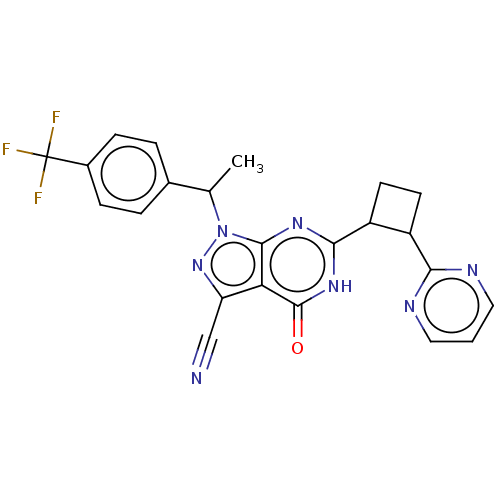
(US10934294, Example 19 | US10934294, Example 20 | ...)Show SMILES CC(c1ccc(cc1)C(F)(F)F)n1nc(C#N)c2c1nc([nH]c2=O)C1CCC1c1ncccn1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Assays for PDE 1 through 11 were performed in parallel at room temperature in 384-well microtiter plates with an incubation volume of 20.2 μL. S... |
US Patent US11028092 (2021)
BindingDB Entry DOI: 10.7270/Q2XS5ZH4 |
More data for this
Ligand-Target Pair | |
Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)
(Homo sapiens (Human)) | BDBM484529

(US10934294, Example 50 | US10934294, Example 51 | ...)Show SMILES CC(c1ccc(nc1)C1CC1)n1nc(C#N)c2c1nc([nH]c2=O)C1CCC1c1ncccn1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... |
US Patent US10934294 (2021)
BindingDB Entry DOI: 10.7270/Q2C250JS |
More data for this
Ligand-Target Pair | |
Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)
(Homo sapiens (Human)) | BDBM484541
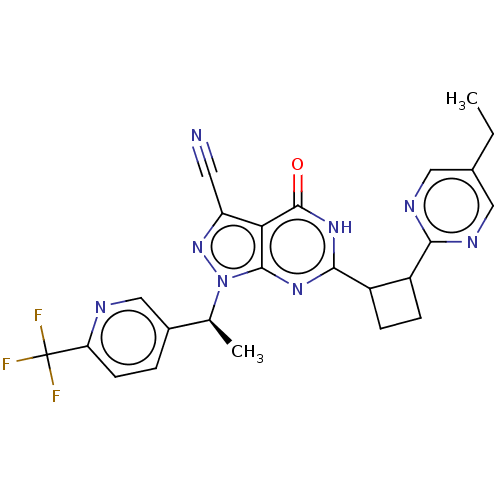
(US10934294, Example 62 | US11028092, Example 63)Show SMILES CCc1cnc(nc1)C1CCC1c1nc2n(nc(C#N)c2c(=O)[nH]1)[C@@H](C)c1ccc(nc1)C(F)(F)F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... |
US Patent US10934294 (2021)
BindingDB Entry DOI: 10.7270/Q2C250JS |
More data for this
Ligand-Target Pair | |
Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)
(Homo sapiens (Human)) | BDBM484570
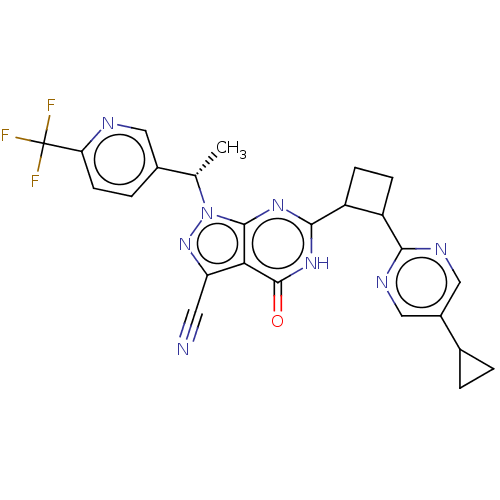
(US10934294, Example 91 | US10934294, Example 92 | ...)Show SMILES C[C@@H](c1ccc(nc1)C(F)(F)F)n1nc(C#N)c2c1nc([nH]c2=O)C1CCC1c1ncc(cn1)C1CC1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... |
US Patent US10934294 (2021)
BindingDB Entry DOI: 10.7270/Q2C250JS |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573157
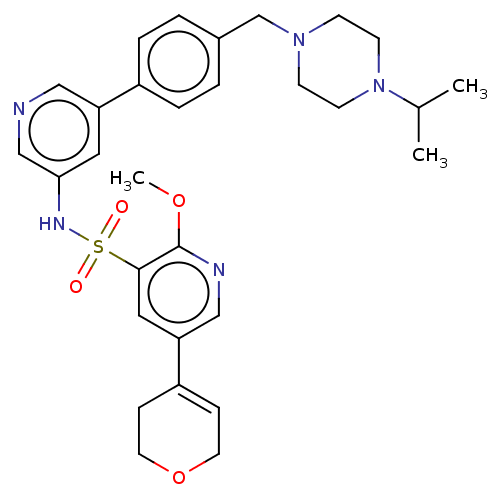
(CHEMBL4850297)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1cncc(c1)-c1ccc(CN2CCN(CC2)C(C)C)cc1)C1=CCOCC1 |t:38| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573166
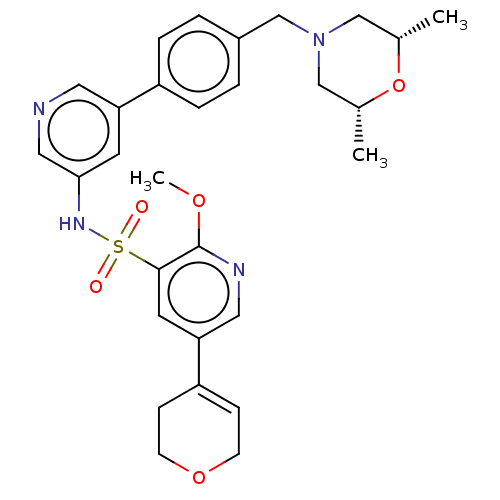
(CHEMBL4869783)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1cncc(c1)-c1ccc(CN2C[C@H](C)O[C@H](C)C2)cc1)C1=CCOCC1 |r,t:37| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50066789

(3-((S)-1-Azetidin-2-ylmethoxy)-5-iodo-pyridine | A...)Show InChI InChI=1S/C9H11IN2O/c10-7-3-9(5-11-4-7)13-6-8-1-2-12-8/h3-5,8,12H,1-2,6H2/t8-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 642-9 (2000)
Article DOI: 10.1124/mol.57.3.642
BindingDB Entry DOI: 10.7270/Q21C1VF8 |
More data for this
Ligand-Target Pair | |
Son of sevenless homolog 1 [564-1049]
() | BDBM609451

(US11702418, Example 10-10)Show SMILES C[C@@H](Nc1nnc(C)c2ccc(cc12)N1CCN(CC1)c1cnn(C)c1)c1cccc(c1C)C(F)(F)F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2C251J6 |
More data for this
Ligand-Target Pair | |
Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)
(Homo sapiens (Human)) | BDBM484497

(US10934294, Example 19 | US10934294, Example 20 | ...)Show SMILES CC(c1ccc(cc1)C(F)(F)F)n1nc(C#N)c2c1nc([nH]c2=O)C1CCC1c1ncccn1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... |
US Patent US10934294 (2021)
BindingDB Entry DOI: 10.7270/Q2C250JS |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM484529

(US10934294, Example 50 | US10934294, Example 51 | ...)Show SMILES CC(c1ccc(nc1)C1CC1)n1nc(C#N)c2c1nc([nH]c2=O)C1CCC1c1ncccn1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Assays for PDE 1 through 11 were performed in parallel at room temperature in 384-well microtiter plates with an incubation volume of 20.2 μL. S... |
US Patent US11028092 (2021)
BindingDB Entry DOI: 10.7270/Q2XS5ZH4 |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM484541

(US10934294, Example 62 | US11028092, Example 63)Show SMILES CCc1cnc(nc1)C1CCC1c1nc2n(nc(C#N)c2c(=O)[nH]1)[C@@H](C)c1ccc(nc1)C(F)(F)F |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Assays for PDE 1 through 11 were performed in parallel at room temperature in 384-well microtiter plates with an incubation volume of 20.2 μL. S... |
US Patent US11028092 (2021)
BindingDB Entry DOI: 10.7270/Q2XS5ZH4 |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM484570

(US10934294, Example 91 | US10934294, Example 92 | ...)Show SMILES C[C@@H](c1ccc(nc1)C(F)(F)F)n1nc(C#N)c2c1nc([nH]c2=O)C1CCC1c1ncc(cn1)C1CC1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Assays for PDE 1 through 11 were performed in parallel at room temperature in 384-well microtiter plates with an incubation volume of 20.2 μL. S... |
US Patent US11028092 (2021)
BindingDB Entry DOI: 10.7270/Q2XS5ZH4 |
More data for this
Ligand-Target Pair | |
Son of sevenless homolog 1 [564-1049]
() | BDBM609522

((R)-(4-(1-methyl-4-((1-(2-methyl-3-(trifluoromethy...)Show SMILES C[C@@H](Nc1nnc(C)c2ccc(cc12)N1CCN(CC1)C(=O)C1COC1)c1cccc(c1C)C(F)(F)F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2C251J6 |
More data for this
Ligand-Target Pair | |
Son of sevenless homolog 1 [564-1049]
() | BDBM609523

(US11702418, Example 11-2)Show SMILES C[C@@H](Nc1nnc(C)c2ccc(cc12)N1CCN(CC1)C(=O)C1CCOCC1)c1cccc(c1C)C(F)(F)F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2C251J6 |
More data for this
Ligand-Target Pair | |
Son of sevenless homolog 1 [564-1049]
() | BDBM609524
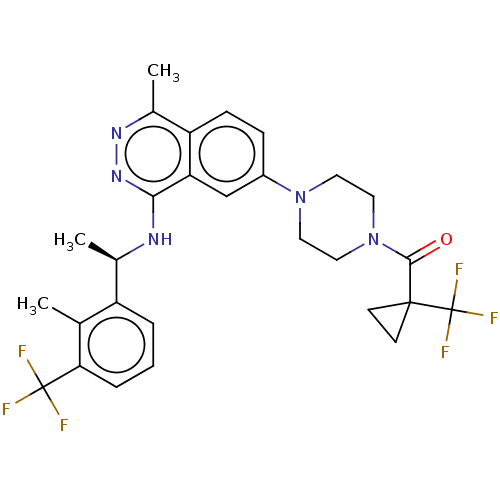
(US11702418, Example 11-3)Show SMILES C[C@@H](Nc1nnc(C)c2ccc(cc12)N1CCN(CC1)C(=O)C1(CC1)C(F)(F)F)c1cccc(c1C)C(F)(F)F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2C251J6 |
More data for this
Ligand-Target Pair | |
Son of sevenless homolog 1 [564-1049]
() | BDBM609525

(US11702418, Example 11-4)Show SMILES C[C@@H](Nc1nnc(C)c2ccc(cc12)N1CCN(CC1)C(=O)[C@@H]1CCOC1)c1cccc(c1C)C(F)(F)F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2C251J6 |
More data for this
Ligand-Target Pair | |
Son of sevenless homolog 1 [564-1049]
() | BDBM609526

(US11702418, Example 11-5)Show SMILES C[C@@H](Nc1nnc(C)c2ccc(cc12)N1CCN(CC1)C(=O)[C@H]1CCOC1)c1cccc(c1C)C(F)(F)F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2C251J6 |
More data for this
Ligand-Target Pair | |
Son of sevenless homolog 1 [564-1049]
() | BDBM609420
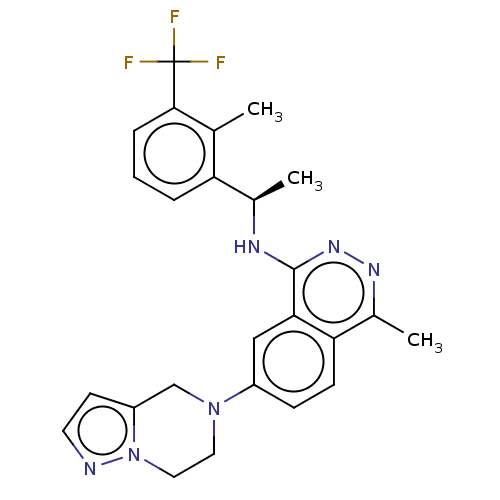
(US11702418, Example 6-10)Show SMILES C[C@@H](Nc1nnc(C)c2ccc(cc12)N1CCn2nccc2C1)c1cccc(c1C)C(F)(F)F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2C251J6 |
More data for this
Ligand-Target Pair | |
Son of sevenless homolog 1 [564-1049]
() | BDBM609446

(US11702418, Example 10-5)Show SMILES C[C@@H](Nc1nnc(C)c2ccc(cc12)N1CCNCC(F)(F)C1)c1cccc(c1C)C(F)(F)F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2C251J6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573177

(CHEMBL4167702)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1ccnc(c1)-c1ccc(CN2CCN(CC2)C(C)C)cc1F)N1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573167
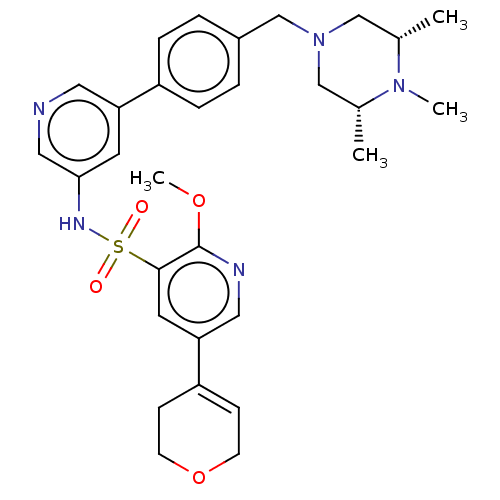
(CHEMBL4858875)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1cncc(c1)-c1ccc(CN2C[C@H](C)N(C)[C@H](C)C2)cc1)C1=CCOCC1 |r,t:38| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Son of sevenless homolog 1 [564-1049]
() | BDBM609413
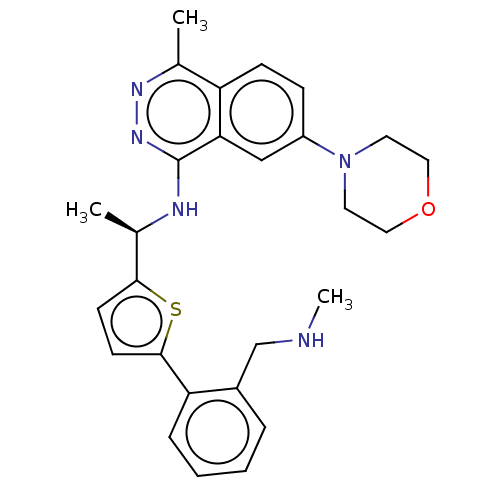
(US11702418, Example 6-3)Show SMILES CNCc1ccccc1-c1ccc(s1)[C@@H](C)Nc1nnc(C)c2ccc(cc12)N1CCOCC1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2C251J6 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50100712
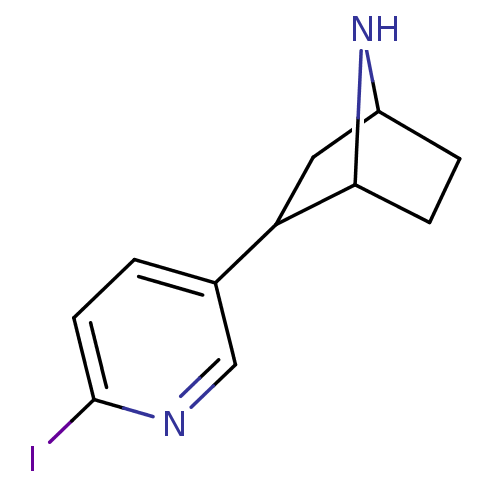
(2-(6-Iodo-pyridin-3-yl)-7-aza-bicyclo[2.2.1]heptan...)Show InChI InChI=1S/C11H13IN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 642-9 (2000)
Article DOI: 10.1124/mol.57.3.642
BindingDB Entry DOI: 10.7270/Q21C1VF8 |
More data for this
Ligand-Target Pair | |
Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)
(Homo sapiens (Human)) | BDBM484551

(US10934294, Example 72 | US10934294, Example 73 | ...)Show SMILES CC(c1ccc(nc1)C1CC1)n1nc(C#N)c2c1nc([nH]c2=O)C1CCC1c1ncc(F)cn1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... |
US Patent US10934294 (2021)
BindingDB Entry DOI: 10.7270/Q2C250JS |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50475330

(CHEMBL442297)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(F)cc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6]-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](=O)-[#7](-[#7])-[#7])-[#6](=O)-[#7]-[#7] Show InChI InChI=1S/C82H142FN35O20/c1-44(104-63(123)42-103-79(138)66(46(3)120)116-76(135)58(37-48-24-26-49(83)27-25-48)106-64(124)41-102-62(122)40-98-39-50(87)36-47-16-5-4-6-17-47)67(126)107-54(21-13-33-99-80(89)90)70(129)111-53(20-9-12-32-86)74(133)115-60(43-119)77(136)105-45(2)68(127)108-55(22-14-34-100-81(91)92)71(130)109-51(18-7-10-30-84)69(128)112-56(23-15-35-101-82(93)94)72(131)110-52(19-8-11-31-85)73(132)114-59(38-61(88)121)75(134)113-57(78(137)117-95)28-29-65(125)118(96)97/h4-6,16-17,24-27,44-46,50-60,66,98,119-120H,7-15,18-23,28-43,84-87,95-97H2,1-3H3,(H2,88,121)(H,102,122)(H,103,138)(H,104,123)(H,105,136)(H,106,124)(H,107,126)(H,108,127)(H,109,130)(H,110,131)(H,111,129)(H,112,128)(H,113,134)(H,114,132)(H,115,133)(H,116,135)(H,117,137)(H4,89,90,99)(H4,91,92,100)(H4,93,94,101)/t44-,45-,46+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,66-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Receptor binding affinity for recombinant human N/OFQ peptide receptor (NOP) expressed in chinese hamster ovary cells |
J Med Chem 48: 1421-7 (2005)
Article DOI: 10.1021/jm040106v
BindingDB Entry DOI: 10.7270/Q2J38WBW |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM484551

(US10934294, Example 72 | US10934294, Example 73 | ...)Show SMILES CC(c1ccc(nc1)C1CC1)n1nc(C#N)c2c1nc([nH]c2=O)C1CCC1c1ncc(F)cn1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Assays for PDE 1 through 11 were performed in parallel at room temperature in 384-well microtiter plates with an incubation volume of 20.2 μL. S... |
US Patent US11028092 (2021)
BindingDB Entry DOI: 10.7270/Q2XS5ZH4 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM86660

(OFQ/N UFP-102)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(F)cc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C73H123FN28O17/c1-41(92-57(107)39-91-70(119)59(43(3)104)102-68(117)53(34-44-24-26-46(74)27-25-44)94-58(108)38-90-56(106)37-89-55(105)36-85-35-45-16-5-4-6-17-45)61(110)96-50(21-13-31-86-71(79)80)65(114)99-49(20-9-12-30-77)67(116)101-54(40-103)69(118)93-42(2)62(111)97-51(22-14-32-87-72(81)82)66(115)98-48(19-8-11-29-76)64(113)100-52(23-15-33-88-73(83)84)63(112)95-47(60(78)109)18-7-10-28-75/h4-6,16-17,24-27,41-43,47-54,59,85,103-104H,7-15,18-23,28-40,75-77H2,1-3H3,(H2,78,109)(H,89,105)(H,90,106)(H,91,119)(H,92,107)(H,93,118)(H,94,108)(H,95,112)(H,96,110)(H,97,111)(H,98,115)(H,99,114)(H,100,113)(H,101,116)(H,102,117)(H4,79,80,86)(H4,81,82,87)(H4,83,84,88)/t41-,42-,43+,47-,48-,49-,50-,51-,52-,53-,54-,59-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Section of Pharmacology
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 1114-23 (2005)
Article DOI: 10.1124/jpet.104.077339
BindingDB Entry DOI: 10.7270/Q2222SC4 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of Escherichia coli |
J Med Chem 28: 303-11 (1985)
BindingDB Entry DOI: 10.7270/Q2J966Z0 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50475333
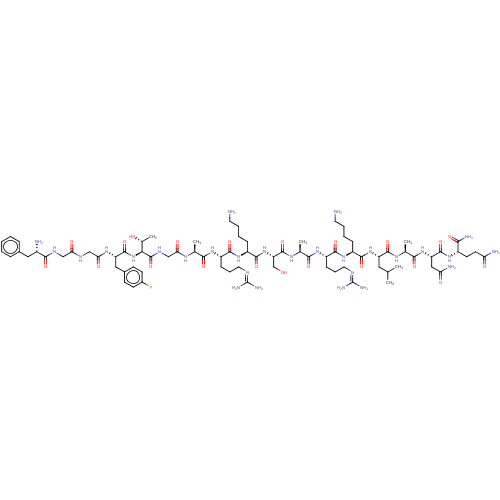
(CHEMBL264846)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(F)cc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#7])=O Show InChI InChI=1S/C79H129FN28O21/c1-40(2)32-54(73(125)97-43(5)67(119)105-56(35-59(85)112)74(126)100-49(64(86)116)26-27-58(84)111)106-71(123)50(18-10-12-28-81)103-70(122)53(21-15-31-92-79(89)90)102-66(118)42(4)98-76(128)57(39-109)107-72(124)51(19-11-13-29-82)104-69(121)52(20-14-30-91-78(87)88)101-65(117)41(3)96-61(114)38-95-77(129)63(44(6)110)108-75(127)55(34-46-22-24-47(80)25-23-46)99-62(115)37-93-60(113)36-94-68(120)48(83)33-45-16-8-7-9-17-45/h7-9,16-17,22-25,40-44,48-57,63,109-110H,10-15,18-21,26-39,81-83H2,1-6H3,(H2,84,111)(H2,85,112)(H2,86,116)(H,93,113)(H,94,120)(H,95,129)(H,96,114)(H,97,125)(H,98,128)(H,99,115)(H,100,126)(H,101,117)(H,102,118)(H,103,122)(H,104,121)(H,105,119)(H,106,123)(H,107,124)(H,108,127)(H4,87,88,91)(H4,89,90,92)/t41-,42-,43-,44+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Receptor binding affinity for recombinant human N/OFQ peptide receptor (NOP) expressed in chinese hamster ovary cells |
J Med Chem 48: 1421-7 (2005)
Article DOI: 10.1021/jm040106v
BindingDB Entry DOI: 10.7270/Q2J38WBW |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026300

(6-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C19H26N4O5/c1-26-14-9-12(8-13-11-22-19(21)23-18(13)20)10-15(17(14)27-2)28-7-5-3-4-6-16(24)25/h9-11H,3-8H2,1-2H3,(H,24,25)(H4,20,21,22,23) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of Escherichia coli |
J Med Chem 28: 303-11 (1985)
BindingDB Entry DOI: 10.7270/Q2J966Z0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026300

(6-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C19H26N4O5/c1-26-14-9-12(8-13-11-22-19(21)23-18(13)20)10-15(17(14)27-2)28-7-5-3-4-6-16(24)25/h9-11H,3-8H2,1-2H3,(H,24,25)(H4,20,21,22,23) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for E. coli Dihydrofolate reductase |
J Med Chem 25: 1120-2 (1983)
BindingDB Entry DOI: 10.7270/Q28G8JRF |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50475331
(CHEMBL411649)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(F)cc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](=O)-[#7](-[#7])-[#7])-[#6](=O)-[#7]-[#7] Show InChI InChI=1S/C82H140FN35O21/c1-44(104-63(124)42-103-79(139)66(46(3)120)116-76(136)57(36-47-24-26-49(83)27-25-47)106-64(125)41-102-62(123)40-101-61(122)39-97-38-48-16-5-4-6-17-48)67(127)107-53(21-13-33-98-80(88)89)70(130)111-52(20-9-12-32-86)74(134)115-59(43-119)77(137)105-45(2)68(128)108-54(22-14-34-99-81(90)91)71(131)109-50(18-7-10-30-84)69(129)112-55(23-15-35-100-82(92)93)72(132)110-51(19-8-11-31-85)73(133)114-58(37-60(87)121)75(135)113-56(78(138)117-94)28-29-65(126)118(95)96/h4-6,16-17,24-27,44-46,50-59,66,97,119-120H,7-15,18-23,28-43,84-86,94-96H2,1-3H3,(H2,87,121)(H,101,122)(H,102,123)(H,103,139)(H,104,124)(H,105,137)(H,106,125)(H,107,127)(H,108,128)(H,109,131)(H,110,132)(H,111,130)(H,112,129)(H,113,135)(H,114,133)(H,115,134)(H,116,136)(H,117,138)(H4,88,89,98)(H4,90,91,99)(H4,92,93,100)/t44-,45-,46+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,66-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Receptor binding affinity for recombinant human N/OFQ peptide receptor (NOP) expressed in chinese hamster ovary cells |
J Med Chem 48: 1421-7 (2005)
Article DOI: 10.1021/jm040106v
BindingDB Entry DOI: 10.7270/Q2J38WBW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573182
(CHEMBL4175571)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1ccnc(c1)-c1ccc(CN2CCN(CC2)C(C)(C)C)cc1F)N1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573181
(CHEMBL4165185)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1ccnc(c1)-c1ccc(CN2CCN(CC2)C(C)(C)C)cc1)N1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573164

(CHEMBL4873390)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1cncc(c1)-c1ccc(CN2CCN(CC2)C(C)C)nc1)C1=CCOCC1 |t:38| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)
(Homo sapiens (Human)) | BDBM484572

(US10934294, Example 93)Show SMILES C[C@@H](c1cnc(nc1)C1CC1)n1nc(C#N)c2c1nc([nH]c2=O)[C@H]1CC[C@H]1c1ncccn1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... |
US Patent US10934294 (2021)
BindingDB Entry DOI: 10.7270/Q2C250JS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data