

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Androgen receptor (Homo sapiens (Human)) | BDBM18656 ((2R,5S)-4-[4-cyano-3-(trifluoromethyl)phenyl]-2,5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd] where [L] is the concentration of labeled ligand a... | J Med Chem 49: 716-26 (2006) Article DOI: 10.1021/jm050293c BindingDB Entry DOI: 10.7270/Q2P84952 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18656 ((2R,5S)-4-[4-cyano-3-(trifluoromethyl)phenyl]-2,5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd] where [L] is the concentration of labeled ligand a... | J Med Chem 49: 716-26 (2006) Article DOI: 10.1021/jm050293c BindingDB Entry DOI: 10.7270/Q2P84952 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18525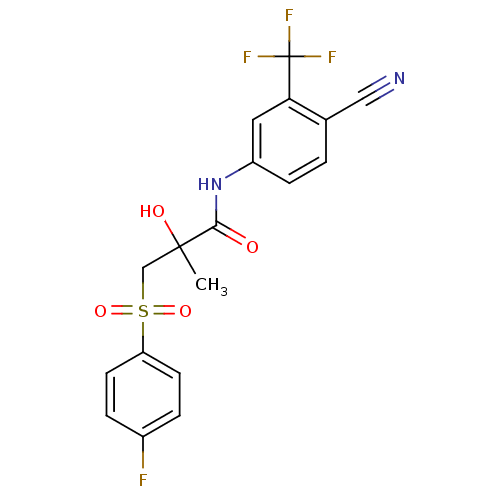 (Bicalutamide | CHEMBL409 | N-[4-cyano-3-(trifluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd] where [L] is the concentration of labeled ligand a... | J Med Chem 49: 716-26 (2006) Article DOI: 10.1021/jm050293c BindingDB Entry DOI: 10.7270/Q2P84952 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18525 (Bicalutamide | CHEMBL409 | N-[4-cyano-3-(trifluoro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd] where [L] is the concentration of labeled ligand a... | J Med Chem 49: 716-26 (2006) Article DOI: 10.1021/jm050293c BindingDB Entry DOI: 10.7270/Q2P84952 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50175277 (CHEMBL3809675) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Pharmaceutical Sciences, Kyoto University, Sakyo-ku, Kyoto 606-8501, Japan. Electronic address: hohno@pharm.kyoto-u.ac.jp. Curated by ChEMBL | Assay Description Inhibition of recombinant human SphK1 expressed in baculovirus infected sf9 cells using D-erythro-sphingosine as substrate in presence of [gamma-33P]... | Bioorg Med Chem 25: 3046-3052 (2017) Article DOI: 10.1016/j.bmc.2017.03.059 BindingDB Entry DOI: 10.7270/Q2XD144Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM174110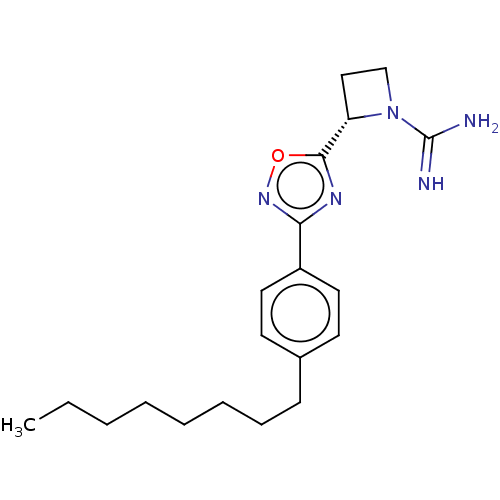 ((S)-2-(3-(4-octylphenyl)-1,2,4- oxadiazol-5-yl)aze...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Pharmaceutical Sciences, Kyoto University, Sakyo-ku, Kyoto 606-8501, Japan. Electronic address: hohno@pharm.kyoto-u.ac.jp. Curated by ChEMBL | Assay Description Inhibition of recombinant human SphK2 expressed in baculovirus infected sf9 cells using D-erythro-sphingosine as substrate after 20 mins in presence ... | Bioorg Med Chem 25: 3046-3052 (2017) Article DOI: 10.1016/j.bmc.2017.03.059 BindingDB Entry DOI: 10.7270/Q2XD144Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Rattus norvegicus) | BDBM18656 ((2R,5S)-4-[4-cyano-3-(trifluoromethyl)phenyl]-2,5-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd] where [L] is the concentration of labeled ligand a... | J Med Chem 49: 716-26 (2006) Article DOI: 10.1021/jm050293c BindingDB Entry DOI: 10.7270/Q2P84952 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Rattus norvegicus) | BDBM18525 (Bicalutamide | CHEMBL409 | N-[4-cyano-3-(trifluoro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd] where [L] is the concentration of labeled ligand a... | J Med Chem 49: 716-26 (2006) Article DOI: 10.1021/jm050293c BindingDB Entry DOI: 10.7270/Q2P84952 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM174110 ((S)-2-(3-(4-octylphenyl)-1,2,4- oxadiazol-5-yl)aze...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Pharmaceutical Sciences, Kyoto University, Sakyo-ku, Kyoto 606-8501, Japan. Electronic address: hohno@pharm.kyoto-u.ac.jp. Curated by ChEMBL | Assay Description Inhibition of recombinant human SphK1 expressed in baculovirus infected sf9 cells using D-erythro-sphingosine as substrate after 20 mins in presence ... | Bioorg Med Chem 25: 3046-3052 (2017) Article DOI: 10.1016/j.bmc.2017.03.059 BindingDB Entry DOI: 10.7270/Q2XD144Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Mus musculus (Mouse)) | BDBM50175277 (CHEMBL3809675) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Pharmaceutical Sciences, Kyoto University, Sakyo-ku, Kyoto 606-8501, Japan. Electronic address: hohno@pharm.kyoto-u.ac.jp. Curated by ChEMBL | Assay Description Inhibition of recombinant mouse SphK2 expressed in baculovirus infected sf9 cells using D-erythro-sphingosine as substrate in presence of [gamma-33P]... | Bioorg Med Chem 25: 3046-3052 (2017) Article DOI: 10.1016/j.bmc.2017.03.059 BindingDB Entry DOI: 10.7270/Q2XD144Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50041978 (CHEMBL3134157) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Pharmaceutical Sciences, Kyoto University, Sakyo-ku, Kyoto 606-8501, Japan. Electronic address: hohno@pharm.kyoto-u.ac.jp. Curated by ChEMBL | Assay Description Inhibition of recombinant C-terminal His6-tagged human SphK1 expressed in baculovirus infected sf21 cells using FITC-sphingosine as substrate after 1... | Bioorg Med Chem 25: 3046-3052 (2017) Article DOI: 10.1016/j.bmc.2017.03.059 BindingDB Entry DOI: 10.7270/Q2XD144Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50375019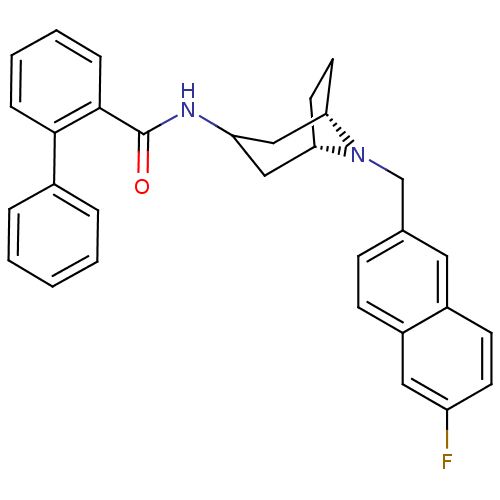 (CHEMBL258996) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR3 expressed in B300-19 cells assessed as inhibition of eotaxin-induced calcium influx | Bioorg Med Chem 16: 144-56 (2008) Article DOI: 10.1016/j.bmc.2007.10.003 BindingDB Entry DOI: 10.7270/Q2445NBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM35353 (indole-2-carboxamide derivative, 25e (R-isomer)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Astellas Pharma Inc. | Assay Description The activity of recombinant human liver GPa in the forward direction was measured by monitoring the production of NADPH. Enzyme activity was assayed... | Bioorg Med Chem 16: 10001-12 (2008) Article DOI: 10.1016/j.bmc.2008.10.021 BindingDB Entry DOI: 10.7270/Q2K072MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50375019 (CHEMBL258996) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR3 expressed in B300-19 cells assessed as inhibition of eotaxin-induced calcium influx | Bioorg Med Chem 16: 144-56 (2008) Article DOI: 10.1016/j.bmc.2007.10.003 BindingDB Entry DOI: 10.7270/Q2445NBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM35351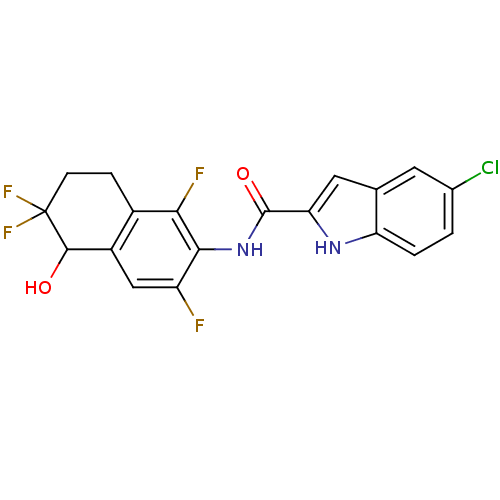 (indole-2-carboxamide derivative, 25e) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Astellas Pharma Inc. | Assay Description The activity of recombinant human liver GPa in the forward direction was measured by monitoring the production of NADPH. Enzyme activity was assayed... | Bioorg Med Chem 16: 10001-12 (2008) Article DOI: 10.1016/j.bmc.2008.10.021 BindingDB Entry DOI: 10.7270/Q2K072MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50225349 (CHEMBL409830 | N-(1-((6-fluoronaphthalen-2-yl)meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR3 expressed in B300-19 cells assessed as inhibition of eotaxin-induced calcium influx | Bioorg Med Chem 16: 144-56 (2008) Article DOI: 10.1016/j.bmc.2007.10.003 BindingDB Entry DOI: 10.7270/Q2445NBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM35350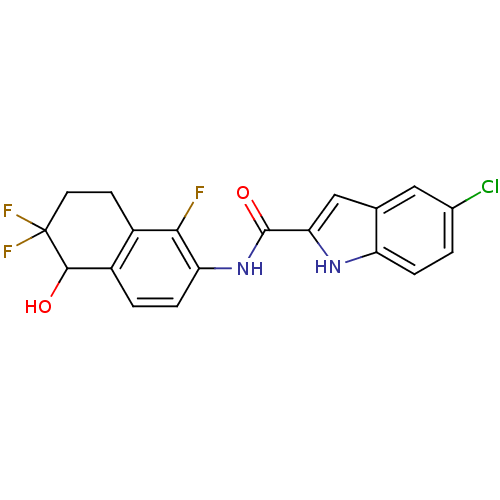 (indole-2-carboxamide derivative, 25d) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Astellas Pharma Inc. | Assay Description The activity of recombinant human liver GPa in the forward direction was measured by monitoring the production of NADPH. Enzyme activity was assayed... | Bioorg Med Chem 16: 10001-12 (2008) Article DOI: 10.1016/j.bmc.2008.10.021 BindingDB Entry DOI: 10.7270/Q2K072MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM35349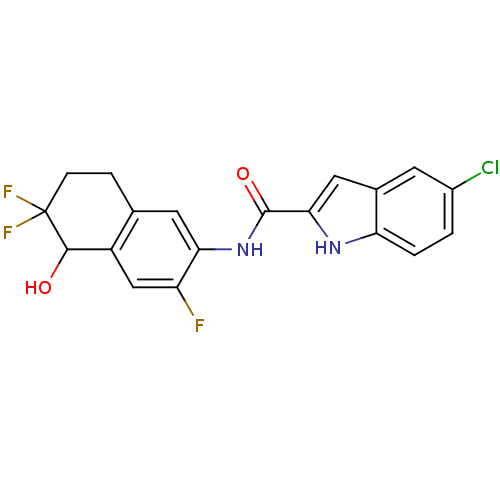 (indole-2-carboxamide derivative, 25c) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Astellas Pharma Inc. | Assay Description The activity of recombinant human liver GPa in the forward direction was measured by monitoring the production of NADPH. Enzyme activity was assayed... | Bioorg Med Chem 16: 10001-12 (2008) Article DOI: 10.1016/j.bmc.2008.10.021 BindingDB Entry DOI: 10.7270/Q2K072MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM35345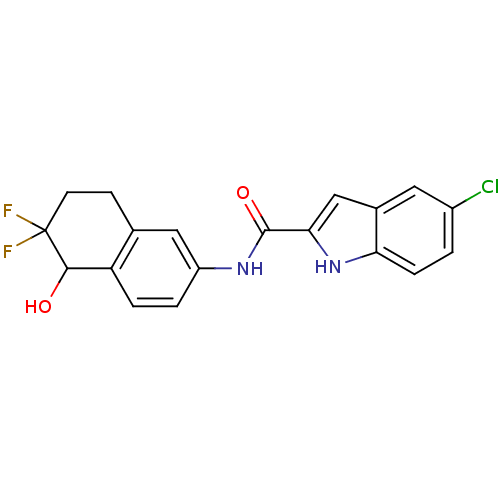 (indole-2-carboxamide derivative, 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Astellas Pharma Inc. | Assay Description The activity of recombinant human liver GPa in the forward direction was measured by monitoring the production of NADPH. Enzyme activity was assayed... | Bioorg Med Chem 16: 10001-12 (2008) Article DOI: 10.1016/j.bmc.2008.10.021 BindingDB Entry DOI: 10.7270/Q2K072MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50375015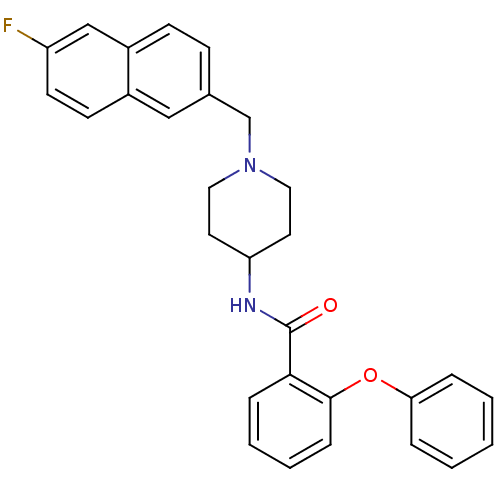 (CHEMBL410041) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR3 expressed in B300-19 cells assessed as inhibition of eotaxin-induced calcium influx | Bioorg Med Chem 16: 144-56 (2008) Article DOI: 10.1016/j.bmc.2007.10.003 BindingDB Entry DOI: 10.7270/Q2445NBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18656 ((2R,5S)-4-[4-cyano-3-(trifluoromethyl)phenyl]-2,5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Astellas Pharma Inc. | Assay Description Activities of the test compounds to inhibit androgen receptor mediated transcription induced by DHT were evaluated using stable transfected AR/CHO#3 ... | J Med Chem 49: 716-26 (2006) Article DOI: 10.1021/jm050293c BindingDB Entry DOI: 10.7270/Q2P84952 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18636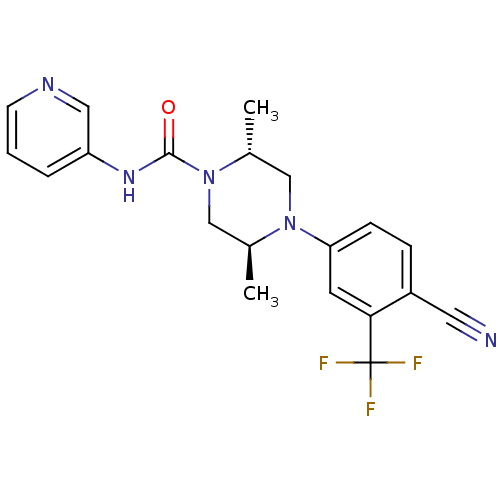 ((2R,5S)-4-[4-cyano-3-(trifluoromethyl)phenyl]-2,5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Astellas Pharma Inc. | Assay Description Activities of the test compounds to inhibit androgen receptor mediated transcription induced by DHT were evaluated using stable transfected AR/CHO#3 ... | J Med Chem 49: 716-26 (2006) Article DOI: 10.1021/jm050293c BindingDB Entry DOI: 10.7270/Q2P84952 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18645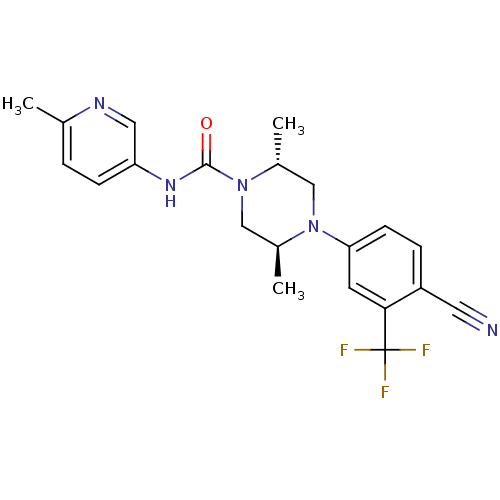 ((2R,5S)-4-[4-cyano-3-(trifluoromethyl)phenyl]-2,5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Astellas Pharma Inc. | Assay Description Activities of the test compounds to inhibit androgen receptor mediated transcription induced by DHT were evaluated using stable transfected AR/CHO#3 ... | J Med Chem 49: 716-26 (2006) Article DOI: 10.1021/jm050293c BindingDB Entry DOI: 10.7270/Q2P84952 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18637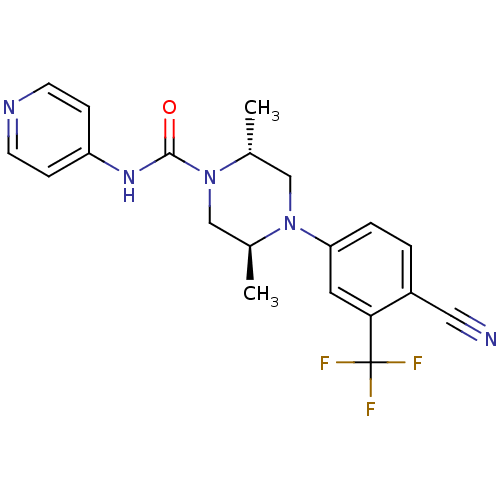 ((2R,5S)-4-[4-cyano-3-(trifluoromethyl)phenyl]-2,5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Astellas Pharma Inc. | Assay Description Activities of the test compounds to inhibit androgen receptor mediated transcription induced by DHT were evaluated using stable transfected AR/CHO#3 ... | J Med Chem 49: 716-26 (2006) Article DOI: 10.1021/jm050293c BindingDB Entry DOI: 10.7270/Q2P84952 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM35352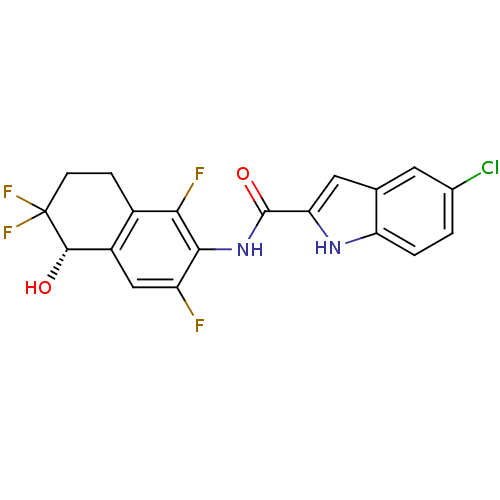 (indole-2-carboxamide derivative, 25e (S-isomer)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Astellas Pharma Inc. | Assay Description The activity of recombinant human liver GPa in the forward direction was measured by monitoring the production of NADPH. Enzyme activity was assayed... | Bioorg Med Chem 16: 10001-12 (2008) Article DOI: 10.1016/j.bmc.2008.10.021 BindingDB Entry DOI: 10.7270/Q2K072MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18649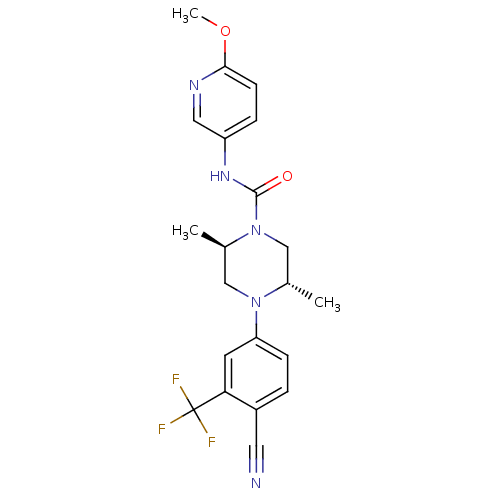 ((2R,5S)-4-[4-cyano-3-(trifluoromethyl)phenyl]-N-(6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Astellas Pharma Inc. | Assay Description Activities of the test compounds to inhibit androgen receptor mediated transcription induced by DHT were evaluated using stable transfected AR/CHO#3 ... | J Med Chem 49: 716-26 (2006) Article DOI: 10.1021/jm050293c BindingDB Entry DOI: 10.7270/Q2P84952 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18646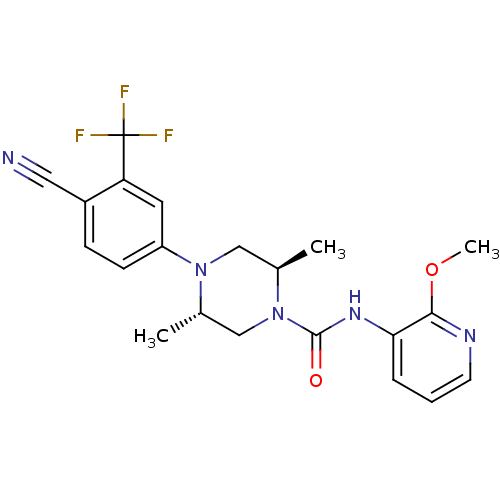 ((2R,5S)-4-[4-cyano-3-(trifluoromethyl)phenyl]-N-(2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Astellas Pharma Inc. | Assay Description Activities of the test compounds to inhibit androgen receptor mediated transcription induced by DHT were evaluated using stable transfected AR/CHO#3 ... | J Med Chem 49: 716-26 (2006) Article DOI: 10.1021/jm050293c BindingDB Entry DOI: 10.7270/Q2P84952 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18654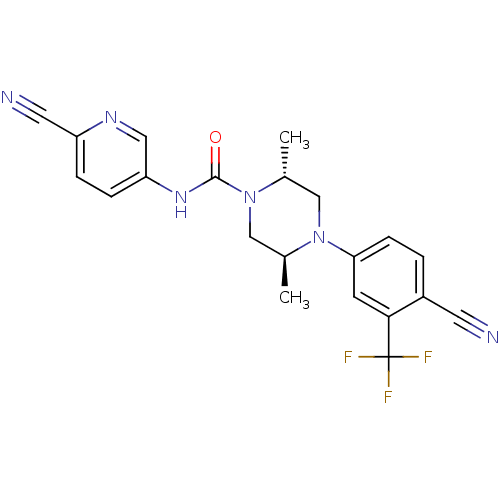 ((2R,5S)-4-[4-cyano-3-(trifluoromethyl)phenyl]-N-(6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Astellas Pharma Inc. | Assay Description Activities of the test compounds to inhibit androgen receptor mediated transcription induced by DHT were evaluated using stable transfected AR/CHO#3 ... | J Med Chem 49: 716-26 (2006) Article DOI: 10.1021/jm050293c BindingDB Entry DOI: 10.7270/Q2P84952 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18634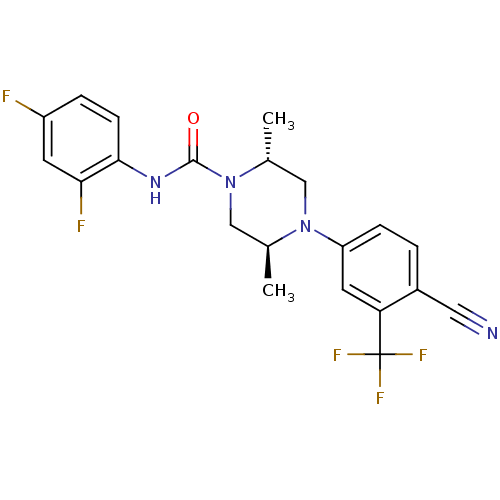 ((2R,5S)-4-[4-cyano-3-(trifluoromethyl)phenyl]-N-(2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Astellas Pharma Inc. | Assay Description Activities of the test compounds to inhibit androgen receptor mediated transcription induced by DHT were evaluated using stable transfected AR/CHO#3 ... | J Med Chem 49: 716-26 (2006) Article DOI: 10.1021/jm050293c BindingDB Entry DOI: 10.7270/Q2P84952 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18655 (4-[4-cyano-3-(trifluoromethyl)phenyl]-2,5-dimethyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Astellas Pharma Inc. | Assay Description Activities of the test compounds to inhibit androgen receptor mediated transcription induced by DHT were evaluated using stable transfected AR/CHO#3 ... | J Med Chem 49: 716-26 (2006) Article DOI: 10.1021/jm050293c BindingDB Entry DOI: 10.7270/Q2P84952 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50251418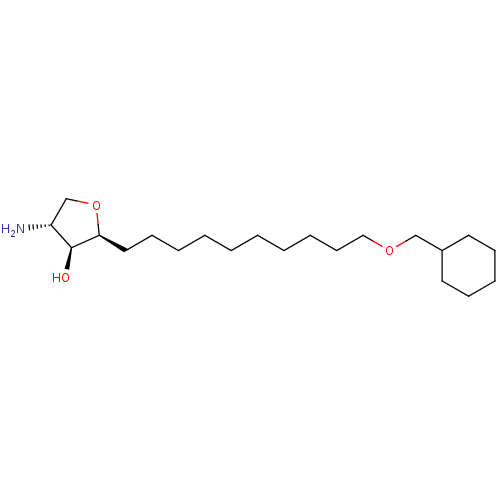 (CHEMBL4075031) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Pharmaceutical Sciences, Kyoto University, Sakyo-ku, Kyoto 606-8501, Japan. Electronic address: hohno@pharm.kyoto-u.ac.jp. Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal GST-tagged human SphK1 (1 to 384 residues) expressed in baculovirus expression system using sphingosine as subst... | Bioorg Med Chem 25: 3046-3052 (2017) Article DOI: 10.1016/j.bmc.2017.03.059 BindingDB Entry DOI: 10.7270/Q2XD144Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18650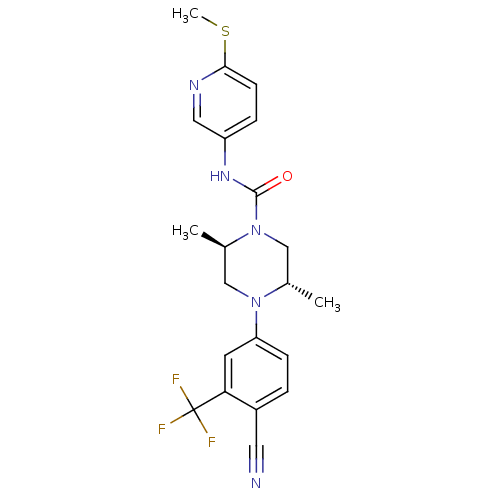 ((2R,5S)-4-[4-cyano-3-(trifluoromethyl)phenyl]-2,5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Astellas Pharma Inc. | Assay Description Activities of the test compounds to inhibit androgen receptor mediated transcription induced by DHT were evaluated using stable transfected AR/CHO#3 ... | J Med Chem 49: 716-26 (2006) Article DOI: 10.1021/jm050293c BindingDB Entry DOI: 10.7270/Q2P84952 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50251418 (CHEMBL4075031) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Pharmaceutical Sciences, Kyoto University, Sakyo-ku, Kyoto 606-8501, Japan. Electronic address: hohno@pharm.kyoto-u.ac.jp. Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal GST-tagged human SphK2 (1 to 618 residues) expressed in baculovirus expression system using sphingosine as subst... | Bioorg Med Chem 25: 3046-3052 (2017) Article DOI: 10.1016/j.bmc.2017.03.059 BindingDB Entry DOI: 10.7270/Q2XD144Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18644 ((2R,5S)-4-[4-cyano-3-(trifluoromethyl)phenyl]-2,5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Astellas Pharma Inc. | Assay Description Activities of the test compounds to inhibit androgen receptor mediated transcription induced by DHT were evaluated using stable transfected AR/CHO#3 ... | J Med Chem 49: 716-26 (2006) Article DOI: 10.1021/jm050293c BindingDB Entry DOI: 10.7270/Q2P84952 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM35339 (indole-2-carboxamide derivative, 5b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Astellas Pharma Inc. | Assay Description The activity of recombinant human liver GPa in the forward direction was measured by monitoring the production of NADPH. Enzyme activity was assayed... | Bioorg Med Chem 16: 10001-12 (2008) Article DOI: 10.1016/j.bmc.2008.10.021 BindingDB Entry DOI: 10.7270/Q2K072MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18643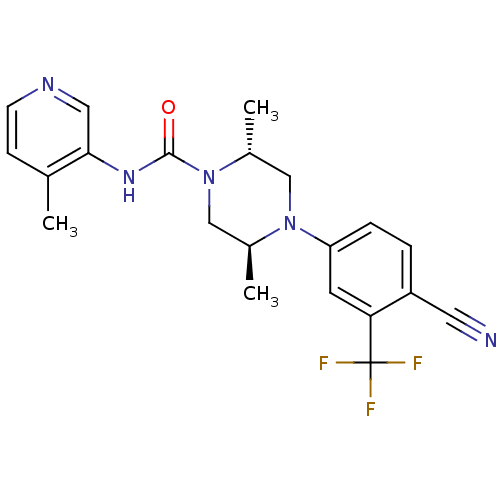 ((2R,5S)-4-[4-cyano-3-(trifluoromethyl)phenyl]-2,5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Astellas Pharma Inc. | Assay Description Activities of the test compounds to inhibit androgen receptor mediated transcription induced by DHT were evaluated using stable transfected AR/CHO#3 ... | J Med Chem 49: 716-26 (2006) Article DOI: 10.1021/jm050293c BindingDB Entry DOI: 10.7270/Q2P84952 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50041978 (CHEMBL3134157) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Pharmaceutical Sciences, Kyoto University, Sakyo-ku, Kyoto 606-8501, Japan. Electronic address: hohno@pharm.kyoto-u.ac.jp. Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal His6 tagged SphK2 expressed in baculovirus infected sf21 cells using FITC-sphingosine as substrate after 1... | Bioorg Med Chem 25: 3046-3052 (2017) Article DOI: 10.1016/j.bmc.2017.03.059 BindingDB Entry DOI: 10.7270/Q2XD144Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18659 ((2R,5S)-N-(6-aminopyridin-3-yl)-4-[4-cyano-3-(trif...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Astellas Pharma Inc. | Assay Description Activities of the test compounds to inhibit androgen receptor mediated transcription induced by DHT were evaluated using stable transfected AR/CHO#3 ... | J Med Chem 49: 716-26 (2006) Article DOI: 10.1021/jm050293c BindingDB Entry DOI: 10.7270/Q2P84952 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50251433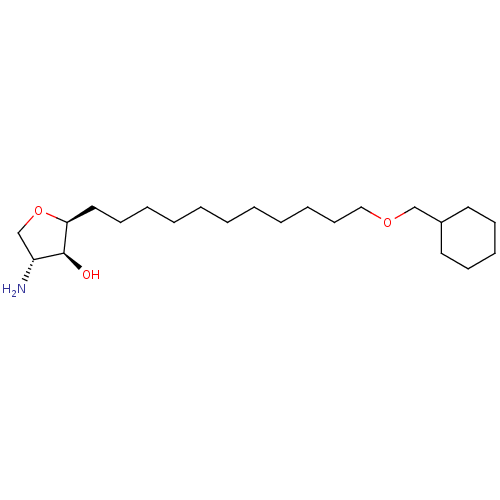 (CHEMBL4076550) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Pharmaceutical Sciences, Kyoto University, Sakyo-ku, Kyoto 606-8501, Japan. Electronic address: hohno@pharm.kyoto-u.ac.jp. Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal GST-tagged human SphK1 (1 to 384 residues) expressed in baculovirus expression system using sphingosine as subst... | Bioorg Med Chem 25: 3046-3052 (2017) Article DOI: 10.1016/j.bmc.2017.03.059 BindingDB Entry DOI: 10.7270/Q2XD144Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50251435 (CHEMBL4081791) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Pharmaceutical Sciences, Kyoto University, Sakyo-ku, Kyoto 606-8501, Japan. Electronic address: hohno@pharm.kyoto-u.ac.jp. Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal GST-tagged human SphK1 (1 to 384 residues) expressed in baculovirus expression system using sphingosine as subst... | Bioorg Med Chem 25: 3046-3052 (2017) Article DOI: 10.1016/j.bmc.2017.03.059 BindingDB Entry DOI: 10.7270/Q2XD144Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM35338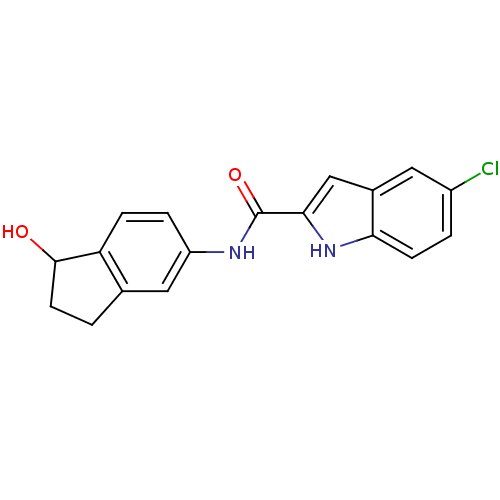 (indole-2-carboxamide derivative, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Astellas Pharma Inc. | Assay Description The activity of recombinant human liver GPa in the forward direction was measured by monitoring the production of NADPH. Enzyme activity was assayed... | Bioorg Med Chem 16: 10001-12 (2008) Article DOI: 10.1016/j.bmc.2008.10.021 BindingDB Entry DOI: 10.7270/Q2K072MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18648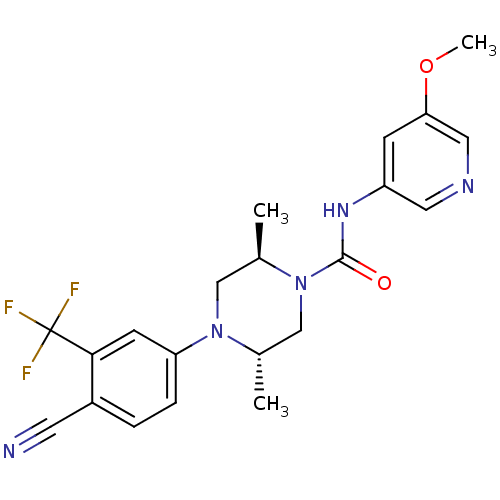 ((2R,5S)-4-[4-cyano-3-(trifluoromethyl)phenyl]-N-(5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Astellas Pharma Inc. | Assay Description Activities of the test compounds to inhibit androgen receptor mediated transcription induced by DHT were evaluated using stable transfected AR/CHO#3 ... | J Med Chem 49: 716-26 (2006) Article DOI: 10.1021/jm050293c BindingDB Entry DOI: 10.7270/Q2P84952 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50251419 (CHEMBL4101713) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Pharmaceutical Sciences, Kyoto University, Sakyo-ku, Kyoto 606-8501, Japan. Electronic address: hohno@pharm.kyoto-u.ac.jp. Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal GST-tagged human SphK2 (1 to 618 residues) expressed in baculovirus expression system using sphingosine as subst... | Bioorg Med Chem 25: 3046-3052 (2017) Article DOI: 10.1016/j.bmc.2017.03.059 BindingDB Entry DOI: 10.7270/Q2XD144Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18652 (N-Arylpiperazine-1-carboxamide Derivative, 30 | me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Astellas Pharma Inc. | Assay Description Activities of the test compounds to inhibit androgen receptor mediated transcription induced by DHT were evaluated using stable transfected AR/CHO#3 ... | J Med Chem 49: 716-26 (2006) Article DOI: 10.1021/jm050293c BindingDB Entry DOI: 10.7270/Q2P84952 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18653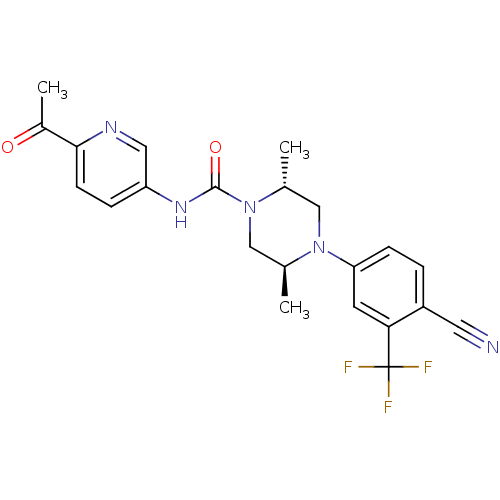 ((2R,5S)-4-[4-cyano-3-(trifluoromethyl)phenyl]-N-(6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Astellas Pharma Inc. | Assay Description Activities of the test compounds to inhibit androgen receptor mediated transcription induced by DHT were evaluated using stable transfected AR/CHO#3 ... | J Med Chem 49: 716-26 (2006) Article DOI: 10.1021/jm050293c BindingDB Entry DOI: 10.7270/Q2P84952 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50251403 (CHEMBL4098301) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Pharmaceutical Sciences, Kyoto University, Sakyo-ku, Kyoto 606-8501, Japan. Electronic address: hohno@pharm.kyoto-u.ac.jp. Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal GST-tagged human SphK2 (1 to 618 residues) expressed in baculovirus expression system using sphingosine as subst... | Bioorg Med Chem 25: 3046-3052 (2017) Article DOI: 10.1016/j.bmc.2017.03.059 BindingDB Entry DOI: 10.7270/Q2XD144Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM35348 (indole-2-carboxamide derivative, 25b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Astellas Pharma Inc. | Assay Description The activity of recombinant human liver GPa in the forward direction was measured by monitoring the production of NADPH. Enzyme activity was assayed... | Bioorg Med Chem 16: 10001-12 (2008) Article DOI: 10.1016/j.bmc.2008.10.021 BindingDB Entry DOI: 10.7270/Q2K072MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50375014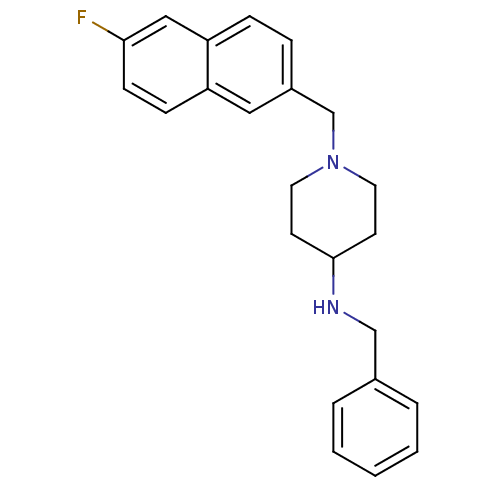 (CHEMBL408368) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR3 expressed in B300-19 cells assessed as inhibition of eotaxin-induced calcium influx | Bioorg Med Chem 16: 144-56 (2008) Article DOI: 10.1016/j.bmc.2007.10.003 BindingDB Entry DOI: 10.7270/Q2445NBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50251394 (CHEMBL1819205) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Pharmaceutical Sciences, Kyoto University, Sakyo-ku, Kyoto 606-8501, Japan. Electronic address: hohno@pharm.kyoto-u.ac.jp. Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal GST-tagged human SphK2 (1 to 618 residues) expressed in baculovirus expression system using sphingosine as subst... | Bioorg Med Chem 25: 3046-3052 (2017) Article DOI: 10.1016/j.bmc.2017.03.059 BindingDB Entry DOI: 10.7270/Q2XD144Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50225353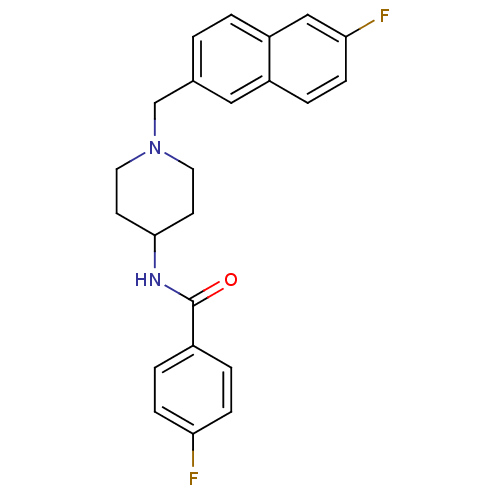 (4-fluoro-N-(1-((6-fluoronaphthalen-2-yl)methyl)pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR3 expressed in B300-19 cells assessed as inhibition of eotaxin-induced calcium influx | Bioorg Med Chem 16: 144-56 (2008) Article DOI: 10.1016/j.bmc.2007.10.003 BindingDB Entry DOI: 10.7270/Q2445NBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 137 total ) | Next | Last >> |