 Found 355 hits with Last Name = 'hampton' and Initial = 'a'
Found 355 hits with Last Name = 'hampton' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenylate kinase 2, mitochondrial
(Rattus norvegicus) | BDBM50367049
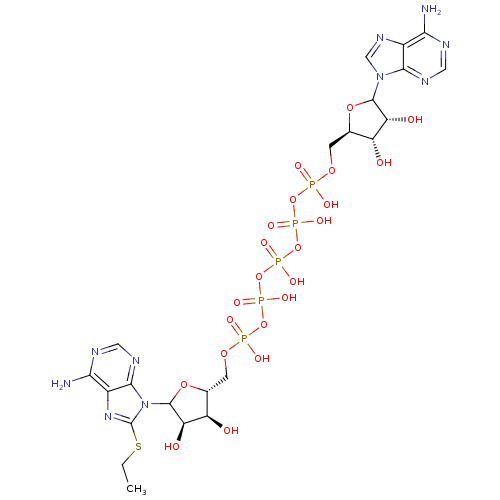
(CHEMBL604399)Show SMILES CCSc1nc2c(N)ncnc2n1C1O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(=O)OP(O)(=O)OP(O)(=O)OC[C@H]2OC([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C22H33N10O22P5S/c1-2-60-22-30-11-17(24)26-6-28-19(11)32(22)21-15(36)13(34)9(50-21)4-48-56(39,40)52-58(43,44)54-59(45,46)53-57(41,42)51-55(37,38)47-3-8-12(33)14(35)20(49-8)31-7-29-10-16(23)25-5-27-18(10)31/h5-9,12-15,20-21,33-36H,2-4H2,1H3,(H,37,38)(H,39,40)(H,41,42)(H,43,44)(H,45,46)(H2,23,25,27)(H2,24,26,28)/t8-,9-,12-,13-,14-,15-,20?,21?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat adenylate kinase II was determined in the presence of AMP, competitive inhibition |
J Med Chem 25: 1179-84 (1983)
BindingDB Entry DOI: 10.7270/Q25D8SD7 |
More data for this
Ligand-Target Pair | |
Adenylate kinase 2, mitochondrial
(Rattus norvegicus) | BDBM50367049

(CHEMBL604399)Show SMILES CCSc1nc2c(N)ncnc2n1C1O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(=O)OP(O)(=O)OP(O)(=O)OC[C@H]2OC([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C22H33N10O22P5S/c1-2-60-22-30-11-17(24)26-6-28-19(11)32(22)21-15(36)13(34)9(50-21)4-48-56(39,40)52-58(43,44)54-59(45,46)53-57(41,42)51-55(37,38)47-3-8-12(33)14(35)20(49-8)31-7-29-10-16(23)25-5-27-18(10)31/h5-9,12-15,20-21,33-36H,2-4H2,1H3,(H,37,38)(H,39,40)(H,41,42)(H,43,44)(H,45,46)(H2,23,25,27)(H2,24,26,28)/t8-,9-,12-,13-,14-,15-,20?,21?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat adenylate kinase II was determined in the presence of AMP, competitive inhibition |
J Med Chem 25: 1179-84 (1983)
BindingDB Entry DOI: 10.7270/Q25D8SD7 |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50228186
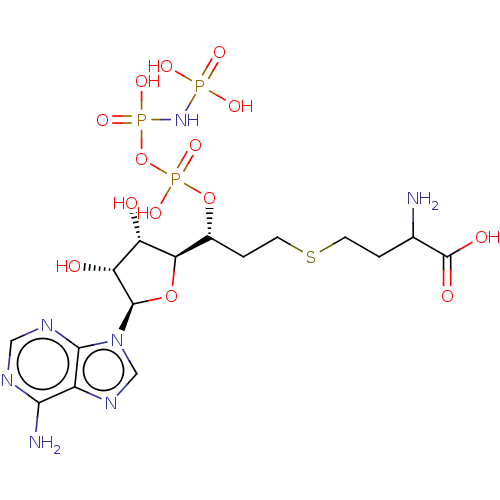
(CHEMBL3706404)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)[C@@H](CCSCCC(N)C(O)=O)OP(O)(=O)OP(O)(=O)NP(O)(O)=O |r| Show InChI InChI=1S/C16H28N7O14P3S/c17-7(16(26)27)1-3-41-4-2-8(36-40(33,34)37-39(31,32)22-38(28,29)30)12-10(24)11(25)15(35-12)23-6-21-9-13(18)19-5-20-14(9)23/h5-8,10-12,15,24-25H,1-4,17H2,(H,26,27)(H,33,34)(H2,18,19,20)(H4,22,28,29,30,31,32)/t7?,8-,10+,11-,12-,15-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Cancer Center
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition constant with kidney form of M-2 variants of Rat methionine adenosyltransferase when ATP was the variable substrat... |
J Med Chem 32: 885-90 (1989)
BindingDB Entry DOI: 10.7270/Q2K939Q8 |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1
(Rattus norvegicus) | BDBM50368140

(CHEMBL3706401 | CHEMBL611854)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(SCCCC)ncnc12)[C@H](CSCC[C@H](N)C(O)=O)OP(O)(=O)OP(O)(=O)NP(O)(O)=O |r| Show InChI InChI=1S/C19H33N6O14P3S2/c1-2-3-5-44-17-12-16(21-8-22-17)25(9-23-12)18-14(27)13(26)15(37-18)11(7-43-6-4-10(20)19(28)29)38-42(35,36)39-41(33,34)24-40(30,31)32/h8-11,13-15,18,26-27H,2-7,20H2,1H3,(H,28,29)(H,35,36)(H4,24,30,31,32,33,34)/t10?,11-,13-,14+,15+,18?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition constants with Met (L-methionine) substrate site of M-2 (kidney form) variant of rat Methionine adenosyltransferase |
J Med Chem 33: 2545-51 (1990)
BindingDB Entry DOI: 10.7270/Q2SJ1M6S |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1
(Rattus norvegicus) | BDBM50368140

(CHEMBL3706401 | CHEMBL611854)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(SCCCC)ncnc12)[C@H](CSCC[C@H](N)C(O)=O)OP(O)(=O)OP(O)(=O)NP(O)(O)=O |r| Show InChI InChI=1S/C19H33N6O14P3S2/c1-2-3-5-44-17-12-16(21-8-22-17)25(9-23-12)18-14(27)13(26)15(37-18)11(7-43-6-4-10(20)19(28)29)38-42(35,36)39-41(33,34)24-40(30,31)32/h8-11,13-15,18,26-27H,2-7,20H2,1H3,(H,28,29)(H,35,36)(H4,24,30,31,32,33,34)/t10?,11-,13-,14+,15+,18?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition constants against ATP substrate site of M-T (Novikoff Ascitic Hepatoma form) variant of rat Methionine adenosyltransferase |
J Med Chem 33: 2545-51 (1990)
BindingDB Entry DOI: 10.7270/Q2SJ1M6S |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50227269
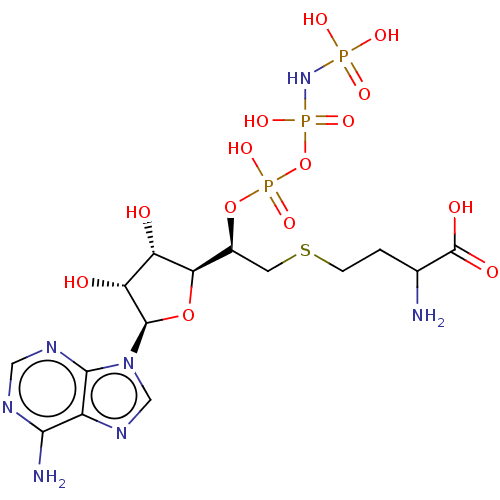
(CHEMBL3706402)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)[C@H](CSCCC(N)C(O)=O)OP(O)(=O)OP(O)(=O)NP(O)(O)=O |r| Show InChI InChI=1S/C15H26N7O14P3S/c16-6(15(25)26)1-2-40-3-7(35-39(32,33)36-38(30,31)21-37(27,28)29)11-9(23)10(24)14(34-11)22-5-20-8-12(17)18-4-19-13(8)22/h4-7,9-11,14,23-24H,1-3,16H2,(H,25,26)(H,32,33)(H2,17,18,19)(H4,21,27,28,29,30,31)/t6?,7-,9-,10+,11+,14+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition constant of ATP varied compound was measured on M-2 variate Methionine adenosyltransferase in rat kidney |
J Med Chem 31: 384-9 (1988)
BindingDB Entry DOI: 10.7270/Q2736T3C |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50227269

(CHEMBL3706402)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)[C@H](CSCCC(N)C(O)=O)OP(O)(=O)OP(O)(=O)NP(O)(O)=O |r| Show InChI InChI=1S/C15H26N7O14P3S/c16-6(15(25)26)1-2-40-3-7(35-39(32,33)36-38(30,31)21-37(27,28)29)11-9(23)10(24)14(34-11)22-5-20-8-12(17)18-4-19-13(8)22/h4-7,9-11,14,23-24H,1-3,16H2,(H,25,26)(H,32,33)(H2,17,18,19)(H4,21,27,28,29,30,31)/t6?,7-,9-,10+,11+,14+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Cancer Center
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition constant with kidney form of M-2 variants of Rat methionine adenosyltransferase when ATP was the variable substrat... |
J Med Chem 32: 885-90 (1989)
BindingDB Entry DOI: 10.7270/Q2K939Q8 |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50226974
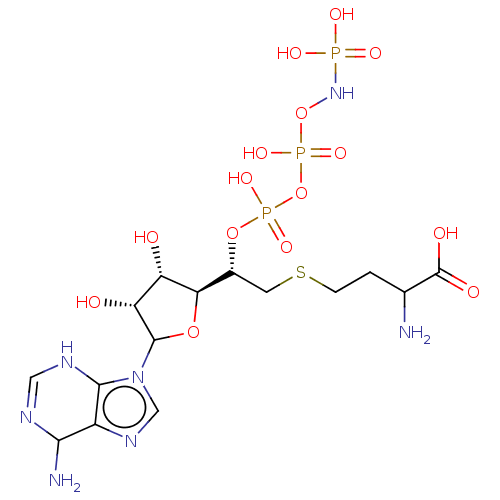
(CHEMBL603986)Show SMILES [H][C@@]1(OC([C@H](O)[C@@H]1O)n1cnc2C(N)N=CNc12)[C@@H](CSCCC(N)C(O)=O)OP(O)(=O)OP(O)(=O)ONP(O)(O)=O |r,c:15| Show InChI InChI=1S/C15H28N7O15P3S/c16-6(15(25)26)1-2-41-3-7(35-39(30,31)37-40(32,33)36-21-38(27,28)29)11-9(23)10(24)14(34-11)22-5-20-8-12(17)18-4-19-13(8)22/h4-7,9-12,14,23-24H,1-3,16-17H2,(H,18,19)(H,25,26)(H,30,31)(H,32,33)(H3,21,27,28,29)/t6?,7-,9+,10-,11-,12?,14?/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition constants of M-2 Forms of rat methionine adenosyltransferase using ATP as the variable substrate; Inhibition is competitive type |
J Med Chem 30: 1599-603 (1987)
BindingDB Entry DOI: 10.7270/Q2CV4KZR |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1
(Rattus norvegicus) | BDBM50227269

(CHEMBL3706402)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)[C@H](CSCCC(N)C(O)=O)OP(O)(=O)OP(O)(=O)NP(O)(O)=O |r| Show InChI InChI=1S/C15H26N7O14P3S/c16-6(15(25)26)1-2-40-3-7(35-39(32,33)36-38(30,31)21-37(27,28)29)11-9(23)10(24)14(34-11)22-5-20-8-12(17)18-4-19-13(8)22/h4-7,9-11,14,23-24H,1-3,16H2,(H,25,26)(H,32,33)(H2,17,18,19)(H4,21,27,28,29,30,31)/t6?,7-,9-,10+,11+,14+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition constants against ATP substrate site of M-2 (kidney form) variant of rat Methionine adenosyltransferase |
J Med Chem 33: 2545-51 (1990)
BindingDB Entry DOI: 10.7270/Q2SJ1M6S |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50228186

(CHEMBL3706404)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)[C@@H](CCSCCC(N)C(O)=O)OP(O)(=O)OP(O)(=O)NP(O)(O)=O |r| Show InChI InChI=1S/C16H28N7O14P3S/c17-7(16(26)27)1-3-41-4-2-8(36-40(33,34)37-39(31,32)22-38(28,29)30)12-10(24)11(25)15(35-12)23-6-21-9-13(18)19-5-20-14(9)23/h5-8,10-12,15,24-25H,1-4,17H2,(H,26,27)(H,33,34)(H2,18,19,20)(H4,22,28,29,30,31,32)/t7?,8-,10+,11-,12-,15-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Cancer Center
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition constant with kidney form of M-T variants of Rat methionine adenosyltransferase when ATP was the variable substrat... |
J Med Chem 32: 885-90 (1989)
BindingDB Entry DOI: 10.7270/Q2K939Q8 |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1
(Rattus norvegicus) | BDBM50227269

(CHEMBL3706402)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)[C@H](CSCCC(N)C(O)=O)OP(O)(=O)OP(O)(=O)NP(O)(O)=O |r| Show InChI InChI=1S/C15H26N7O14P3S/c16-6(15(25)26)1-2-40-3-7(35-39(32,33)36-38(30,31)21-37(27,28)29)11-9(23)10(24)14(34-11)22-5-20-8-12(17)18-4-19-13(8)22/h4-7,9-11,14,23-24H,1-3,16H2,(H,25,26)(H,32,33)(H2,17,18,19)(H4,21,27,28,29,30,31)/t6?,7-,9-,10+,11+,14+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition constants against Met (L-methionine)P substrate site of M-T (Novikoff Ascitic Hepatoma form) variant of rat Methionine adenosyltransferase |
J Med Chem 33: 2545-51 (1990)
BindingDB Entry DOI: 10.7270/Q2SJ1M6S |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50227269

(CHEMBL3706402)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)[C@H](CSCCC(N)C(O)=O)OP(O)(=O)OP(O)(=O)NP(O)(O)=O |r| Show InChI InChI=1S/C15H26N7O14P3S/c16-6(15(25)26)1-2-40-3-7(35-39(32,33)36-38(30,31)21-37(27,28)29)11-9(23)10(24)14(34-11)22-5-20-8-12(17)18-4-19-13(8)22/h4-7,9-11,14,23-24H,1-3,16H2,(H,25,26)(H,32,33)(H2,17,18,19)(H4,21,27,28,29,30,31)/t6?,7-,9-,10+,11+,14+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Cancer Center
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition constant with kidney form of M-T variants of Rat methionine adenosyltransferase when ATP was the variable substrat... |
J Med Chem 32: 885-90 (1989)
BindingDB Entry DOI: 10.7270/Q2K939Q8 |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50227269

(CHEMBL3706402)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)[C@H](CSCCC(N)C(O)=O)OP(O)(=O)OP(O)(=O)NP(O)(O)=O |r| Show InChI InChI=1S/C15H26N7O14P3S/c16-6(15(25)26)1-2-40-3-7(35-39(32,33)36-38(30,31)21-37(27,28)29)11-9(23)10(24)14(34-11)22-5-20-8-12(17)18-4-19-13(8)22/h4-7,9-11,14,23-24H,1-3,16H2,(H,25,26)(H,32,33)(H2,17,18,19)(H4,21,27,28,29,30,31)/t6?,7-,9-,10+,11+,14+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition constant of ATP varied compound was measured on MT variant methionine adenosyltransferase in rat ascitic hepatoma cells |
J Med Chem 31: 384-9 (1988)
BindingDB Entry DOI: 10.7270/Q2736T3C |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50226974

(CHEMBL603986)Show SMILES [H][C@@]1(OC([C@H](O)[C@@H]1O)n1cnc2C(N)N=CNc12)[C@@H](CSCCC(N)C(O)=O)OP(O)(=O)OP(O)(=O)ONP(O)(O)=O |r,c:15| Show InChI InChI=1S/C15H28N7O15P3S/c16-6(15(25)26)1-2-41-3-7(35-39(30,31)37-40(32,33)36-21-38(27,28)29)11-9(23)10(24)14(34-11)22-5-20-8-12(17)18-4-19-13(8)22/h4-7,9-12,14,23-24H,1-3,16-17H2,(H,18,19)(H,25,26)(H,30,31)(H,32,33)(H3,21,27,28,29)/t6?,7-,9+,10-,11-,12?,14?/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition constants of MT Forms of rat Novikoff Ascitic Hepatomae using ATP as the variable substrate; Inhibition is competitive type |
J Med Chem 30: 1599-603 (1987)
BindingDB Entry DOI: 10.7270/Q2CV4KZR |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50228185
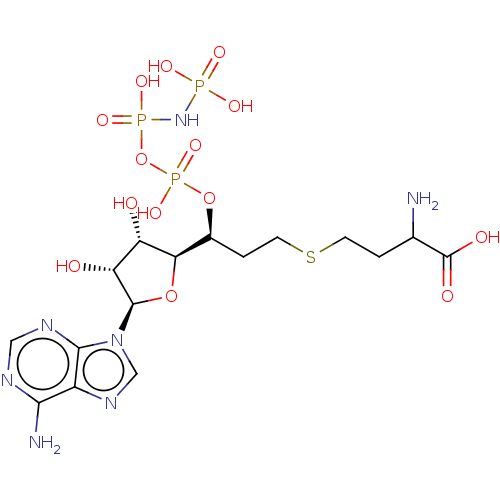
(CHEMBL3706405)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)[C@H](CCSCCC(N)C(O)=O)OP(O)(=O)OP(O)(=O)NP(O)(O)=O |r| Show InChI InChI=1S/C16H28N7O14P3S/c17-7(16(26)27)1-3-41-4-2-8(36-40(33,34)37-39(31,32)22-38(28,29)30)12-10(24)11(25)15(35-12)23-6-21-9-13(18)19-5-20-14(9)23/h5-8,10-12,15,24-25H,1-4,17H2,(H,26,27)(H,33,34)(H2,18,19,20)(H4,22,28,29,30,31,32)/t7?,8-,10-,11+,12+,15+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Cancer Center
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition constant with kidney form of M-2 variants of Rat methionine adenosyltransferase when ATP was the variable substrat... |
J Med Chem 32: 885-90 (1989)
BindingDB Entry DOI: 10.7270/Q2K939Q8 |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50226770

(CHEMBL609078)Show SMILES [H][C@@]1(OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)[C@H](CNP(O)(=O)OP(O)(=O)OP(O)(O)=O)SCCC(N)C(O)=O |r| Show InChI InChI=1S/C15H26N7O14P3S/c16-6(15(25)26)1-2-40-7(3-21-37(27,28)35-39(32,33)36-38(29,30)31)11-9(23)10(24)14(34-11)22-5-20-8-12(17)18-4-19-13(8)22/h4-7,9-11,14,23-24H,1-3,16H2,(H,25,26)(H,32,33)(H2,17,18,19)(H2,21,27,28)(H2,29,30,31)/t6?,7-,9-,10+,11+,14?/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition constant of compound with Novikoff ascitic hepatoma (M-T) form of rat methionine adenosyltransferase when ATP was used as variable substra... |
J Med Chem 30: 888-94 (1987)
BindingDB Entry DOI: 10.7270/Q22J6F29 |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50226972

(CHEMBL606493)Show SMILES [H][C@@]1(OC([C@H](O)[C@@H]1O)n1cnc2C(N)N=CNc12)[C@H](CNP(O)(=O)OP(O)(=O)OP(O)(O)=O)SCCC(N)C(O)=O |r,c:15| Show InChI InChI=1S/C15H28N7O14P3S/c16-6(15(25)26)1-2-40-7(3-21-37(27,28)35-39(32,33)36-38(29,30)31)11-9(23)10(24)14(34-11)22-5-20-8-12(17)18-4-19-13(8)22/h4-7,9-12,14,23-24H,1-3,16-17H2,(H,18,19)(H,25,26)(H,32,33)(H2,21,27,28)(H2,29,30,31)/t6?,7-,9-,10+,11+,12?,14?/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition constants of MT Forms of rat Novikoff Ascitic Hepatomae using ATP as the variable substrate; Inhibition is competitive type |
J Med Chem 30: 1599-603 (1987)
BindingDB Entry DOI: 10.7270/Q2CV4KZR |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50226768
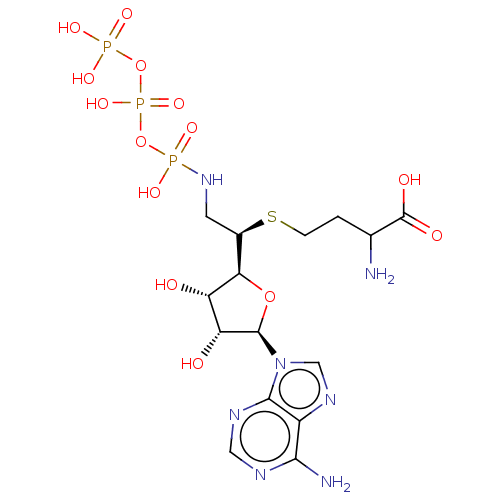
(CHEMBL1791426)Show SMILES [H][C@](CNP(O)(=O)OP(O)(=O)OP(O)(O)=O)(SCCC(N)C(O)=O)[C@@]1([H])O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C15H26N7O14P3S/c16-6(15(25)26)1-2-40-7(3-21-37(27,28)35-39(32,33)36-38(29,30)31)11-9(23)10(24)14(34-11)22-5-20-8-12(17)18-4-19-13(8)22/h4-7,9-11,14,23-24H,1-3,16H2,(H,25,26)(H,32,33)(H2,17,18,19)(H2,21,27,28)(H2,29,30,31)/t6?,7-,9+,10-,11-,14-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition constant of ATP varied compound was measured on MT variant methionine adenosyltransferase in rat ascitic hepatoma cells |
J Med Chem 31: 384-9 (1988)
BindingDB Entry DOI: 10.7270/Q2736T3C |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50226770

(CHEMBL609078)Show SMILES [H][C@@]1(OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)[C@H](CNP(O)(=O)OP(O)(=O)OP(O)(O)=O)SCCC(N)C(O)=O |r| Show InChI InChI=1S/C15H26N7O14P3S/c16-6(15(25)26)1-2-40-7(3-21-37(27,28)35-39(32,33)36-38(29,30)31)11-9(23)10(24)14(34-11)22-5-20-8-12(17)18-4-19-13(8)22/h4-7,9-11,14,23-24H,1-3,16H2,(H,25,26)(H,32,33)(H2,17,18,19)(H2,21,27,28)(H2,29,30,31)/t6?,7-,9-,10+,11+,14?/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition constant of compound with kidney (M-2) form of rat methionine adenosyltransferase when ATP was used as variable substrate;Competitive type... |
J Med Chem 30: 888-94 (1987)
BindingDB Entry DOI: 10.7270/Q22J6F29 |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50226768

(CHEMBL1791426)Show SMILES [H][C@](CNP(O)(=O)OP(O)(=O)OP(O)(O)=O)(SCCC(N)C(O)=O)[C@@]1([H])O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C15H26N7O14P3S/c16-6(15(25)26)1-2-40-7(3-21-37(27,28)35-39(32,33)36-38(29,30)31)11-9(23)10(24)14(34-11)22-5-20-8-12(17)18-4-19-13(8)22/h4-7,9-11,14,23-24H,1-3,16H2,(H,25,26)(H,32,33)(H2,17,18,19)(H2,21,27,28)(H2,29,30,31)/t6?,7-,9+,10-,11-,14-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition constant of ATP varied compound was measured on M-2 variate methionine adenosyltransferase in rat kidney |
J Med Chem 31: 384-9 (1988)
BindingDB Entry DOI: 10.7270/Q2736T3C |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50226972

(CHEMBL606493)Show SMILES [H][C@@]1(OC([C@H](O)[C@@H]1O)n1cnc2C(N)N=CNc12)[C@H](CNP(O)(=O)OP(O)(=O)OP(O)(O)=O)SCCC(N)C(O)=O |r,c:15| Show InChI InChI=1S/C15H28N7O14P3S/c16-6(15(25)26)1-2-40-7(3-21-37(27,28)35-39(32,33)36-38(29,30)31)11-9(23)10(24)14(34-11)22-5-20-8-12(17)18-4-19-13(8)22/h4-7,9-12,14,23-24H,1-3,16-17H2,(H,18,19)(H,25,26)(H,32,33)(H2,21,27,28)(H2,29,30,31)/t6?,7-,9-,10+,11+,12?,14?/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition constants of M-2 Forms of rat methionine adenosyltransferase using ATP as the variable substrate; Inhibition is competitive type |
J Med Chem 30: 1599-603 (1987)
BindingDB Entry DOI: 10.7270/Q2CV4KZR |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50227270
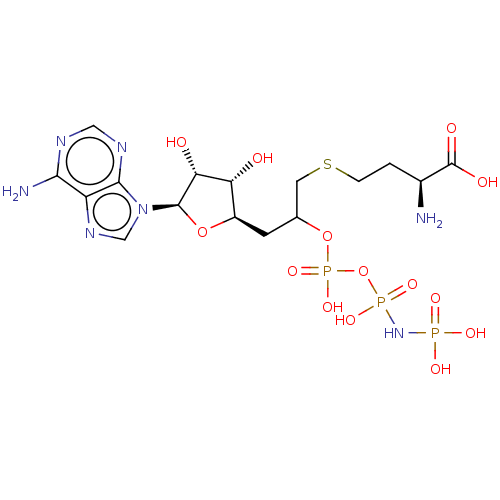
(CHEMBL3706403)Show SMILES N[C@@H](CCSCC(C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)OP(O)(=O)OP(O)(=O)NP(O)(O)=O)C(O)=O |r| Show InChI InChI=1S/C16H28N7O14P3S/c17-8(16(26)27)1-2-41-4-7(36-40(33,34)37-39(31,32)22-38(28,29)30)3-9-11(24)12(25)15(35-9)23-6-21-10-13(18)19-5-20-14(10)23/h5-9,11-12,15,24-25H,1-4,17H2,(H,26,27)(H,33,34)(H2,18,19,20)(H4,22,28,29,30,31,32)/t7?,8-,9+,11+,12+,15+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition constant of ATP varied compound was measured on M-2 variate methionine adenosyltransferase in rat kidney |
J Med Chem 31: 384-9 (1988)
BindingDB Entry DOI: 10.7270/Q2736T3C |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50228185

(CHEMBL3706405)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)[C@H](CCSCCC(N)C(O)=O)OP(O)(=O)OP(O)(=O)NP(O)(O)=O |r| Show InChI InChI=1S/C16H28N7O14P3S/c17-7(16(26)27)1-3-41-4-2-8(36-40(33,34)37-39(31,32)22-38(28,29)30)12-10(24)11(25)15(35-12)23-6-21-9-13(18)19-5-20-14(9)23/h5-8,10-12,15,24-25H,1-4,17H2,(H,26,27)(H,33,34)(H2,18,19,20)(H4,22,28,29,30,31,32)/t7?,8-,10-,11+,12+,15+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Cancer Center
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition constant with kidney form of M-T variants of Rat methionine adenosyltransferase when ATP was the variable substrat... |
J Med Chem 32: 885-90 (1989)
BindingDB Entry DOI: 10.7270/Q2K939Q8 |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50227271
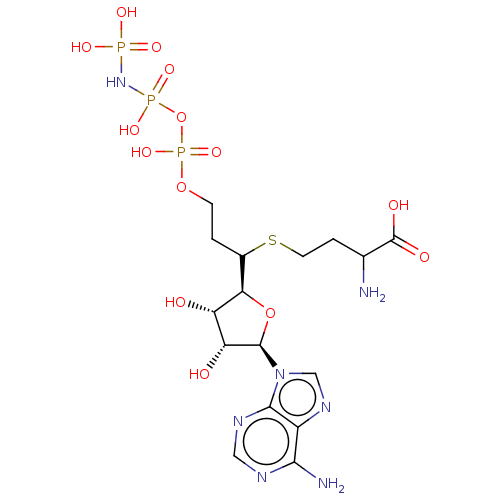
(CHEMBL1791425)Show SMILES NC(CCSC(CCOP(O)(=O)OP(O)(=O)NP(O)(O)=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| Show InChI InChI=1S/C16H28N7O14P3S/c17-7(16(26)27)2-4-41-8(1-3-35-40(33,34)37-39(31,32)22-38(28,29)30)12-10(24)11(25)15(36-12)23-6-21-9-13(18)19-5-20-14(9)23/h5-8,10-12,15,24-25H,1-4,17H2,(H,26,27)(H,33,34)(H2,18,19,20)(H4,22,28,29,30,31,32)/t7?,8?,10-,11+,12+,15+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition constant of ATP varied compound was measured on M-2 variate methionine adenosyltransferase in rat kidney |
J Med Chem 31: 384-9 (1988)
BindingDB Entry DOI: 10.7270/Q2736T3C |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50227270

(CHEMBL3706403)Show SMILES N[C@@H](CCSCC(C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)OP(O)(=O)OP(O)(=O)NP(O)(O)=O)C(O)=O |r| Show InChI InChI=1S/C16H28N7O14P3S/c17-8(16(26)27)1-2-41-4-7(36-40(33,34)37-39(31,32)22-38(28,29)30)3-9-11(24)12(25)15(35-9)23-6-21-10-13(18)19-5-20-14(10)23/h5-9,11-12,15,24-25H,1-4,17H2,(H,26,27)(H,33,34)(H2,18,19,20)(H4,22,28,29,30,31,32)/t7?,8-,9+,11+,12+,15+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition constant of ATP varied compound was measured on MT variant methionine adenosyltransferase in rat ascitic hepatoma cells |
J Med Chem 31: 384-9 (1988)
BindingDB Entry DOI: 10.7270/Q2736T3C |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50227269

(CHEMBL3706402)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)[C@H](CSCCC(N)C(O)=O)OP(O)(=O)OP(O)(=O)NP(O)(O)=O |r| Show InChI InChI=1S/C15H26N7O14P3S/c16-6(15(25)26)1-2-40-3-7(35-39(32,33)36-38(30,31)21-37(27,28)29)11-9(23)10(24)14(34-11)22-5-20-8-12(17)18-4-19-13(8)22/h4-7,9-11,14,23-24H,1-3,16H2,(H,25,26)(H,32,33)(H2,17,18,19)(H4,21,27,28,29,30,31)/t6?,7-,9-,10+,11+,14+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition constant of Met varied compound was measured on M-2 variant Methionine adenosyltransferase in rat kidney |
J Med Chem 31: 384-9 (1988)
BindingDB Entry DOI: 10.7270/Q2736T3C |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50227269

(CHEMBL3706402)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)[C@H](CSCCC(N)C(O)=O)OP(O)(=O)OP(O)(=O)NP(O)(O)=O |r| Show InChI InChI=1S/C15H26N7O14P3S/c16-6(15(25)26)1-2-40-3-7(35-39(32,33)36-38(30,31)21-37(27,28)29)11-9(23)10(24)14(34-11)22-5-20-8-12(17)18-4-19-13(8)22/h4-7,9-11,14,23-24H,1-3,16H2,(H,25,26)(H,32,33)(H2,17,18,19)(H4,21,27,28,29,30,31)/t6?,7-,9-,10+,11+,14+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Cancer Center
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition constant with kidney form of M-2 variants of Rat methionine adenosyltransferase when Methionine was the variable s... |
J Med Chem 32: 885-90 (1989)
BindingDB Entry DOI: 10.7270/Q2K939Q8 |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50226974

(CHEMBL603986)Show SMILES [H][C@@]1(OC([C@H](O)[C@@H]1O)n1cnc2C(N)N=CNc12)[C@@H](CSCCC(N)C(O)=O)OP(O)(=O)OP(O)(=O)ONP(O)(O)=O |r,c:15| Show InChI InChI=1S/C15H28N7O15P3S/c16-6(15(25)26)1-2-41-3-7(35-39(30,31)37-40(32,33)36-21-38(27,28)29)11-9(23)10(24)14(34-11)22-5-20-8-12(17)18-4-19-13(8)22/h4-7,9-12,14,23-24H,1-3,16-17H2,(H,18,19)(H,25,26)(H,30,31)(H,32,33)(H3,21,27,28,29)/t6?,7-,9+,10-,11-,12?,14?/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition constants of M-2 Forms of rat methionine adenosyltransferase using Methionine as the variable substrate; Inhibition is non competitive typ... |
J Med Chem 30: 1599-603 (1987)
BindingDB Entry DOI: 10.7270/Q2CV4KZR |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1
(Rattus norvegicus) | BDBM50227269

(CHEMBL3706402)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)[C@H](CSCCC(N)C(O)=O)OP(O)(=O)OP(O)(=O)NP(O)(O)=O |r| Show InChI InChI=1S/C15H26N7O14P3S/c16-6(15(25)26)1-2-40-3-7(35-39(32,33)36-38(30,31)21-37(27,28)29)11-9(23)10(24)14(34-11)22-5-20-8-12(17)18-4-19-13(8)22/h4-7,9-11,14,23-24H,1-3,16H2,(H,25,26)(H,32,33)(H2,17,18,19)(H4,21,27,28,29,30,31)/t6?,7-,9-,10+,11+,14+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition constants with Met (L-methionine) substrate site of M-2 (kidney form) variant of rat Methionine adenosyltransferase |
J Med Chem 33: 2545-51 (1990)
BindingDB Entry DOI: 10.7270/Q2SJ1M6S |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50227269

(CHEMBL3706402)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)[C@H](CSCCC(N)C(O)=O)OP(O)(=O)OP(O)(=O)NP(O)(O)=O |r| Show InChI InChI=1S/C15H26N7O14P3S/c16-6(15(25)26)1-2-40-3-7(35-39(32,33)36-38(30,31)21-37(27,28)29)11-9(23)10(24)14(34-11)22-5-20-8-12(17)18-4-19-13(8)22/h4-7,9-11,14,23-24H,1-3,16H2,(H,25,26)(H,32,33)(H2,17,18,19)(H4,21,27,28,29,30,31)/t6?,7-,9-,10+,11+,14+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition constant of Met varied compound was measured on MT variant methionine adenosyltransferase in rat ascitic hepatoma cells |
J Med Chem 31: 384-9 (1988)
BindingDB Entry DOI: 10.7270/Q2736T3C |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50227269

(CHEMBL3706402)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)[C@H](CSCCC(N)C(O)=O)OP(O)(=O)OP(O)(=O)NP(O)(O)=O |r| Show InChI InChI=1S/C15H26N7O14P3S/c16-6(15(25)26)1-2-40-3-7(35-39(32,33)36-38(30,31)21-37(27,28)29)11-9(23)10(24)14(34-11)22-5-20-8-12(17)18-4-19-13(8)22/h4-7,9-11,14,23-24H,1-3,16H2,(H,25,26)(H,32,33)(H2,17,18,19)(H4,21,27,28,29,30,31)/t6?,7-,9-,10+,11+,14+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Cancer Center
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition constant with kidney form of M-T variants of Rat methionine adenosyltransferase when Methionine was the variable s... |
J Med Chem 32: 885-90 (1989)
BindingDB Entry DOI: 10.7270/Q2K939Q8 |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50226974

(CHEMBL603986)Show SMILES [H][C@@]1(OC([C@H](O)[C@@H]1O)n1cnc2C(N)N=CNc12)[C@@H](CSCCC(N)C(O)=O)OP(O)(=O)OP(O)(=O)ONP(O)(O)=O |r,c:15| Show InChI InChI=1S/C15H28N7O15P3S/c16-6(15(25)26)1-2-41-3-7(35-39(30,31)37-40(32,33)36-21-38(27,28)29)11-9(23)10(24)14(34-11)22-5-20-8-12(17)18-4-19-13(8)22/h4-7,9-12,14,23-24H,1-3,16-17H2,(H,18,19)(H,25,26)(H,30,31)(H,32,33)(H3,21,27,28,29)/t6?,7-,9+,10-,11-,12?,14?/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition constants of MT Forms of rat Novikoff Ascitic Hepatomae using Methionine as the variable substrate; Inhibition is non competitive type |
J Med Chem 30: 1599-603 (1987)
BindingDB Entry DOI: 10.7270/Q2CV4KZR |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1
(Rattus norvegicus) | BDBM50227269

(CHEMBL3706402)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)[C@H](CSCCC(N)C(O)=O)OP(O)(=O)OP(O)(=O)NP(O)(O)=O |r| Show InChI InChI=1S/C15H26N7O14P3S/c16-6(15(25)26)1-2-40-3-7(35-39(32,33)36-38(30,31)21-37(27,28)29)11-9(23)10(24)14(34-11)22-5-20-8-12(17)18-4-19-13(8)22/h4-7,9-11,14,23-24H,1-3,16H2,(H,25,26)(H,32,33)(H2,17,18,19)(H4,21,27,28,29,30,31)/t6?,7-,9-,10+,11+,14+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition constants against Met (L-methionine)P substrate site of M-T (Novikoff Ascitic Hepatoma form) variant of rat Methionine adenosyltransferase |
J Med Chem 33: 2545-51 (1990)
BindingDB Entry DOI: 10.7270/Q2SJ1M6S |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1
(Rattus norvegicus) | BDBM50368140

(CHEMBL3706401 | CHEMBL611854)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(SCCCC)ncnc12)[C@H](CSCC[C@H](N)C(O)=O)OP(O)(=O)OP(O)(=O)NP(O)(O)=O |r| Show InChI InChI=1S/C19H33N6O14P3S2/c1-2-3-5-44-17-12-16(21-8-22-17)25(9-23-12)18-14(27)13(26)15(37-18)11(7-43-6-4-10(20)19(28)29)38-42(35,36)39-41(33,34)24-40(30,31)32/h8-11,13-15,18,26-27H,2-7,20H2,1H3,(H,28,29)(H,35,36)(H4,24,30,31,32,33,34)/t10?,11-,13-,14+,15+,18?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition constants against Met (L-methionine)P substrate site of M-T (Novikoff Ascitic Hepatoma form) variant of rat Methionine adenosyltransferase |
J Med Chem 33: 2545-51 (1990)
BindingDB Entry DOI: 10.7270/Q2SJ1M6S |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50227271

(CHEMBL1791425)Show SMILES NC(CCSC(CCOP(O)(=O)OP(O)(=O)NP(O)(O)=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| Show InChI InChI=1S/C16H28N7O14P3S/c17-7(16(26)27)2-4-41-8(1-3-35-40(33,34)37-39(31,32)22-38(28,29)30)12-10(24)11(25)15(36-12)23-6-21-9-13(18)19-5-20-14(9)23/h5-8,10-12,15,24-25H,1-4,17H2,(H,26,27)(H,33,34)(H2,18,19,20)(H4,22,28,29,30,31,32)/t7?,8?,10-,11+,12+,15+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition constant of ATP varied compound was measured on MT variant methionine adenosyltransferase in rat ascitic hepatoma cells |
J Med Chem 31: 384-9 (1988)
BindingDB Entry DOI: 10.7270/Q2736T3C |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50228186

(CHEMBL3706404)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)[C@@H](CCSCCC(N)C(O)=O)OP(O)(=O)OP(O)(=O)NP(O)(O)=O |r| Show InChI InChI=1S/C16H28N7O14P3S/c17-7(16(26)27)1-3-41-4-2-8(36-40(33,34)37-39(31,32)22-38(28,29)30)12-10(24)11(25)15(35-12)23-6-21-9-13(18)19-5-20-14(9)23/h5-8,10-12,15,24-25H,1-4,17H2,(H,26,27)(H,33,34)(H2,18,19,20)(H4,22,28,29,30,31,32)/t7?,8-,10+,11-,12-,15-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Cancer Center
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition constant with kidney form of M-T variants of Rat methionine adenosyltransferase when Methionine was the variable s... |
J Med Chem 32: 885-90 (1989)
BindingDB Entry DOI: 10.7270/Q2K939Q8 |
More data for this
Ligand-Target Pair | |
Adenylate kinase 2, mitochondrial
(Rattus norvegicus) | BDBM50367044
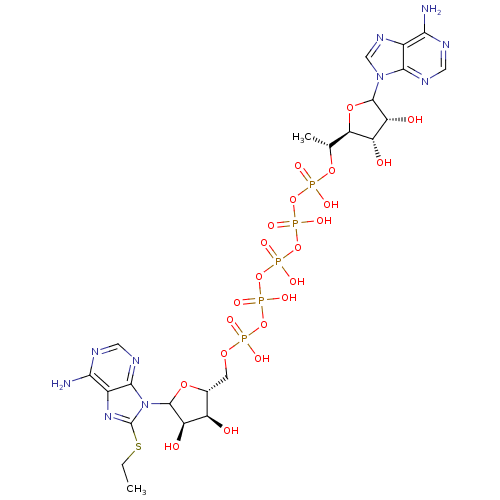
(CHEMBL605639)Show SMILES CCSc1nc2c(N)ncnc2n1C1O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(=O)OP(O)(=O)OP(O)(=O)O[C@H](C)[C@H]2OC([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C23H35N10O22P5S/c1-3-61-23-31-11-18(25)27-6-29-20(11)33(23)22-14(36)12(34)9(49-22)4-48-56(38,39)52-58(42,43)54-60(46,47)55-59(44,45)53-57(40,41)51-8(2)16-13(35)15(37)21(50-16)32-7-30-10-17(24)26-5-28-19(10)32/h5-9,12-16,21-22,34-37H,3-4H2,1-2H3,(H,38,39)(H,40,41)(H,42,43)(H,44,45)(H,46,47)(H2,24,26,28)(H2,25,27,29)/t8-,9-,12-,13+,14-,15-,16-,21?,22?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat Adenylate kinase M isoenzyme in the presence of AMP non competitive inhibition |
J Med Chem 25: 1179-84 (1983)
BindingDB Entry DOI: 10.7270/Q25D8SD7 |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50227271

(CHEMBL1791425)Show SMILES NC(CCSC(CCOP(O)(=O)OP(O)(=O)NP(O)(O)=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| Show InChI InChI=1S/C16H28N7O14P3S/c17-7(16(26)27)2-4-41-8(1-3-35-40(33,34)37-39(31,32)22-38(28,29)30)12-10(24)11(25)15(36-12)23-6-21-9-13(18)19-5-20-14(9)23/h5-8,10-12,15,24-25H,1-4,17H2,(H,26,27)(H,33,34)(H2,18,19,20)(H4,22,28,29,30,31,32)/t7?,8?,10-,11+,12+,15+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition constant of Met varied compound was measured on MT variant methionine adenosyltransferase in rat ascitic hepatoma cells |
J Med Chem 31: 384-9 (1988)
BindingDB Entry DOI: 10.7270/Q2736T3C |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50228186

(CHEMBL3706404)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)[C@@H](CCSCCC(N)C(O)=O)OP(O)(=O)OP(O)(=O)NP(O)(O)=O |r| Show InChI InChI=1S/C16H28N7O14P3S/c17-7(16(26)27)1-3-41-4-2-8(36-40(33,34)37-39(31,32)22-38(28,29)30)12-10(24)11(25)15(35-12)23-6-21-9-13(18)19-5-20-14(9)23/h5-8,10-12,15,24-25H,1-4,17H2,(H,26,27)(H,33,34)(H2,18,19,20)(H4,22,28,29,30,31,32)/t7?,8-,10+,11-,12-,15-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Cancer Center
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition constant with kidney form of M-2 variants of Rat methionine adenosyltransferase when Methionine was the variable s... |
J Med Chem 32: 885-90 (1989)
BindingDB Entry DOI: 10.7270/Q2K939Q8 |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50227271

(CHEMBL1791425)Show SMILES NC(CCSC(CCOP(O)(=O)OP(O)(=O)NP(O)(O)=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| Show InChI InChI=1S/C16H28N7O14P3S/c17-7(16(26)27)2-4-41-8(1-3-35-40(33,34)37-39(31,32)22-38(28,29)30)12-10(24)11(25)15(36-12)23-6-21-9-13(18)19-5-20-14(9)23/h5-8,10-12,15,24-25H,1-4,17H2,(H,26,27)(H,33,34)(H2,18,19,20)(H4,22,28,29,30,31,32)/t7?,8?,10-,11+,12+,15+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition constant of Met varied compound was measured on M-2 variant methionine adenosyltransferase in rat kidney |
J Med Chem 31: 384-9 (1988)
BindingDB Entry DOI: 10.7270/Q2736T3C |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50227270

(CHEMBL3706403)Show SMILES N[C@@H](CCSCC(C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)OP(O)(=O)OP(O)(=O)NP(O)(O)=O)C(O)=O |r| Show InChI InChI=1S/C16H28N7O14P3S/c17-8(16(26)27)1-2-41-4-7(36-40(33,34)37-39(31,32)22-38(28,29)30)3-9-11(24)12(25)15(35-9)23-6-21-10-13(18)19-5-20-14(10)23/h5-9,11-12,15,24-25H,1-4,17H2,(H,26,27)(H,33,34)(H2,18,19,20)(H4,22,28,29,30,31,32)/t7?,8-,9+,11+,12+,15+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition constant of Met varied compound was measured on M-2 variant methionine adenosyltransferase in rat kidney |
J Med Chem 31: 384-9 (1988)
BindingDB Entry DOI: 10.7270/Q2736T3C |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50226768

(CHEMBL1791426)Show SMILES [H][C@](CNP(O)(=O)OP(O)(=O)OP(O)(O)=O)(SCCC(N)C(O)=O)[C@@]1([H])O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C15H26N7O14P3S/c16-6(15(25)26)1-2-40-7(3-21-37(27,28)35-39(32,33)36-38(29,30)31)11-9(23)10(24)14(34-11)22-5-20-8-12(17)18-4-19-13(8)22/h4-7,9-11,14,23-24H,1-3,16H2,(H,25,26)(H,32,33)(H2,17,18,19)(H2,21,27,28)(H2,29,30,31)/t6?,7-,9+,10-,11-,14-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition constant of Met varied compound was measured on MT variant methionine adenosyltransferase in rat ascitic hepatoma cells |
J Med Chem 31: 384-9 (1988)
BindingDB Entry DOI: 10.7270/Q2736T3C |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50226770

(CHEMBL609078)Show SMILES [H][C@@]1(OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)[C@H](CNP(O)(=O)OP(O)(=O)OP(O)(O)=O)SCCC(N)C(O)=O |r| Show InChI InChI=1S/C15H26N7O14P3S/c16-6(15(25)26)1-2-40-7(3-21-37(27,28)35-39(32,33)36-38(29,30)31)11-9(23)10(24)14(34-11)22-5-20-8-12(17)18-4-19-13(8)22/h4-7,9-11,14,23-24H,1-3,16H2,(H,25,26)(H,32,33)(H2,17,18,19)(H2,21,27,28)(H2,29,30,31)/t6?,7-,9-,10+,11+,14?/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition constant of compound with Novikoff ascitic hepatoma (M-T) form of rat methionine adenosyltransferase when methionine was used as variable ... |
J Med Chem 30: 888-94 (1987)
BindingDB Entry DOI: 10.7270/Q22J6F29 |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50226972

(CHEMBL606493)Show SMILES [H][C@@]1(OC([C@H](O)[C@@H]1O)n1cnc2C(N)N=CNc12)[C@H](CNP(O)(=O)OP(O)(=O)OP(O)(O)=O)SCCC(N)C(O)=O |r,c:15| Show InChI InChI=1S/C15H28N7O14P3S/c16-6(15(25)26)1-2-40-7(3-21-37(27,28)35-39(32,33)36-38(29,30)31)11-9(23)10(24)14(34-11)22-5-20-8-12(17)18-4-19-13(8)22/h4-7,9-12,14,23-24H,1-3,16-17H2,(H,18,19)(H,25,26)(H,32,33)(H2,21,27,28)(H2,29,30,31)/t6?,7-,9-,10+,11+,12?,14?/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition constants of MT Forms of rat Novikoff Ascitic Hepatomae using Methionine as the variable substrate; Inhibition is non competitive type |
J Med Chem 30: 1599-603 (1987)
BindingDB Entry DOI: 10.7270/Q2CV4KZR |
More data for this
Ligand-Target Pair | |
Thymidine kinase, cytosolic
(Rattus norvegicus) | BDBM50132297
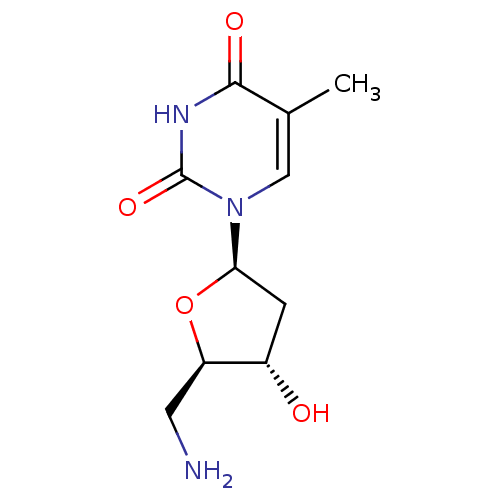
(1-((2R,4S,5R)-5-(aminomethyl)-4-hydroxy-tetrahydro...)Show SMILES Cc1cn([C@H]2C[C@H](O)[C@@H](CN)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C10H15N3O4/c1-5-4-13(10(16)12-9(5)15)8-2-6(14)7(3-11)17-8/h4,6-8,14H,2-3,11H2,1H3,(H,12,15,16)/t6-,7+,8+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition against rat cytoplasmic Thymidine kinase |
J Med Chem 25: 644-9 (1982)
BindingDB Entry DOI: 10.7270/Q2GQ6ZBJ |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50227270

(CHEMBL3706403)Show SMILES N[C@@H](CCSCC(C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)OP(O)(=O)OP(O)(=O)NP(O)(O)=O)C(O)=O |r| Show InChI InChI=1S/C16H28N7O14P3S/c17-8(16(26)27)1-2-41-4-7(36-40(33,34)37-39(31,32)22-38(28,29)30)3-9-11(24)12(25)15(35-9)23-6-21-10-13(18)19-5-20-14(10)23/h5-9,11-12,15,24-25H,1-4,17H2,(H,26,27)(H,33,34)(H2,18,19,20)(H4,22,28,29,30,31,32)/t7?,8-,9+,11+,12+,15+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition constant of Met varied compound was measured on MT variant methionine adenosyltransferase in rat ascitic hepatoma cells |
J Med Chem 31: 384-9 (1988)
BindingDB Entry DOI: 10.7270/Q2736T3C |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50228185

(CHEMBL3706405)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)[C@H](CCSCCC(N)C(O)=O)OP(O)(=O)OP(O)(=O)NP(O)(O)=O |r| Show InChI InChI=1S/C16H28N7O14P3S/c17-7(16(26)27)1-3-41-4-2-8(36-40(33,34)37-39(31,32)22-38(28,29)30)12-10(24)11(25)15(35-12)23-6-21-9-13(18)19-5-20-14(9)23/h5-8,10-12,15,24-25H,1-4,17H2,(H,26,27)(H,33,34)(H2,18,19,20)(H4,22,28,29,30,31,32)/t7?,8-,10-,11+,12+,15+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Cancer Center
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition constant with kidney form of M-T variants of Rat methionine adenosyltransferase when Methionine was the variable s... |
J Med Chem 32: 885-90 (1989)
BindingDB Entry DOI: 10.7270/Q2K939Q8 |
More data for this
Ligand-Target Pair | |
Adenylate kinase 2, mitochondrial
(Rattus norvegicus) | BDBM50367044

(CHEMBL605639)Show SMILES CCSc1nc2c(N)ncnc2n1C1O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(=O)OP(O)(=O)OP(O)(=O)O[C@H](C)[C@H]2OC([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C23H35N10O22P5S/c1-3-61-23-31-11-18(25)27-6-29-20(11)33(23)22-14(36)12(34)9(49-22)4-48-56(38,39)52-58(42,43)54-60(46,47)55-59(44,45)53-57(40,41)51-8(2)16-13(35)15(37)21(50-16)32-7-30-10-17(24)26-5-28-19(10)32/h5-9,12-16,21-22,34-37H,3-4H2,1-2H3,(H,38,39)(H,40,41)(H,42,43)(H,44,45)(H,46,47)(H2,24,26,28)(H2,25,27,29)/t8-,9-,12-,13+,14-,15-,16-,21?,22?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat adenylate kinase II was determined in the presence of AMP, non competitive inhibition |
J Med Chem 25: 1179-84 (1983)
BindingDB Entry DOI: 10.7270/Q25D8SD7 |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1
(Rattus norvegicus) | BDBM50368140

(CHEMBL3706401 | CHEMBL611854)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(SCCCC)ncnc12)[C@H](CSCC[C@H](N)C(O)=O)OP(O)(=O)OP(O)(=O)NP(O)(O)=O |r| Show InChI InChI=1S/C19H33N6O14P3S2/c1-2-3-5-44-17-12-16(21-8-22-17)25(9-23-12)18-14(27)13(26)15(37-18)11(7-43-6-4-10(20)19(28)29)38-42(35,36)39-41(33,34)24-40(30,31)32/h8-11,13-15,18,26-27H,2-7,20H2,1H3,(H,28,29)(H,35,36)(H4,24,30,31,32,33,34)/t10?,11-,13-,14+,15+,18?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition constants against ATP substrate site of M-T (Novikoff Ascitic Hepatoma form) variant of rat Methionine adenosyltransferase |
J Med Chem 33: 2545-51 (1990)
BindingDB Entry DOI: 10.7270/Q2SJ1M6S |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-1/type-2
(Rattus norvegicus) | BDBM50226770

(CHEMBL609078)Show SMILES [H][C@@]1(OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)[C@H](CNP(O)(=O)OP(O)(=O)OP(O)(O)=O)SCCC(N)C(O)=O |r| Show InChI InChI=1S/C15H26N7O14P3S/c16-6(15(25)26)1-2-40-7(3-21-37(27,28)35-39(32,33)36-38(29,30)31)11-9(23)10(24)14(34-11)22-5-20-8-12(17)18-4-19-13(8)22/h4-7,9-11,14,23-24H,1-3,16H2,(H,25,26)(H,32,33)(H2,17,18,19)(H2,21,27,28)(H2,29,30,31)/t6?,7-,9-,10+,11+,14?/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition constant of compound with kidney (M-2) form of rat methionine adenosyltransferase when methionine was used as variable substrate;Simple no... |
J Med Chem 30: 888-94 (1987)
BindingDB Entry DOI: 10.7270/Q22J6F29 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data