

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50112815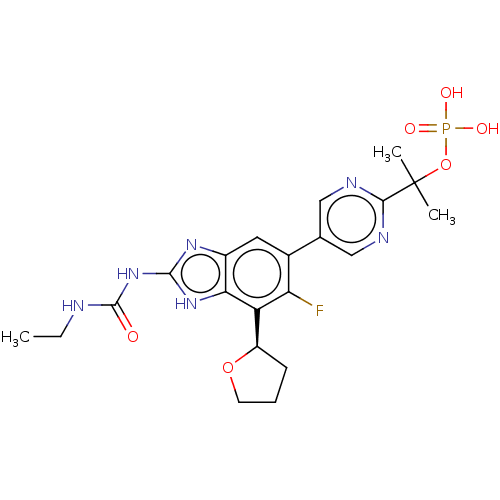 (CHEMBL2221212) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | <17.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyrase | ACS Med Chem Lett 6: 822-6 (2015) Article DOI: 10.1021/acsmedchemlett.5b00196 BindingDB Entry DOI: 10.7270/Q2J67JPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50393079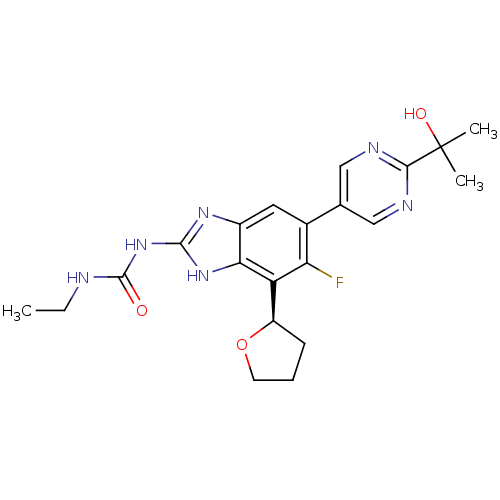 (CHEMBL2152855 | US9040542, 23) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | <21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyrase | ACS Med Chem Lett 6: 822-6 (2015) Article DOI: 10.1021/acsmedchemlett.5b00196 BindingDB Entry DOI: 10.7270/Q2J67JPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Staphylococcus aureus) | BDBM50393079 (CHEMBL2152855 | US9040542, 23) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA topoisomerase 4 | ACS Med Chem Lett 6: 822-6 (2015) Article DOI: 10.1021/acsmedchemlett.5b00196 BindingDB Entry DOI: 10.7270/Q2J67JPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Staphylococcus aureus) | BDBM50112815 (CHEMBL2221212) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA topoisomerase 4 | ACS Med Chem Lett 6: 822-6 (2015) Article DOI: 10.1021/acsmedchemlett.5b00196 BindingDB Entry DOI: 10.7270/Q2J67JPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50326056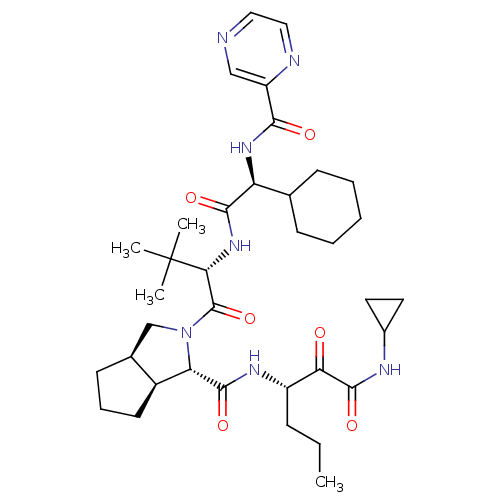 ((1S,3aR,6aS)-2-((S)-2-((S)-2-cyclohexyl-2-(pyrazin...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3 protease V36L mutant expressed in Escherichia coli BL21 (DE3) | Antimicrob Agents Chemother 52: 110-20 (2008) Article DOI: 10.1128/aac.00863-07 BindingDB Entry DOI: 10.7270/Q2T43WXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50326056 ((1S,3aR,6aS)-2-((S)-2-((S)-2-cyclohexyl-2-(pyrazin...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3 protease V36M mutant expressed in Escherichia coli BL21 (DE3) | Antimicrob Agents Chemother 52: 110-20 (2008) Article DOI: 10.1128/aac.00863-07 BindingDB Entry DOI: 10.7270/Q2T43WXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50326056 ((1S,3aR,6aS)-2-((S)-2-((S)-2-cyclohexyl-2-(pyrazin...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of HCV 1a NS3-4A R155T mutant protease after 60 mins | J Biol Chem 282: 22619-28 (2007) Article DOI: 10.1074/jbc.m610207200 BindingDB Entry DOI: 10.7270/Q2DZ0C3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50326056 ((1S,3aR,6aS)-2-((S)-2-((S)-2-cyclohexyl-2-(pyrazin...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of HCV 1a NS3-4A R155K mutant protease after 60 mins | J Biol Chem 282: 22619-28 (2007) Article DOI: 10.1074/jbc.m610207200 BindingDB Entry DOI: 10.7270/Q2DZ0C3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50326056 ((1S,3aR,6aS)-2-((S)-2-((S)-2-cyclohexyl-2-(pyrazin...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of HCV 1a NS3-4A R155I mutant protease after 60 mins | J Biol Chem 282: 22619-28 (2007) Article DOI: 10.1074/jbc.m610207200 BindingDB Entry DOI: 10.7270/Q2DZ0C3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50326056 ((1S,3aR,6aS)-2-((S)-2-((S)-2-cyclohexyl-2-(pyrazin...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of HCV 1a NS3-4A protease R155S mutant after 60 mins | J Biol Chem 282: 22619-28 (2007) Article DOI: 10.1074/jbc.m610207200 BindingDB Entry DOI: 10.7270/Q2DZ0C3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50326056 ((1S,3aR,6aS)-2-((S)-2-((S)-2-cyclohexyl-2-(pyrazin...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3 protease V36M/R155K double mutant expressed in Escherichia coli BL21 (DE3) | Antimicrob Agents Chemother 52: 110-20 (2008) Article DOI: 10.1128/aac.00863-07 BindingDB Entry DOI: 10.7270/Q2T43WXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||