

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50124714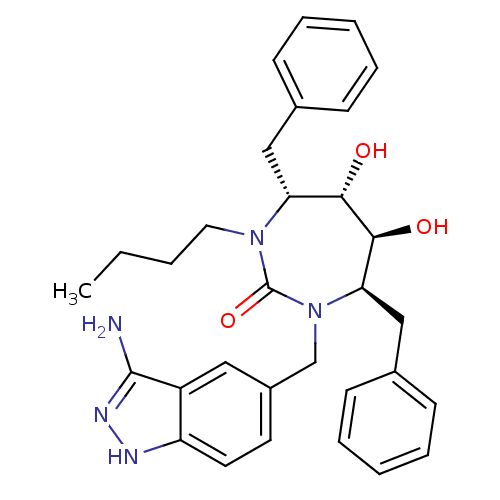 ((4R,5S,6S,7R)-1-(3-Amino-1H-indazol-5-ylmethyl)-4,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity of the compound against HIV-Protease was determined | Bioorg Med Chem Lett 13: 605-8 (2003) BindingDB Entry DOI: 10.7270/Q27D2THB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50124721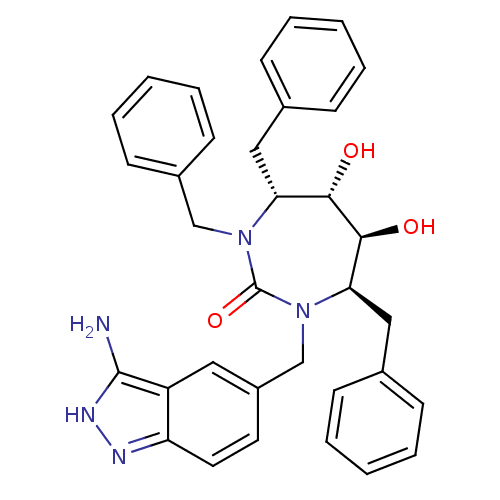 ((4R,5S,6S,7R)-1-(3-Amino-1H-indazol-5-ylmethyl)-3,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity of the compound against HIV-Protease was determined | Bioorg Med Chem Lett 13: 605-8 (2003) BindingDB Entry DOI: 10.7270/Q27D2THB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50124716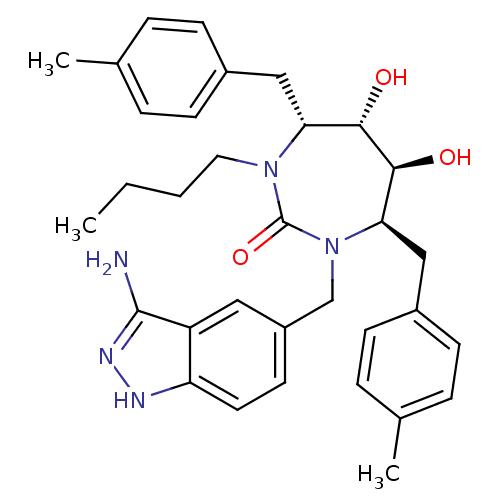 ((4R,5S,6S,7R)-1-(3-Amino-1H-indazol-5-ylmethyl)-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity of the compound against HIV-Protease was determined | Bioorg Med Chem Lett 13: 605-8 (2003) BindingDB Entry DOI: 10.7270/Q27D2THB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50124715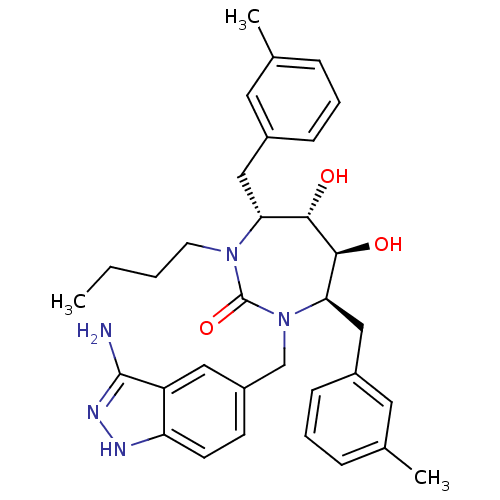 ((4R,5S,6S,7R)-1-(3-Amino-1H-indazol-5-ylmethyl)-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity of the compound against HIV-Protease was determined | Bioorg Med Chem Lett 13: 605-8 (2003) BindingDB Entry DOI: 10.7270/Q27D2THB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50124722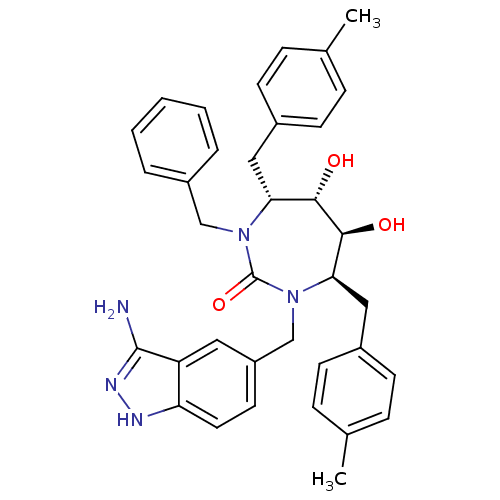 ((4R,5S,6S,7R)-1-(3-Amino-1H-indazol-5-ylmethyl)-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity of the compound against HIV-Protease was determined | Bioorg Med Chem Lett 13: 605-8 (2003) BindingDB Entry DOI: 10.7270/Q27D2THB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50124718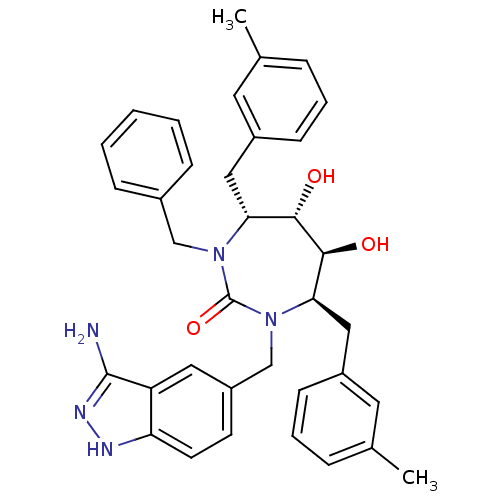 ((4R,5S,6S,7R)-1-(3-Amino-1H-indazol-5-ylmethyl)-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity of the compound against HIV-Protease was determined | Bioorg Med Chem Lett 13: 605-8 (2003) BindingDB Entry DOI: 10.7270/Q27D2THB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50124723 ((4R,5S,6S,7R)-1-(3-Amino-1H-indazol-5-ylmethyl)-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity of the compound against HIV-Protease was determined | Bioorg Med Chem Lett 13: 605-8 (2003) BindingDB Entry DOI: 10.7270/Q27D2THB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50124717 ((4R,5S,6S,7R)-1-(3-Amino-1H-indazol-5-ylmethyl)-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity of the compound against HIV-Protease was determined | Bioorg Med Chem Lett 13: 605-8 (2003) BindingDB Entry DOI: 10.7270/Q27D2THB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibition of [3H]prazosin binding Alpha-1 adrenergic receptor of crude rat brain membrane. | J Med Chem 25: 75-81 (1982) BindingDB Entry DOI: 10.7270/Q2TM7DBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50124719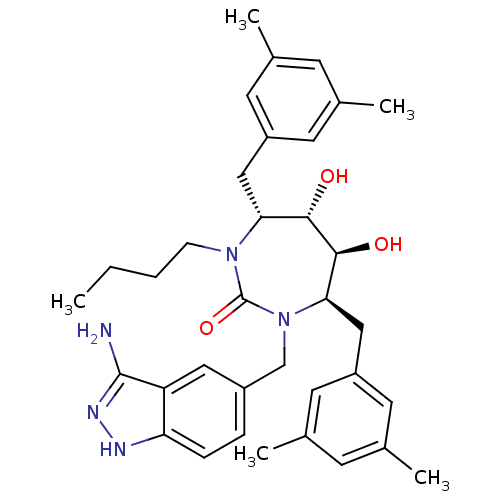 ((4R,5S,6S,7R)-1-(3-Amino-1H-indazol-5-ylmethyl)-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity of the compound against HIV-Protease was determined | Bioorg Med Chem Lett 13: 605-8 (2003) BindingDB Entry DOI: 10.7270/Q27D2THB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50124720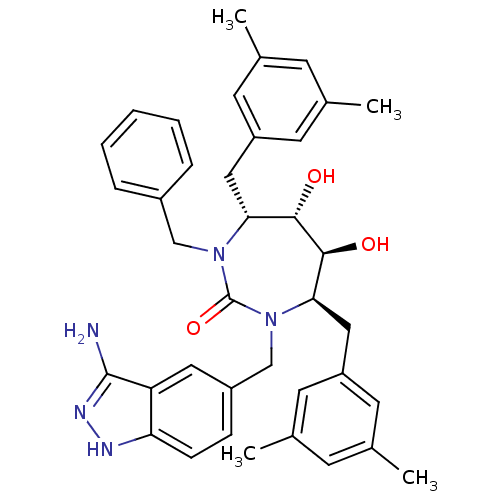 ((4R,5S,6S,7R)-1-(3-Amino-1H-indazol-5-ylmethyl)-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity of the compound against HIV-Protease was determined | Bioorg Med Chem Lett 13: 605-8 (2003) BindingDB Entry DOI: 10.7270/Q27D2THB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM50049736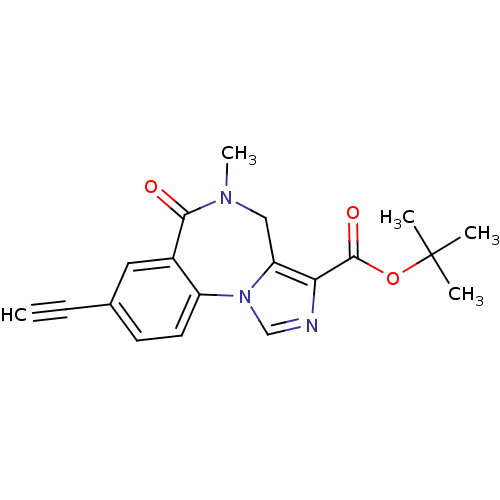 (8-Ethynyl-5-methyl-6-oxo-5,6-dihydro-4H-2,5,10b-tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Moltech Corporation Curated by ChEMBL | Assay Description Binding affinity to GABAA alpha-5-beta-3-gamma-2 receptor | J Med Chem 51: 3788-803 (2008) Article DOI: 10.1021/jm701433b BindingDB Entry DOI: 10.7270/Q2FQ9XH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM50244227 (CHEMBL458147 | tert-butyl 7-chloro-4-methyl-5-oxo-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Moltech Corporation Curated by ChEMBL | Assay Description Binding affinity to GABAA alpha-5-beta-2-gamma-2 receptor | J Med Chem 51: 3788-803 (2008) Article DOI: 10.1021/jm701433b BindingDB Entry DOI: 10.7270/Q2FQ9XH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM27337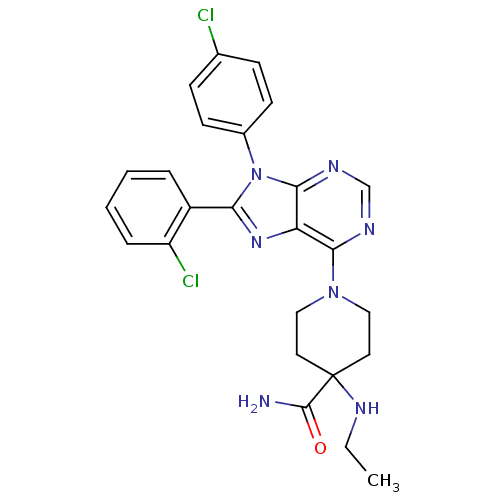 (1-[8-(2-chlorophenyl)-9-(4-chlorophenyl)-9H-purin-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 expressed in CHOK1 cell membranes | J Med Chem 61: 4370-4385 (2018) Article DOI: 10.1021/acs.jmedchem.7b01820 BindingDB Entry DOI: 10.7270/Q20004QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM50244195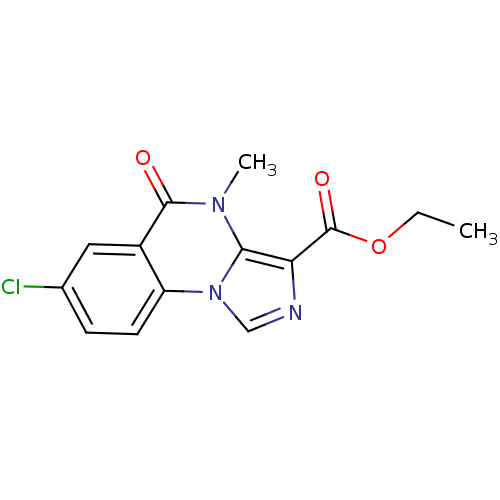 (CHEMBL453251 | ethyl 7-chloro-4-methyl-5-oxo-4,5-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Moltech Corporation Curated by ChEMBL | Assay Description Binding affinity to GABAA alpha-5-beta-2-gamma-2 receptor | J Med Chem 51: 3788-803 (2008) Article DOI: 10.1021/jm701433b BindingDB Entry DOI: 10.7270/Q2FQ9XH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50461710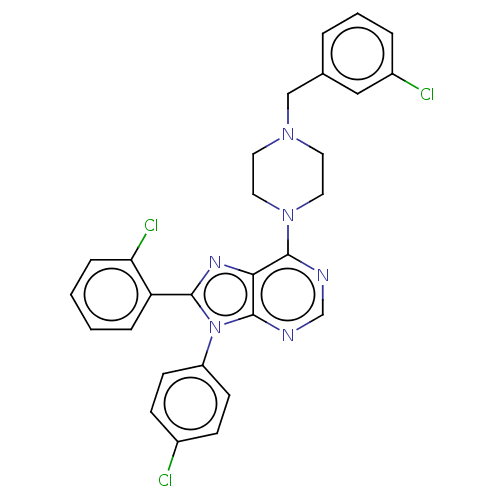 (CHEMBL4225147) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 expressed in CHOK1 cell membranes | J Med Chem 61: 4370-4385 (2018) Article DOI: 10.1021/acs.jmedchem.7b01820 BindingDB Entry DOI: 10.7270/Q20004QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50387125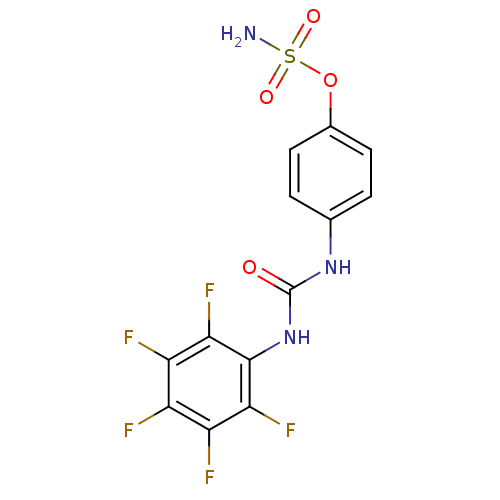 (4-ureidophenyl sulfamate ring derivative 3j | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Nationale Sup£rieure de Chimie de Montpellier Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 12 preincubated for 15 mins measured for 10 to 100 sec using phenol red indicator-based stopped flow assay | Bioorg Med Chem Lett 22: 4681-5 (2012) Article DOI: 10.1016/j.bmcl.2012.05.083 BindingDB Entry DOI: 10.7270/Q20866C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50461703 (CHEMBL4225421) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 expressed in CHOK1 cell membranes | J Med Chem 61: 4370-4385 (2018) Article DOI: 10.1021/acs.jmedchem.7b01820 BindingDB Entry DOI: 10.7270/Q20004QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50461698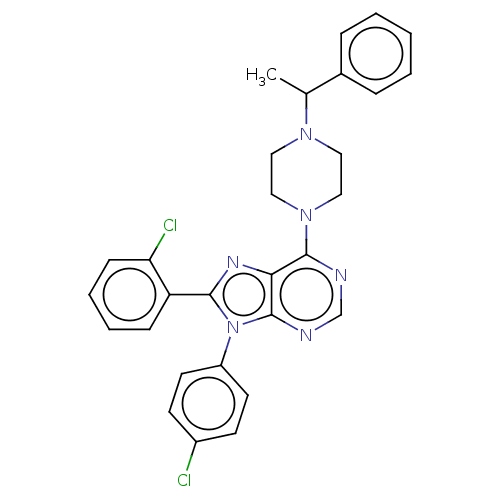 (CHEMBL4227354) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 expressed in CHOK1 cell membranes | J Med Chem 61: 4370-4385 (2018) Article DOI: 10.1021/acs.jmedchem.7b01820 BindingDB Entry DOI: 10.7270/Q20004QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (RAT) | BDBM50051562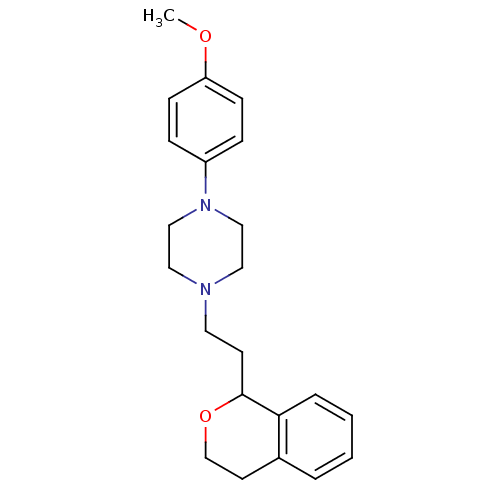 (1-(2-Isochroman-1-yl-ethyl)-4-(4-methoxy-phenyl)-p...) | Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Binding affinity of [3H]-Spiperone towards Dopamine receptor D4 expressed in cultured cells or from rat whole brain. | J Med Chem 39: 2435-7 (1996) Article DOI: 10.1021/jm960084f BindingDB Entry DOI: 10.7270/Q2H1314Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM50244262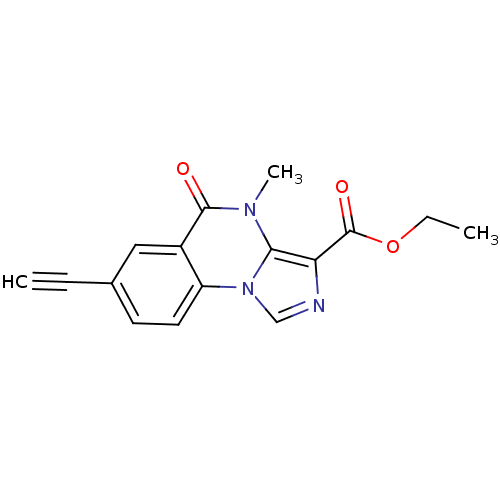 (CHEMBL471978 | ethyl 7-ethynyl-4-methyl-5-oxo-4,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Moltech Corporation Curated by ChEMBL | Assay Description Binding affinity to GABAA alpha-5-beta-3-gamma-2 receptor | J Med Chem 51: 3788-803 (2008) Article DOI: 10.1021/jm701433b BindingDB Entry DOI: 10.7270/Q2FQ9XH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50016897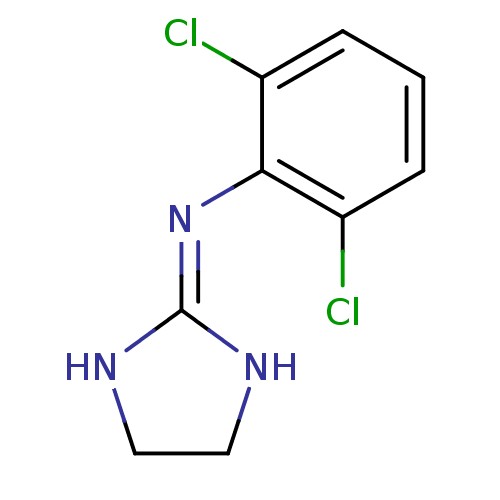 (2-(2,6-dichloroanilino)-1,3-diazacyclopentene-(2) ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibition of [3H]clonidine binding Alpha-2 adrenergic receptor of crude rat brain membrane | J Med Chem 25: 75-81 (1982) BindingDB Entry DOI: 10.7270/Q2TM7DBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50130880 (CHEMBL407196 | NT(1-13) | neurotensin | pGlu-Leu-T...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [125L]NT from rat neurotensin receptor type 1 expressed in CHOK1 cells by radioligand binding assay | Bioorg Med Chem Lett 25: 292-6 (2014) Article DOI: 10.1016/j.bmcl.2014.11.047 BindingDB Entry DOI: 10.7270/Q2M90B9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM85050 (CAS_184162-64-9 | SR 142948A | SR142948 | SR142948...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [125L]NT from rat neurotensin receptor type 1 expressed in CHOK1 cells by radioligand binding assay | Bioorg Med Chem Lett 25: 292-6 (2014) Article DOI: 10.1016/j.bmcl.2014.11.047 BindingDB Entry DOI: 10.7270/Q2M90B9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50461693 (CHEMBL4228096) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 expressed in CHOK1 cell membranes | J Med Chem 61: 4370-4385 (2018) Article DOI: 10.1021/acs.jmedchem.7b01820 BindingDB Entry DOI: 10.7270/Q20004QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50461691 (CHEMBL4224946) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 expressed in CHOK1 cell membranes | J Med Chem 61: 4370-4385 (2018) Article DOI: 10.1021/acs.jmedchem.7b01820 BindingDB Entry DOI: 10.7270/Q20004QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50461686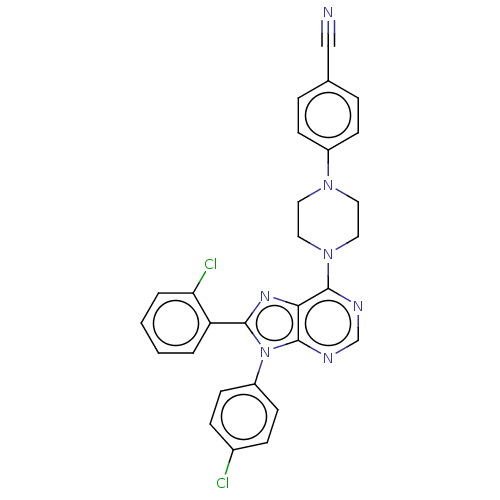 (CHEMBL4228748) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 expressed in CHOK1 cell membranes | J Med Chem 61: 4370-4385 (2018) Article DOI: 10.1021/acs.jmedchem.7b01820 BindingDB Entry DOI: 10.7270/Q20004QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50461712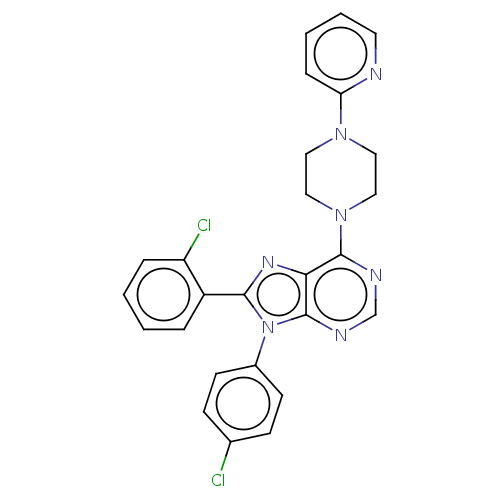 (CHEMBL4229172) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 expressed in CHOK1 cell membranes | J Med Chem 61: 4370-4385 (2018) Article DOI: 10.1021/acs.jmedchem.7b01820 BindingDB Entry DOI: 10.7270/Q20004QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50387129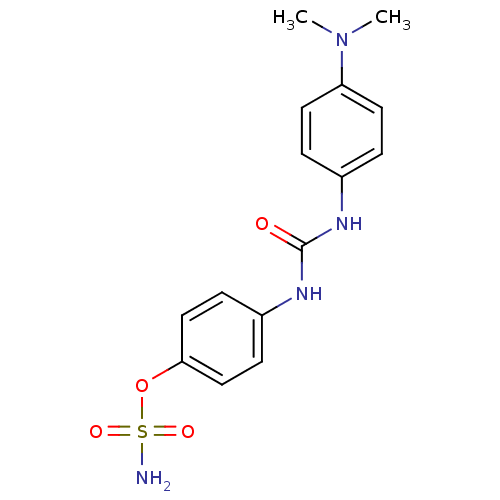 (4-ureidophenyl sulfamate ring derivative 3n | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Nationale Sup£rieure de Chimie de Montpellier Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 12 preincubated for 15 mins measured for 10 to 100 sec using phenol red indicator-based stopped flow assay | Bioorg Med Chem Lett 22: 4681-5 (2012) Article DOI: 10.1016/j.bmcl.2012.05.083 BindingDB Entry DOI: 10.7270/Q20866C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50387131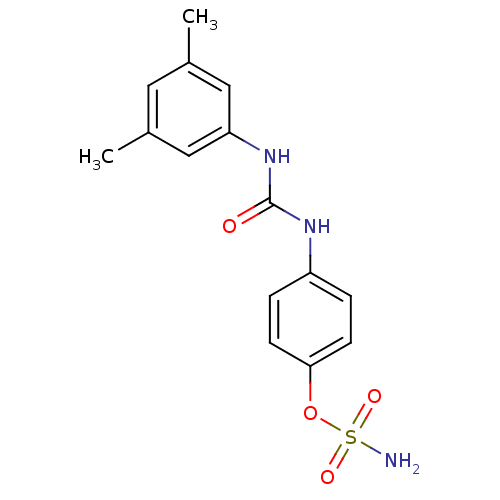 (4-ureidophenyl sulfamate ring derivative 3p | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Nationale Sup£rieure de Chimie de Montpellier Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 12 preincubated for 15 mins measured for 10 to 100 sec using phenol red indicator-based stopped flow assay | Bioorg Med Chem Lett 22: 4681-5 (2012) Article DOI: 10.1016/j.bmcl.2012.05.083 BindingDB Entry DOI: 10.7270/Q20866C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50461688 (CHEMBL4225049) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 expressed in CHOK1 cell membranes | J Med Chem 61: 4370-4385 (2018) Article DOI: 10.1021/acs.jmedchem.7b01820 BindingDB Entry DOI: 10.7270/Q20004QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50387139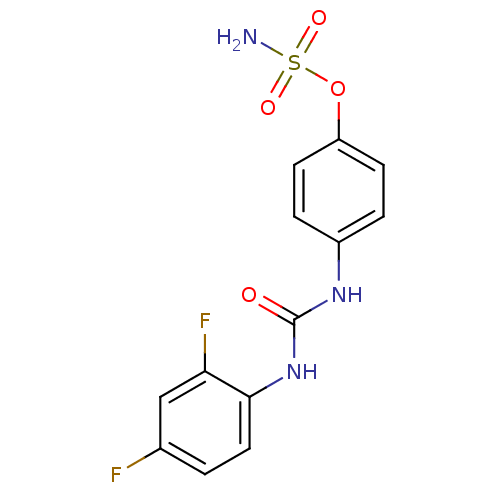 (CHEMBL2047819) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Nationale Sup£rieure de Chimie de Montpellier Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 12 preincubated for 15 mins measured for 10 to 100 sec using phenol red indicator-based stopped flow assay | Bioorg Med Chem Lett 22: 4681-5 (2012) Article DOI: 10.1016/j.bmcl.2012.05.083 BindingDB Entry DOI: 10.7270/Q20866C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50461694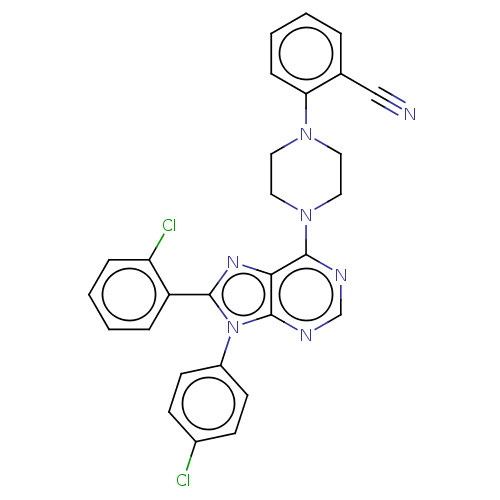 (CHEMBL4225213) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 expressed in CHOK1 cell membranes | J Med Chem 61: 4370-4385 (2018) Article DOI: 10.1021/acs.jmedchem.7b01820 BindingDB Entry DOI: 10.7270/Q20004QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50461695 (CHEMBL4226066) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 expressed in CHOK1 cell membranes | J Med Chem 61: 4370-4385 (2018) Article DOI: 10.1021/acs.jmedchem.7b01820 BindingDB Entry DOI: 10.7270/Q20004QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50461685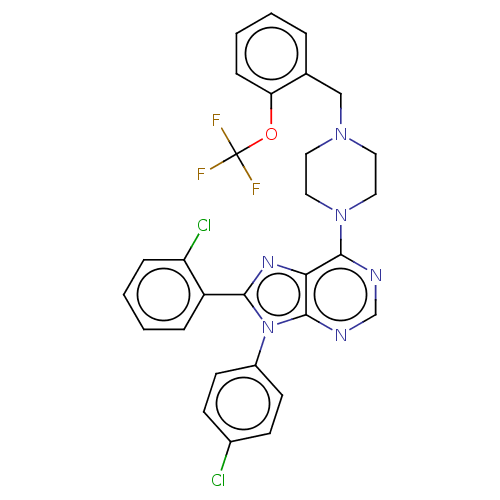 (CHEMBL4227442) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 expressed in CHOK1 cell membranes | J Med Chem 61: 4370-4385 (2018) Article DOI: 10.1021/acs.jmedchem.7b01820 BindingDB Entry DOI: 10.7270/Q20004QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50461705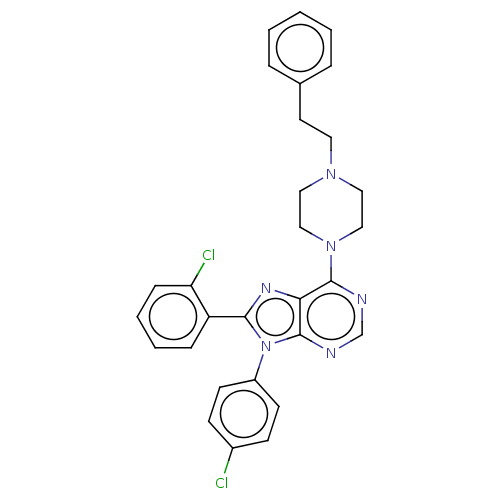 (CHEMBL4226777) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 expressed in CHOK1 cell membranes | J Med Chem 61: 4370-4385 (2018) Article DOI: 10.1021/acs.jmedchem.7b01820 BindingDB Entry DOI: 10.7270/Q20004QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50387161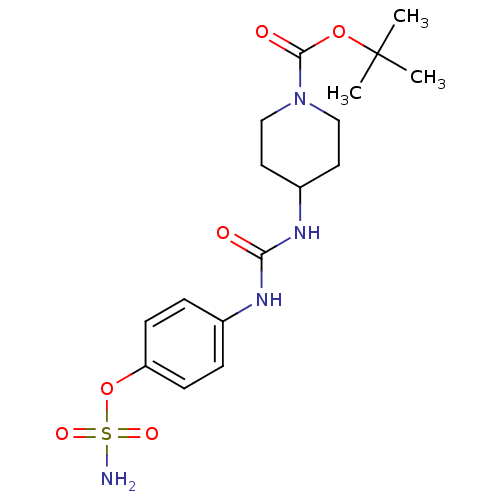 (4-ureidophenyl sulfamate ring derivative 3aw | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Nationale Sup£rieure de Chimie de Montpellier Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 12 preincubated for 15 mins measured for 10 to 100 sec using phenol red indicator-based stopped flow assay | Bioorg Med Chem Lett 22: 4681-5 (2012) Article DOI: 10.1016/j.bmcl.2012.05.083 BindingDB Entry DOI: 10.7270/Q20866C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (RAT) | BDBM50051561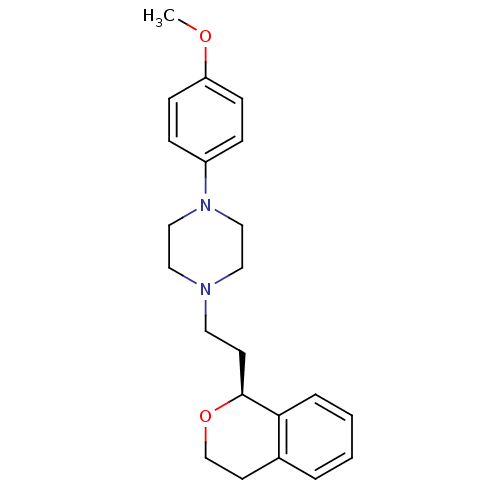 (1-((S)-2-Isochroman-1-yl-ethyl)-4-(4-methoxy-pheny...) | Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Binding affinity of [3H]-Spiperone towards Dopamine receptor D4 expressed in cultured cells or from rat whole brain. | J Med Chem 39: 2435-7 (1996) Article DOI: 10.1021/jm960084f BindingDB Entry DOI: 10.7270/Q2H1314Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-4 (Homo sapiens (Human)) | BDBM50244261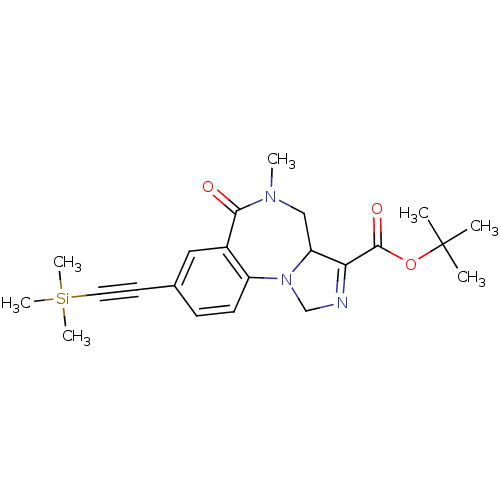 (5-Methyl-6-oxo-8-trimethylsilanylethynyl-3a,4,5,6-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Moltech Corporation Curated by ChEMBL | Assay Description Binding affinity to GABAA alpha-4-beta-3-gamma-2 receptor | J Med Chem 51: 3788-803 (2008) Article DOI: 10.1021/jm701433b BindingDB Entry DOI: 10.7270/Q2FQ9XH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM50244261 (5-Methyl-6-oxo-8-trimethylsilanylethynyl-3a,4,5,6-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Moltech Corporation Curated by ChEMBL | Assay Description Binding affinity to GABAA alpha-5-beta-3-gamma-2 receptor | J Med Chem 51: 3788-803 (2008) Article DOI: 10.1021/jm701433b BindingDB Entry DOI: 10.7270/Q2FQ9XH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM27343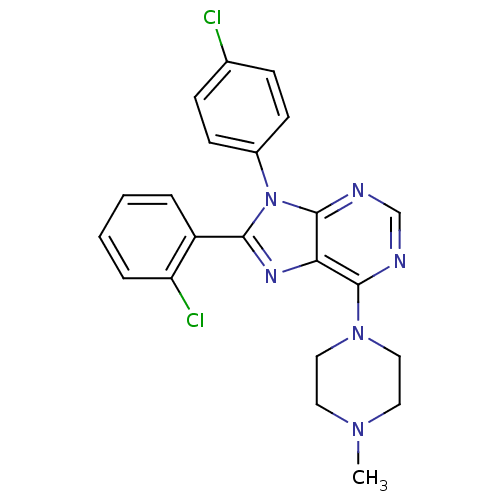 (8-(2-chlorophenyl)-9-(4-chlorophenyl)-6-(4-methylp...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 expressed in CHOK1 cell membranes | J Med Chem 61: 4370-4385 (2018) Article DOI: 10.1021/acs.jmedchem.7b01820 BindingDB Entry DOI: 10.7270/Q20004QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50387160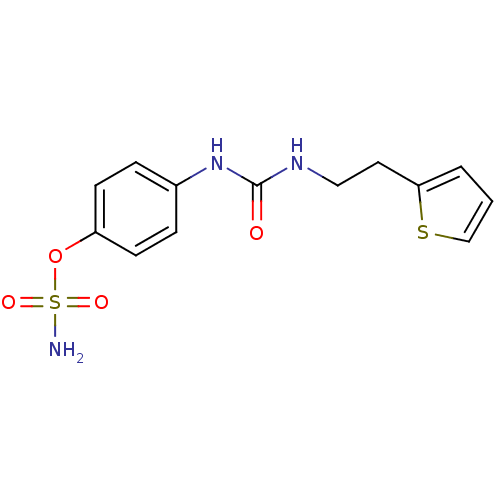 (4-ureidophenyl sulfamate ring derivative 3ay | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Nationale Sup£rieure de Chimie de Montpellier Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 12 preincubated for 15 mins measured for 10 to 100 sec using phenol red indicator-based stopped flow assay | Bioorg Med Chem Lett 22: 4681-5 (2012) Article DOI: 10.1016/j.bmcl.2012.05.083 BindingDB Entry DOI: 10.7270/Q20866C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (RAT) | BDBM85067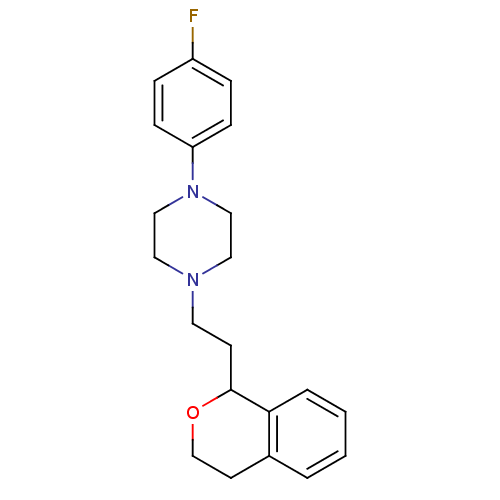 (CAS_170856-41-4 | CHEMBL81330 | PNU 96415E | PNU-9...) | Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Binding affinity of [3H]-Spiperone towards Dopamine receptor D4 expressed in cultured cells or from rat whole brain. | J Med Chem 39: 2435-7 (1996) Article DOI: 10.1021/jm960084f BindingDB Entry DOI: 10.7270/Q2H1314Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50387117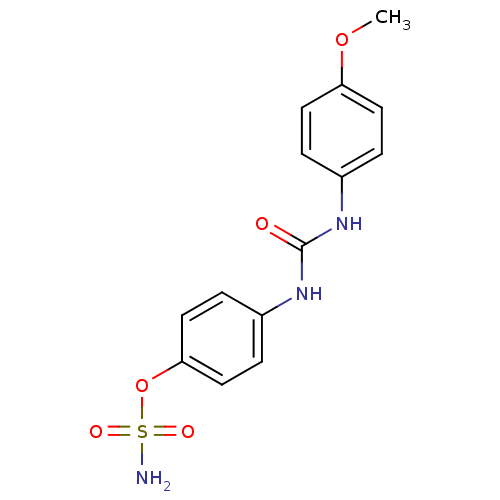 (4-ureidophenyl sulfamate ring derivative 3g | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Nationale Sup£rieure de Chimie de Montpellier Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 12 preincubated for 15 mins measured for 10 to 100 sec using phenol red indicator-based stopped flow assay | Bioorg Med Chem Lett 22: 4681-5 (2012) Article DOI: 10.1016/j.bmcl.2012.05.083 BindingDB Entry DOI: 10.7270/Q20866C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50387130 (4-ureidophenyl sulfamate ring derivative 3o | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Nationale Sup£rieure de Chimie de Montpellier Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 12 preincubated for 15 mins measured for 10 to 100 sec using phenol red indicator-based stopped flow assay | Bioorg Med Chem Lett 22: 4681-5 (2012) Article DOI: 10.1016/j.bmcl.2012.05.083 BindingDB Entry DOI: 10.7270/Q20866C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50461687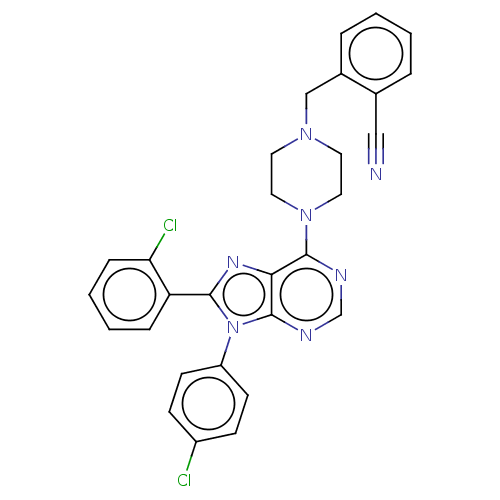 (CHEMBL4226710) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 expressed in CHOK1 cell membranes | J Med Chem 61: 4370-4385 (2018) Article DOI: 10.1021/acs.jmedchem.7b01820 BindingDB Entry DOI: 10.7270/Q20004QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50461696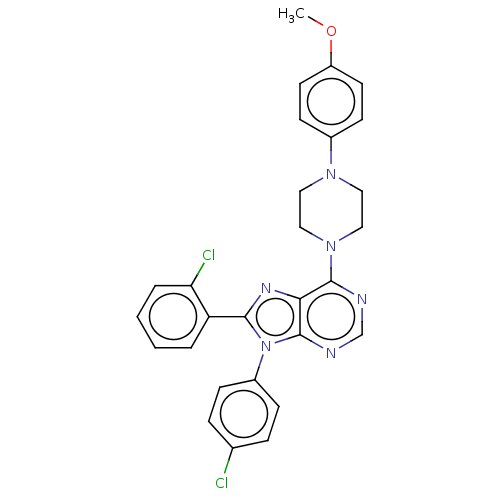 (CHEMBL4225525) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 expressed in CHOK1 cell membranes | J Med Chem 61: 4370-4385 (2018) Article DOI: 10.1021/acs.jmedchem.7b01820 BindingDB Entry DOI: 10.7270/Q20004QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50461700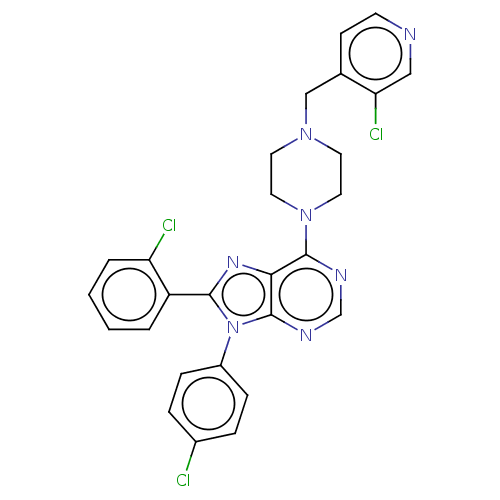 (CHEMBL4229032) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 expressed in CHOK1 cell membranes | J Med Chem 61: 4370-4385 (2018) Article DOI: 10.1021/acs.jmedchem.7b01820 BindingDB Entry DOI: 10.7270/Q20004QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50387155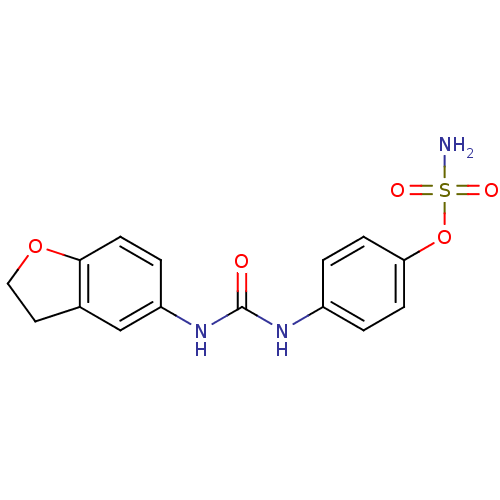 (4-ureidophenyl sulfamate ring derivative 3as | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Nationale Sup£rieure de Chimie de Montpellier Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 12 preincubated for 15 mins measured for 10 to 100 sec using phenol red indicator-based stopped flow assay | Bioorg Med Chem Lett 22: 4681-5 (2012) Article DOI: 10.1016/j.bmcl.2012.05.083 BindingDB Entry DOI: 10.7270/Q20866C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (RAT) | BDBM50051563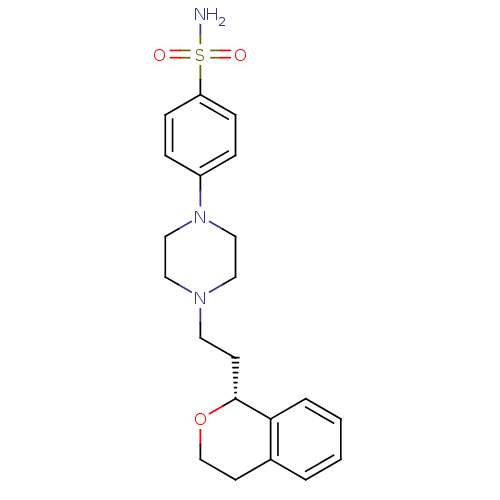 (4-[4-((R)-2-Isochroman-1-yl-ethyl)-piperazin-1-yl]...) | Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn, Inc. Curated by PDSP Ki Database | J Pharmacol Exp Ther 279: 1392-403 (1996) BindingDB Entry DOI: 10.7270/Q2SQ8XX9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2012 total ) | Next | Last >> |