 Found 433 hits with Last Name = 'henderson' and Initial = 'ja'
Found 433 hits with Last Name = 'henderson' and Initial = 'ja' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50044287
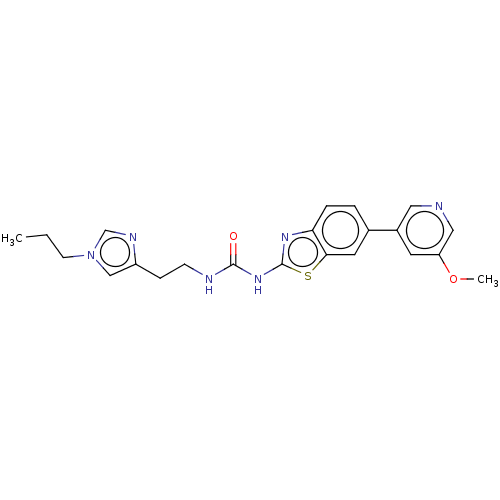
(CHEMBL3356900)Show SMILES CCCn1cnc(CCNC(=O)Nc2nc3ccc(cc3s2)-c2cncc(OC)c2)c1 Show InChI InChI=1S/C22H24N6O2S/c1-3-8-28-13-17(25-14-28)6-7-24-21(29)27-22-26-19-5-4-15(10-20(19)31-22)16-9-18(30-2)12-23-11-16/h4-5,9-14H,3,6-8H2,1-2H3,(H2,24,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274571
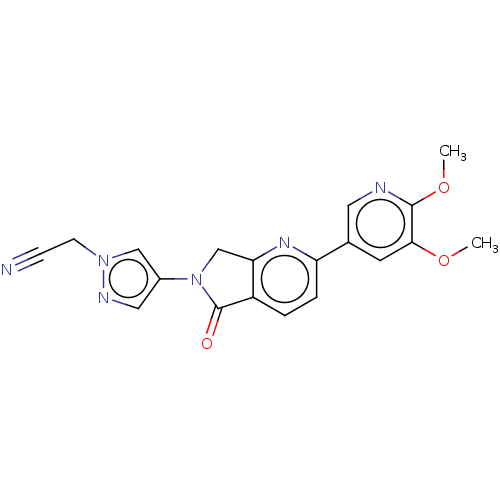
(CHEMBL4127784)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N(Cc2n1)c1cnn(CC#N)c1 Show InChI InChI=1S/C19H16N6O3/c1-27-17-7-12(8-21-18(17)28-2)15-4-3-14-16(23-15)11-25(19(14)26)13-9-22-24(10-13)6-5-20/h3-4,7-10H,6,11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274538
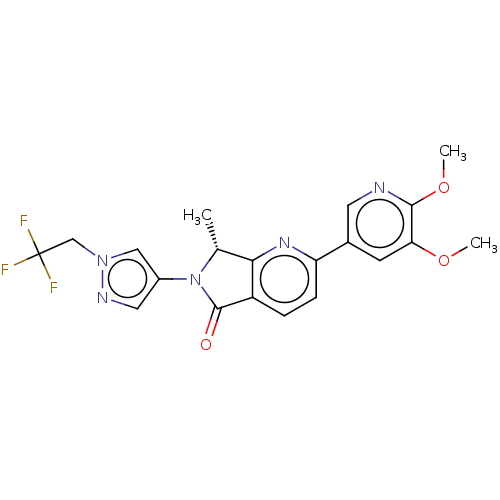
(CHEMBL4126773)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N([C@H](C)c2n1)c1cnn(CC(F)(F)F)c1 |r| Show InChI InChI=1S/C20H18F3N5O3/c1-11-17-14(19(29)28(11)13-8-25-27(9-13)10-20(21,22)23)4-5-15(26-17)12-6-16(30-2)18(31-3)24-7-12/h4-9,11H,10H2,1-3H3/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274537
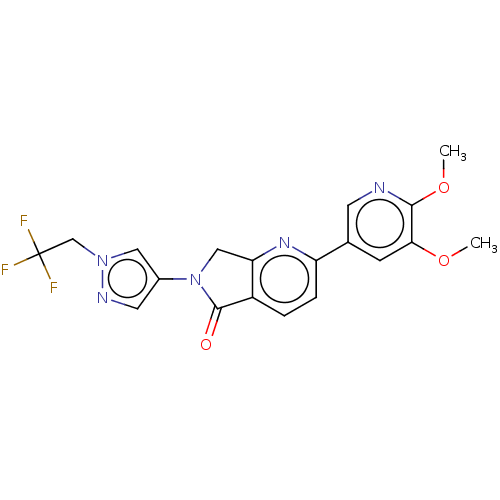
(CHEMBL4129974)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N(Cc2n1)c1cnn(CC(F)(F)F)c1 Show InChI InChI=1S/C19H16F3N5O3/c1-29-16-5-11(6-23-17(16)30-2)14-4-3-13-15(25-14)9-27(18(13)28)12-7-24-26(8-12)10-19(20,21)22/h3-8H,9-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274572
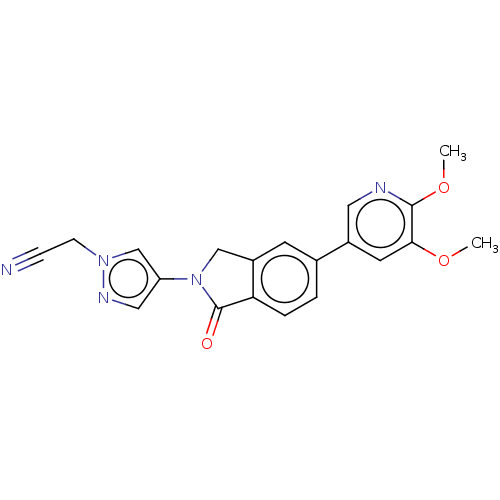
(CHEMBL4129180)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N(Cc2c1)c1cnn(CC#N)c1 Show InChI InChI=1S/C20H17N5O3/c1-27-18-8-14(9-22-19(18)28-2)13-3-4-17-15(7-13)11-25(20(17)26)16-10-23-24(12-16)6-5-21/h3-4,7-10,12H,6,11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274541
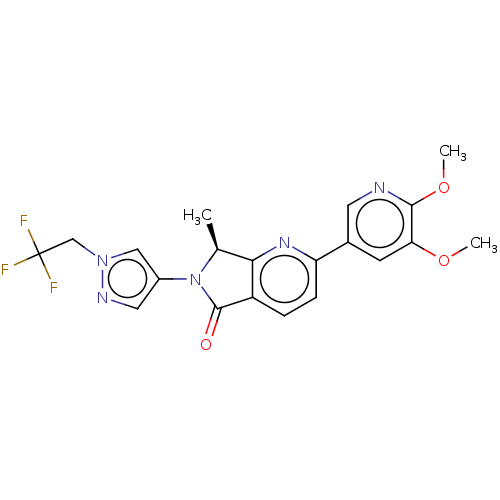
(CHEMBL4130036)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N([C@@H](C)c2n1)c1cnn(CC(F)(F)F)c1 |r| Show InChI InChI=1S/C20H18F3N5O3/c1-11-17-14(19(29)28(11)13-8-25-27(9-13)10-20(21,22)23)4-5-15(26-17)12-6-16(30-2)18(31-3)24-7-12/h4-9,11H,10H2,1-3H3/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274559

(CHEMBL4126707)Show SMILES CCn1cc(cn1)N1Cc2nc(ccc2C1=O)-c1cnc(OC)c(OC)c1 Show InChI InChI=1S/C19H19N5O3/c1-4-23-10-13(9-21-23)24-11-16-14(19(24)25)5-6-15(22-16)12-7-17(26-2)18(27-3)20-8-12/h5-10H,4,11H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274543

(CHEMBL4129251)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N(Cc2n1)c1cnn(CC(F)F)c1 Show InChI InChI=1S/C19H17F2N5O3/c1-28-16-5-11(6-22-18(16)29-2)14-4-3-13-15(24-14)9-26(19(13)27)12-7-23-25(8-12)10-17(20)21/h3-8,17H,9-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274542

(CHEMBL4127853)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N(Cc2n1)c1cnn(CC#C)c1 Show InChI InChI=1S/C20H17N5O3/c1-4-7-24-11-14(10-22-24)25-12-17-15(20(25)26)5-6-16(23-17)13-8-18(27-2)19(28-3)21-9-13/h1,5-6,8-11H,7,12H2,2-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274540

(CHEMBL4128822)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N(c3cnn(CC(F)(F)F)c3)C(C)(C)c2n1 Show InChI InChI=1S/C21H20F3N5O3/c1-20(2)17-14(19(30)29(20)13-9-26-28(10-13)11-21(22,23)24)5-6-15(27-17)12-7-16(31-3)18(32-4)25-8-12/h5-10H,11H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274560
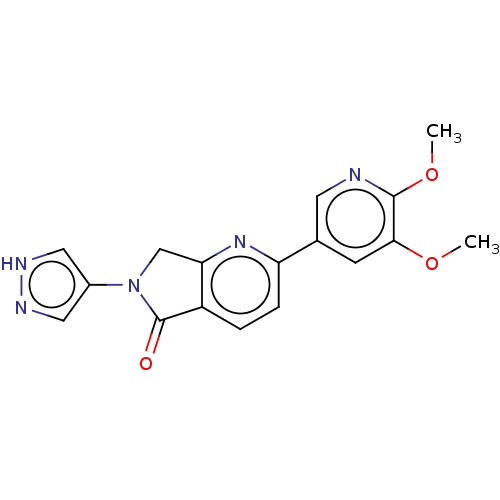
(CHEMBL4125738)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N(Cc2n1)c1cn[nH]c1 Show InChI InChI=1S/C17H15N5O3/c1-24-15-5-10(6-18-16(15)25-2)13-4-3-12-14(21-13)9-22(17(12)23)11-7-19-20-8-11/h3-8H,9H2,1-2H3,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274580

(CHEMBL4128006)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N(Cc2n1)c1cnn(CCC#N)c1 Show InChI InChI=1S/C20H18N6O3/c1-28-18-8-13(9-22-19(18)29-2)16-5-4-15-17(24-16)12-26(20(15)27)14-10-23-25(11-14)7-3-6-21/h4-5,8-11H,3,7,12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274544

(CHEMBL4126620)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N(Cc2n1)c1cnn(c1)C(C)(C)C#N Show InChI InChI=1S/C21H20N6O3/c1-21(2,12-22)27-10-14(9-24-27)26-11-17-15(20(26)28)5-6-16(25-17)13-7-18(29-3)19(30-4)23-8-13/h5-10H,11H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274573
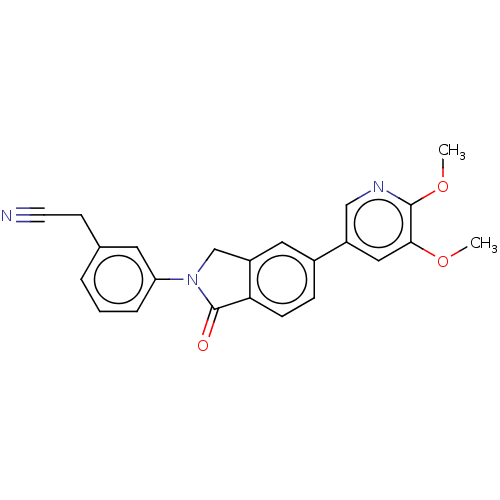
(CHEMBL4126829)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N(Cc2c1)c1cccc(CC#N)c1 Show InChI InChI=1S/C23H19N3O3/c1-28-21-12-17(13-25-22(21)29-2)16-6-7-20-18(11-16)14-26(23(20)27)19-5-3-4-15(10-19)8-9-24/h3-7,10-13H,8,14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274581

(CHEMBL4129025)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N(Cc2n1)c1cnn(C)c1 Show InChI InChI=1S/C18H17N5O3/c1-22-9-12(8-20-22)23-10-15-13(18(23)24)4-5-14(21-15)11-6-16(25-2)17(26-3)19-7-11/h4-9H,10H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274574

(CHEMBL4128211)Show SMILES COc1ccc(cc1OC)-c1ccc2C(=O)N(Cc2c1)c1cccc(CC#N)c1 Show InChI InChI=1S/C24H20N2O3/c1-28-22-9-7-18(14-23(22)29-2)17-6-8-21-19(13-17)15-26(24(21)27)20-5-3-4-16(12-20)10-11-25/h3-9,12-14H,10,15H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
DNA-dependent protein kinase catalytic subunit
(Homo sapiens (Human)) | BDBM50274538

(CHEMBL4126773)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N([C@H](C)c2n1)c1cnn(CC(F)(F)F)c1 |r| Show InChI InChI=1S/C20H18F3N5O3/c1-11-17-14(19(29)28(11)13-8-25-27(9-13)10-20(21,22)23)4-5-15(26-17)12-6-16(30-2)18(31-3)24-7-12/h4-9,11H,10H2,1-3H3/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of DNA-PK (unknown origin) using EPPLSQEAFADLWKKK as substrate after 15 mins in presence of [33P-ATP] by radiometric method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274538

(CHEMBL4126773)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N([C@H](C)c2n1)c1cnn(CC(F)(F)F)c1 |r| Show InChI InChI=1S/C20H18F3N5O3/c1-11-17-14(19(29)28(11)13-8-25-27(9-13)10-20(21,22)23)4-5-15(26-17)12-6-16(30-2)18(31-3)24-7-12/h4-9,11H,10H2,1-3H3/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma in TNFalpha primed human neutrophils assessed as inhibition of fMLP-induced ROS generation preincubated for 30 to 60 mins fol... |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274538

(CHEMBL4126773)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N([C@H](C)c2n1)c1cnn(CC(F)(F)F)c1 |r| Show InChI InChI=1S/C20H18F3N5O3/c1-11-17-14(19(29)28(11)13-8-25-27(9-13)10-20(21,22)23)4-5-15(26-17)12-6-16(30-2)18(31-3)24-7-12/h4-9,11H,10H2,1-3H3/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma in human THP1 cells assessed as inhibition of MCP1-induced Akt phosphorylation at Ser-47 residue preincubated for 1 hr follow... |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274541

(CHEMBL4130036)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N([C@@H](C)c2n1)c1cnn(CC(F)(F)F)c1 |r| Show InChI InChI=1S/C20H18F3N5O3/c1-11-17-14(19(29)28(11)13-8-25-27(9-13)10-20(21,22)23)4-5-15(26-17)12-6-16(30-2)18(31-3)24-7-12/h4-9,11H,10H2,1-3H3/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma in human THP1 cells assessed as inhibition of MCP1-induced Akt phosphorylation at Ser-47 residue preincubated for 1 hr follow... |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol-4,5-bisphosphate 3-kinase
(Rattus norvegicus) | BDBM50274538

(CHEMBL4126773)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N([C@H](C)c2n1)c1cnn(CC(F)(F)F)c1 |r| Show InChI InChI=1S/C20H18F3N5O3/c1-11-17-14(19(29)28(11)13-8-25-27(9-13)10-20(21,22)23)4-5-15(26-17)12-6-16(30-2)18(31-3)24-7-12/h4-9,11H,10H2,1-3H3/t11-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma in rat spleenocytes assessed as inhibition of MCP1-induced Akt phosphorylation at Ser-47 residue preincubated for 1 hr follow... |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274537

(CHEMBL4129974)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N(Cc2n1)c1cnn(CC(F)(F)F)c1 Show InChI InChI=1S/C19H16F3N5O3/c1-29-16-5-11(6-23-17(16)30-2)14-4-3-13-15(25-14)9-27(18(13)28)12-7-24-26(8-12)10-19(20,21)22/h3-8H,9-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma in human THP1 cells assessed as inhibition of MCP1-induced Akt phosphorylation at Ser-47 residue preincubated for 1 hr follow... |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274540

(CHEMBL4128822)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N(c3cnn(CC(F)(F)F)c3)C(C)(C)c2n1 Show InChI InChI=1S/C21H20F3N5O3/c1-20(2)17-14(19(30)29(20)13-9-26-28(10-13)11-21(22,23)24)5-6-15(27-17)12-7-16(31-3)18(32-4)25-8-12/h5-10H,11H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma in human THP1 cells assessed as inhibition of MCP1-induced Akt phosphorylation at Ser-47 residue preincubated for 1 hr follow... |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274538

(CHEMBL4126773)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N([C@H](C)c2n1)c1cnn(CC(F)(F)F)c1 |r| Show InChI InChI=1S/C20H18F3N5O3/c1-11-17-14(19(29)28(11)13-8-25-27(9-13)10-20(21,22)23)4-5-15(26-17)12-6-16(30-2)18(31-3)24-7-12/h4-9,11H,10H2,1-3H3/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma in TNFalpha primed human whole blood assessed as inhibition of fMLP-induced ROS generation preincubated for 30 to 60 mins fol... |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50061235

(CHEMBL3393730)Show SMILES [H][C@]1(C[C@H](C)CN1C(=O)[C@@H](NC(=O)OC)C(C)C)c1ncc([nH]1)-c1ccc(cc1)-c1ccc(s1)-c1cc2[nH]c(nc2s1)[C@]1([H])C[C@H](C)CN1C(=O)[C@@H](NC(=O)OC)C(C)C |r| Show InChI InChI=1S/C42H50N8O6S2/c1-21(2)34(46-41(53)55-7)39(51)49-19-23(5)15-29(49)36-43-18-28(45-36)25-9-11-26(12-10-25)31-13-14-32(57-31)33-17-27-38(58-33)48-37(44-27)30-16-24(6)20-50(30)40(52)35(22(3)4)47-42(54)56-8/h9-14,17-18,21-24,29-30,34-35H,15-16,19-20H2,1-8H3,(H,46,53)(H,47,54)/b28-25-,31-26-,33-32+/t23-,24-,29-,30-,34-,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 25: 940-3 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.044
BindingDB Entry DOI: 10.7270/Q2SX6FWC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061235

(CHEMBL3393730)Show SMILES [H][C@]1(C[C@H](C)CN1C(=O)[C@@H](NC(=O)OC)C(C)C)c1ncc([nH]1)-c1ccc(cc1)-c1ccc(s1)-c1cc2[nH]c(nc2s1)[C@]1([H])C[C@H](C)CN1C(=O)[C@@H](NC(=O)OC)C(C)C |r| Show InChI InChI=1S/C42H50N8O6S2/c1-21(2)34(46-41(53)55-7)39(51)49-19-23(5)15-29(49)36-43-18-28(45-36)25-9-11-26(12-10-25)31-13-14-32(57-31)33-17-27-38(58-33)48-37(44-27)30-16-24(6)20-50(30)40(52)35(22(3)4)47-42(54)56-8/h9-14,17-18,21-24,29-30,34-35H,15-16,19-20H2,1-8H3,(H,46,53)(H,47,54)/b28-25-,31-26-,33-32+/t23-,24-,29-,30-,34-,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by Q-patch assay |
Bioorg Med Chem Lett 25: 940-3 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.044
BindingDB Entry DOI: 10.7270/Q2SX6FWC |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50101586
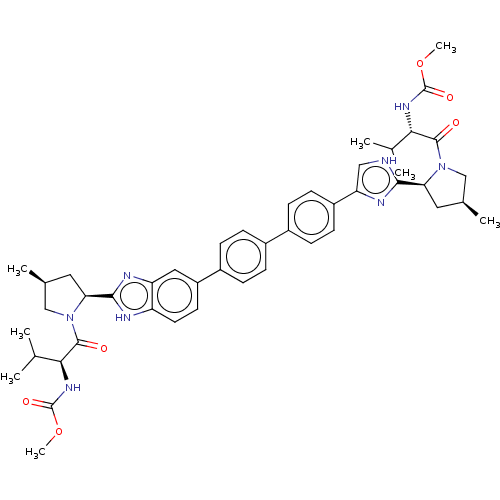
(CHEMBL3394868)Show SMILES COC(=O)N[C@@H](C(C)C)C(=O)N1C[C@@H](C)C[C@H]1c1nc(c[nH]1)-c1ccc(cc1)-c1ccc(cc1)-c1ccc2[nH]c(nc2c1)[C@@H]1C[C@H](C)CN1C(=O)[C@@H](NC(=O)OC)C(C)C |r| Show InChI InChI=1S/C46H54N8O6/c1-25(2)39(51-45(57)59-7)43(55)53-23-27(5)19-37(53)41-47-22-36(50-41)32-15-13-30(14-16-32)29-9-11-31(12-10-29)33-17-18-34-35(21-33)49-42(48-34)38-20-28(6)24-54(38)44(56)40(26(3)4)52-46(58)60-8/h9-18,21-22,25-28,37-40H,19-20,23-24H2,1-8H3,(H,51,57)(H,52,58)/b30-29-,33-31-,36-32-/t27-,28-,37-,38-,39-,40-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 25: 944-7 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.045
BindingDB Entry DOI: 10.7270/Q208673W |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50101586

(CHEMBL3394868)Show SMILES COC(=O)N[C@@H](C(C)C)C(=O)N1C[C@@H](C)C[C@H]1c1nc(c[nH]1)-c1ccc(cc1)-c1ccc(cc1)-c1ccc2[nH]c(nc2c1)[C@@H]1C[C@H](C)CN1C(=O)[C@@H](NC(=O)OC)C(C)C |r| Show InChI InChI=1S/C46H54N8O6/c1-25(2)39(51-45(57)59-7)43(55)53-23-27(5)19-37(53)41-47-22-36(50-41)32-15-13-30(14-16-32)29-9-11-31(12-10-29)33-17-18-34-35(21-33)49-42(48-34)38-20-28(6)24-54(38)44(56)40(26(3)4)52-46(58)60-8/h9-18,21-22,25-28,37-40H,19-20,23-24H2,1-8H3,(H,51,57)(H,52,58)/b30-29-,33-31-,36-32-/t27-,28-,37-,38-,39-,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 25: 944-7 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.045
BindingDB Entry DOI: 10.7270/Q208673W |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50101586

(CHEMBL3394868)Show SMILES COC(=O)N[C@@H](C(C)C)C(=O)N1C[C@@H](C)C[C@H]1c1nc(c[nH]1)-c1ccc(cc1)-c1ccc(cc1)-c1ccc2[nH]c(nc2c1)[C@@H]1C[C@H](C)CN1C(=O)[C@@H](NC(=O)OC)C(C)C |r| Show InChI InChI=1S/C46H54N8O6/c1-25(2)39(51-45(57)59-7)43(55)53-23-27(5)19-37(53)41-47-22-36(50-41)32-15-13-30(14-16-32)29-9-11-31(12-10-29)33-17-18-34-35(21-33)49-42(48-34)38-20-28(6)24-54(38)44(56)40(26(3)4)52-46(58)60-8/h9-18,21-22,25-28,37-40H,19-20,23-24H2,1-8H3,(H,51,57)(H,52,58)/b30-29-,33-31-,36-32-/t27-,28-,37-,38-,39-,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 25: 944-7 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.045
BindingDB Entry DOI: 10.7270/Q208673W |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50061236

(CHEMBL3120454)Show SMILES COC(=O)N[C@@H](C(C)C)C(=O)N1C[C@@H](C)C[C@H]1c1ncc([nH]1)-c1ccc(cc1)-c1ccc(cc1)-c1cc2[nH]c(nc2s1)[C@@H]1C[C@H](C)CN1C(=O)[C@@H](NC(=O)OC)C(C)C |r| Show InChI InChI=1S/C44H52N8O6S/c1-23(2)36(48-43(55)57-7)41(53)51-21-25(5)17-33(51)38-45-20-32(47-38)29-13-9-27(10-14-29)28-11-15-30(16-12-28)35-19-31-40(59-35)50-39(46-31)34-18-26(6)22-52(34)42(54)37(24(3)4)49-44(56)58-8/h9-16,19-20,23-26,33-34,36-37H,17-18,21-22H2,1-8H3,(H,48,55)(H,49,56)/b28-27-,32-29-,35-30+/t25-,26-,33-,34-,36-,37-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 25: 940-3 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.044
BindingDB Entry DOI: 10.7270/Q2SX6FWC |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50061235

(CHEMBL3393730)Show SMILES [H][C@]1(C[C@H](C)CN1C(=O)[C@@H](NC(=O)OC)C(C)C)c1ncc([nH]1)-c1ccc(cc1)-c1ccc(s1)-c1cc2[nH]c(nc2s1)[C@]1([H])C[C@H](C)CN1C(=O)[C@@H](NC(=O)OC)C(C)C |r| Show InChI InChI=1S/C42H50N8O6S2/c1-21(2)34(46-41(53)55-7)39(51)49-19-23(5)15-29(49)36-43-18-28(45-36)25-9-11-26(12-10-25)31-13-14-32(57-31)33-17-27-38(58-33)48-37(44-27)30-16-24(6)20-50(30)40(52)35(22(3)4)47-42(54)56-8/h9-14,17-18,21-24,29-30,34-35H,15-16,19-20H2,1-8H3,(H,46,53)(H,47,54)/b28-25-,31-26-,33-32+/t23-,24-,29-,30-,34-,35-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 25: 940-3 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.044
BindingDB Entry DOI: 10.7270/Q2SX6FWC |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50061236

(CHEMBL3120454)Show SMILES COC(=O)N[C@@H](C(C)C)C(=O)N1C[C@@H](C)C[C@H]1c1ncc([nH]1)-c1ccc(cc1)-c1ccc(cc1)-c1cc2[nH]c(nc2s1)[C@@H]1C[C@H](C)CN1C(=O)[C@@H](NC(=O)OC)C(C)C |r| Show InChI InChI=1S/C44H52N8O6S/c1-23(2)36(48-43(55)57-7)41(53)51-21-25(5)17-33(51)38-45-20-32(47-38)29-13-9-27(10-14-29)28-11-15-30(16-12-28)35-19-31-40(59-35)50-39(46-31)34-18-26(6)22-52(34)42(54)37(24(3)4)49-44(56)58-8/h9-16,19-20,23-26,33-34,36-37H,17-18,21-22H2,1-8H3,(H,48,55)(H,49,56)/b28-27-,32-29-,35-30+/t25-,26-,33-,34-,36-,37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 25: 940-3 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.044
BindingDB Entry DOI: 10.7270/Q2SX6FWC |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50061236

(CHEMBL3120454)Show SMILES COC(=O)N[C@@H](C(C)C)C(=O)N1C[C@@H](C)C[C@H]1c1ncc([nH]1)-c1ccc(cc1)-c1ccc(cc1)-c1cc2[nH]c(nc2s1)[C@@H]1C[C@H](C)CN1C(=O)[C@@H](NC(=O)OC)C(C)C |r| Show InChI InChI=1S/C44H52N8O6S/c1-23(2)36(48-43(55)57-7)41(53)51-21-25(5)17-33(51)38-45-20-32(47-38)29-13-9-27(10-14-29)28-11-15-30(16-12-28)35-19-31-40(59-35)50-39(46-31)34-18-26(6)22-52(34)42(54)37(24(3)4)49-44(56)58-8/h9-16,19-20,23-26,33-34,36-37H,17-18,21-22H2,1-8H3,(H,48,55)(H,49,56)/b28-27-,32-29-,35-30+/t25-,26-,33-,34-,36-,37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 25: 940-3 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.044
BindingDB Entry DOI: 10.7270/Q2SX6FWC |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50101586

(CHEMBL3394868)Show SMILES COC(=O)N[C@@H](C(C)C)C(=O)N1C[C@@H](C)C[C@H]1c1nc(c[nH]1)-c1ccc(cc1)-c1ccc(cc1)-c1ccc2[nH]c(nc2c1)[C@@H]1C[C@H](C)CN1C(=O)[C@@H](NC(=O)OC)C(C)C |r| Show InChI InChI=1S/C46H54N8O6/c1-25(2)39(51-45(57)59-7)43(55)53-23-27(5)19-37(53)41-47-22-36(50-41)32-15-13-30(14-16-32)29-9-11-31(12-10-29)33-17-18-34-35(21-33)49-42(48-34)38-20-28(6)24-54(38)44(56)40(26(3)4)52-46(58)60-8/h9-18,21-22,25-28,37-40H,19-20,23-24H2,1-8H3,(H,51,57)(H,52,58)/b30-29-,33-31-,36-32-/t27-,28-,37-,38-,39-,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
Bioorg Med Chem Lett 25: 944-7 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.045
BindingDB Entry DOI: 10.7270/Q208673W |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50101586

(CHEMBL3394868)Show SMILES COC(=O)N[C@@H](C(C)C)C(=O)N1C[C@@H](C)C[C@H]1c1nc(c[nH]1)-c1ccc(cc1)-c1ccc(cc1)-c1ccc2[nH]c(nc2c1)[C@@H]1C[C@H](C)CN1C(=O)[C@@H](NC(=O)OC)C(C)C |r| Show InChI InChI=1S/C46H54N8O6/c1-25(2)39(51-45(57)59-7)43(55)53-23-27(5)19-37(53)41-47-22-36(50-41)32-15-13-30(14-16-32)29-9-11-31(12-10-29)33-17-18-34-35(21-33)49-42(48-34)38-20-28(6)24-54(38)44(56)40(26(3)4)52-46(58)60-8/h9-18,21-22,25-28,37-40H,19-20,23-24H2,1-8H3,(H,51,57)(H,52,58)/b30-29-,33-31-,36-32-/t27-,28-,37-,38-,39-,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 25: 944-7 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.045
BindingDB Entry DOI: 10.7270/Q208673W |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50101586

(CHEMBL3394868)Show SMILES COC(=O)N[C@@H](C(C)C)C(=O)N1C[C@@H](C)C[C@H]1c1nc(c[nH]1)-c1ccc(cc1)-c1ccc(cc1)-c1ccc2[nH]c(nc2c1)[C@@H]1C[C@H](C)CN1C(=O)[C@@H](NC(=O)OC)C(C)C |r| Show InChI InChI=1S/C46H54N8O6/c1-25(2)39(51-45(57)59-7)43(55)53-23-27(5)19-37(53)41-47-22-36(50-41)32-15-13-30(14-16-32)29-9-11-31(12-10-29)33-17-18-34-35(21-33)49-42(48-34)38-20-28(6)24-54(38)44(56)40(26(3)4)52-46(58)60-8/h9-18,21-22,25-28,37-40H,19-20,23-24H2,1-8H3,(H,51,57)(H,52,58)/b30-29-,33-31-,36-32-/t27-,28-,37-,38-,39-,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel by planar technique |
Bioorg Med Chem Lett 25: 944-7 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.045
BindingDB Entry DOI: 10.7270/Q208673W |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50101586

(CHEMBL3394868)Show SMILES COC(=O)N[C@@H](C(C)C)C(=O)N1C[C@@H](C)C[C@H]1c1nc(c[nH]1)-c1ccc(cc1)-c1ccc(cc1)-c1ccc2[nH]c(nc2c1)[C@@H]1C[C@H](C)CN1C(=O)[C@@H](NC(=O)OC)C(C)C |r| Show InChI InChI=1S/C46H54N8O6/c1-25(2)39(51-45(57)59-7)43(55)53-23-27(5)19-37(53)41-47-22-36(50-41)32-15-13-30(14-16-32)29-9-11-31(12-10-29)33-17-18-34-35(21-33)49-42(48-34)38-20-28(6)24-54(38)44(56)40(26(3)4)52-46(58)60-8/h9-18,21-22,25-28,37-40H,19-20,23-24H2,1-8H3,(H,51,57)(H,52,58)/b30-29-,33-31-,36-32-/t27-,28-,37-,38-,39-,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel by QPatch technique |
Bioorg Med Chem Lett 25: 944-7 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.045
BindingDB Entry DOI: 10.7270/Q208673W |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50061235

(CHEMBL3393730)Show SMILES [H][C@]1(C[C@H](C)CN1C(=O)[C@@H](NC(=O)OC)C(C)C)c1ncc([nH]1)-c1ccc(cc1)-c1ccc(s1)-c1cc2[nH]c(nc2s1)[C@]1([H])C[C@H](C)CN1C(=O)[C@@H](NC(=O)OC)C(C)C |r| Show InChI InChI=1S/C42H50N8O6S2/c1-21(2)34(46-41(53)55-7)39(51)49-19-23(5)15-29(49)36-43-18-28(45-36)25-9-11-26(12-10-25)31-13-14-32(57-31)33-17-27-38(58-33)48-37(44-27)30-16-24(6)20-50(30)40(52)35(22(3)4)47-42(54)56-8/h9-14,17-18,21-24,29-30,34-35H,15-16,19-20H2,1-8H3,(H,46,53)(H,47,54)/b28-25-,31-26-,33-32+/t23-,24-,29-,30-,34-,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 25: 940-3 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.044
BindingDB Entry DOI: 10.7270/Q2SX6FWC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50274538

(CHEMBL4126773)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N([C@H](C)c2n1)c1cnn(CC(F)(F)F)c1 |r| Show InChI InChI=1S/C20H18F3N5O3/c1-11-17-14(19(29)28(11)13-8-25-27(9-13)10-20(21,22)23)4-5-15(26-17)12-6-16(30-2)18(31-3)24-7-12/h4-9,11H,10H2,1-3H3/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061236

(CHEMBL3120454)Show SMILES COC(=O)N[C@@H](C(C)C)C(=O)N1C[C@@H](C)C[C@H]1c1ncc([nH]1)-c1ccc(cc1)-c1ccc(cc1)-c1cc2[nH]c(nc2s1)[C@@H]1C[C@H](C)CN1C(=O)[C@@H](NC(=O)OC)C(C)C |r| Show InChI InChI=1S/C44H52N8O6S/c1-23(2)36(48-43(55)57-7)41(53)51-21-25(5)17-33(51)38-45-20-32(47-38)29-13-9-27(10-14-29)28-11-15-30(16-12-28)35-19-31-40(59-35)50-39(46-31)34-18-26(6)22-52(34)42(54)37(24(3)4)49-44(56)58-8/h9-16,19-20,23-26,33-34,36-37H,17-18,21-22H2,1-8H3,(H,48,55)(H,49,56)/b28-27-,32-29-,35-30+/t25-,26-,33-,34-,36-,37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by Q-patch assay |
Bioorg Med Chem Lett 25: 940-3 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.044
BindingDB Entry DOI: 10.7270/Q2SX6FWC |
More data for this
Ligand-Target Pair | |
DNA damage-binding protein 1/Protein cereblon
(Homo sapiens (Human)) | BDBM575232
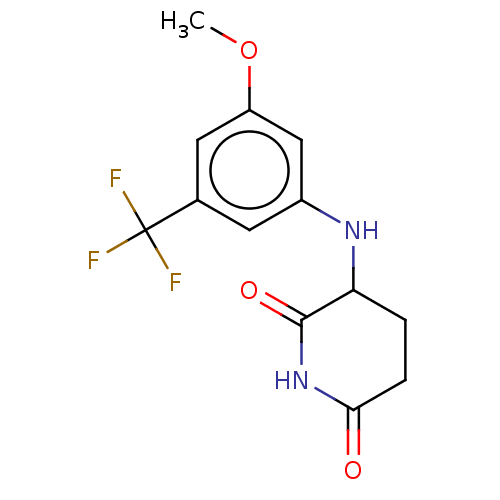
(US11459335, Compound 29 | US20230279023, Compound ...) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20869F6 |
More data for this
Ligand-Target Pair | |
DNA damage-binding protein 1/Protein cereblon
(Homo sapiens (Human)) | BDBM575233
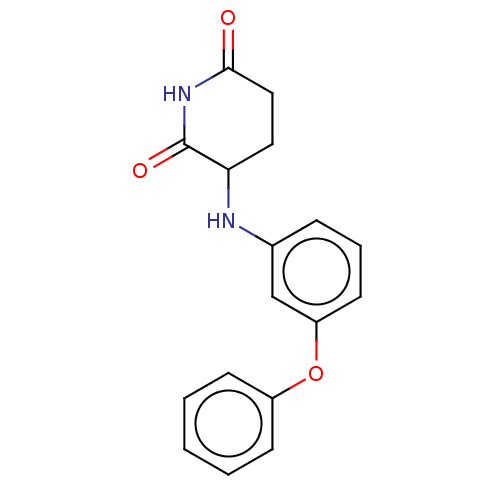
(US11459335, Compound 30 | US20230279023, Compound ...) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20869F6 |
More data for this
Ligand-Target Pair | |
DNA damage-binding protein 1/Protein cereblon
(Homo sapiens (Human)) | BDBM575234

(US11459335, Compound 31 | US20230279023, Compound ...) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20869F6 |
More data for this
Ligand-Target Pair | |
DNA damage-binding protein 1/Protein cereblon
(Homo sapiens (Human)) | BDBM575235
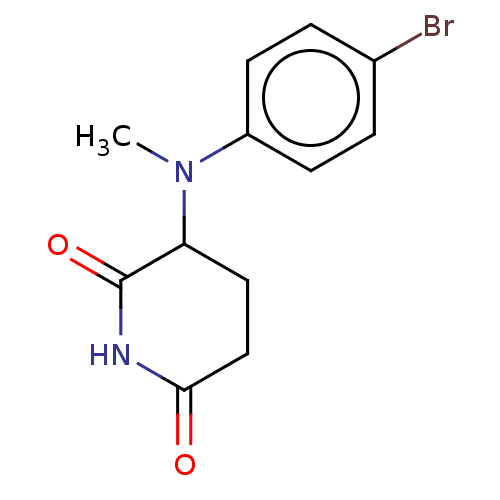
(US11459335, Compound 32 | US20230279023, Compound ...) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20869F6 |
More data for this
Ligand-Target Pair | |
DNA damage-binding protein 1/Protein cereblon
(Homo sapiens (Human)) | BDBM575236
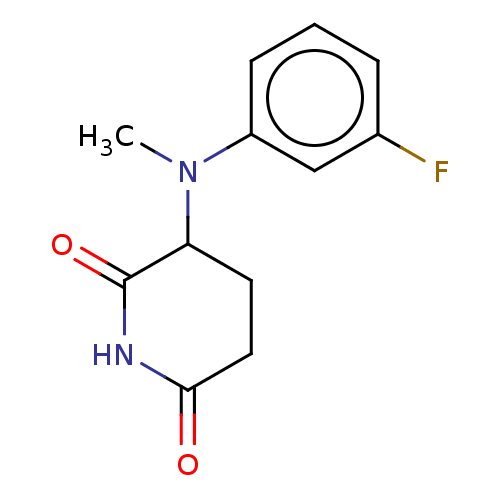
(US11459335, Compound 33 | US20230279023, Compound ...) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20869F6 |
More data for this
Ligand-Target Pair | |
DNA damage-binding protein 1/Protein cereblon
(Homo sapiens (Human)) | BDBM575237
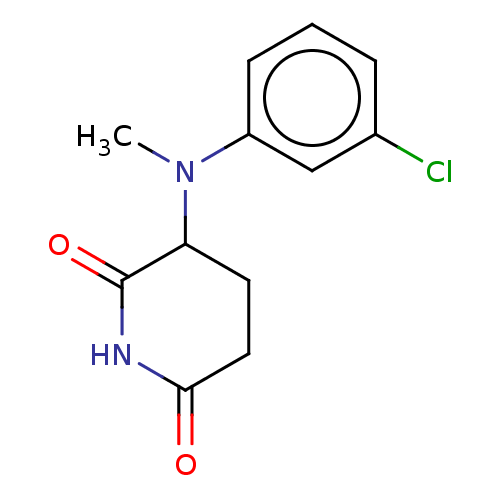
(US11459335, Compound 34 | US20230279023, Compound ...) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20869F6 |
More data for this
Ligand-Target Pair | |
DNA damage-binding protein 1/Protein cereblon
(Homo sapiens (Human)) | BDBM575238

(US11459335, Compound 35 | US20230279023, Compound ...) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20869F6 |
More data for this
Ligand-Target Pair | |
DNA damage-binding protein 1/Protein cereblon
(Homo sapiens (Human)) | BDBM575239
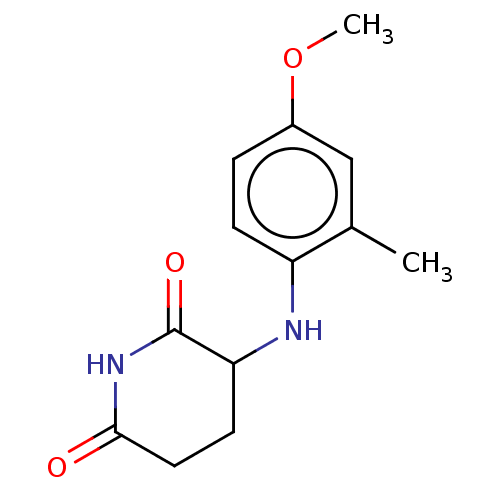
(US11459335, Compound 36 | US20230279023, Compound ...) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20869F6 |
More data for this
Ligand-Target Pair | |
DNA damage-binding protein 1/Protein cereblon
(Homo sapiens (Human)) | BDBM575240
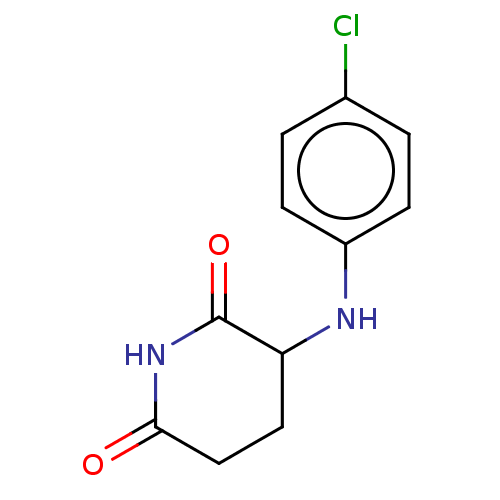
(US11459335, Compound 37 | US20230279023, Compound ...) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20869F6 |
More data for this
Ligand-Target Pair | |
DNA damage-binding protein 1/Protein cereblon
(Homo sapiens (Human)) | BDBM575241
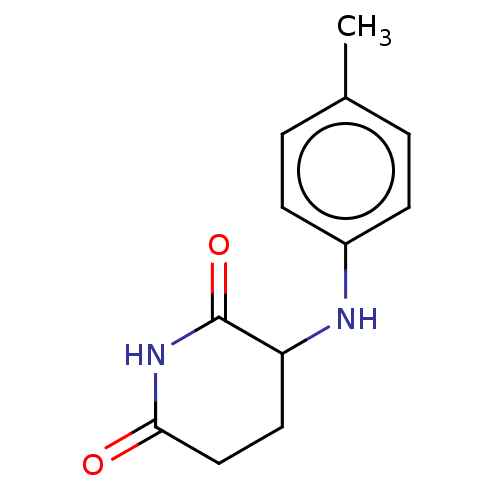
(US11459335, Compound 38 | US20230279023, Compound ...) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20869F6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data