

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50143282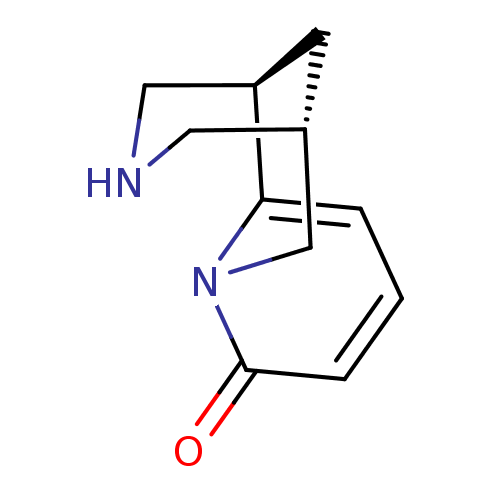 ((-)-cytisine | (1R,5S)-1,2,3,4,5,6-Hexahydro-1,5-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to nicotinic acetylcholine receptor alpha1 beta gamma delta of electroplax | J Med Chem 48: 3474-7 (2005) Article DOI: 10.1021/jm050069n BindingDB Entry DOI: 10.7270/Q2N87BJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50166908 (5,8,14-triazatetracyclo[10.3.1.02,11.04,9]hexadeca...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 322 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to nicotinic acetylcholine receptor alpha-7 subunit in rat GH4C1 cells | J Med Chem 48: 3474-7 (2005) Article DOI: 10.1021/jm050069n BindingDB Entry DOI: 10.7270/Q2N87BJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50166909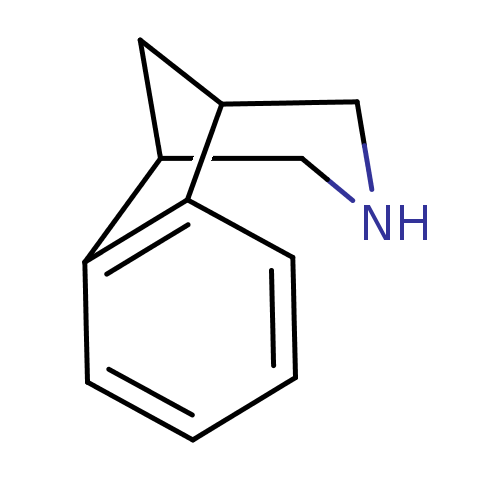 (10-Aza-tricyclo[6.3.1.0*2,7*]dodeca-2(7),3,5-trien...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to nicotinic acetylcholine receptor alpha1 beta gamma delta of electroplax | J Med Chem 48: 3474-7 (2005) Article DOI: 10.1021/jm050069n BindingDB Entry DOI: 10.7270/Q2N87BJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50166907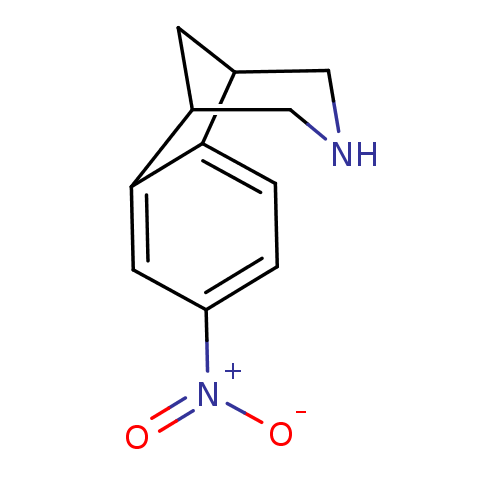 (4-nitro-10-azatricyclo[6.3.1.02,7]dodeca-2(7),3,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to nicotinic acetylcholine receptor alpha1 beta gamma delta of electroplax | J Med Chem 48: 3474-7 (2005) Article DOI: 10.1021/jm050069n BindingDB Entry DOI: 10.7270/Q2N87BJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50166908 (5,8,14-triazatetracyclo[10.3.1.02,11.04,9]hexadeca...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 3.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to nicotinic acetylcholine receptor alpha1 beta gamma delta of electroplax | J Med Chem 48: 3474-7 (2005) Article DOI: 10.1021/jm050069n BindingDB Entry DOI: 10.7270/Q2N87BJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50143282 ((-)-cytisine | (1R,5S)-1,2,3,4,5,6-Hexahydro-1,5-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to nicotinic acetylcholine receptor alpha-7 subunit in rat GH4C1 cells | J Med Chem 48: 3474-7 (2005) Article DOI: 10.1021/jm050069n BindingDB Entry DOI: 10.7270/Q2N87BJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50166909 (10-Aza-tricyclo[6.3.1.0*2,7*]dodeca-2(7),3,5-trien...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to nicotinic acetylcholine receptor alpha-7 subunit in rat GH4C1 cells | J Med Chem 48: 3474-7 (2005) Article DOI: 10.1021/jm050069n BindingDB Entry DOI: 10.7270/Q2N87BJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM82070 (CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 6.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to nicotinic acetylcholine receptor alpha1 beta gamma delta of electroplax | J Med Chem 48: 3474-7 (2005) Article DOI: 10.1021/jm050069n BindingDB Entry DOI: 10.7270/Q2N87BJ7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM82070 (CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 6.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to nicotinic acetylcholine receptor alpha-7 subunit in rat GH4C1 cells | J Med Chem 48: 3474-7 (2005) Article DOI: 10.1021/jm050069n BindingDB Entry DOI: 10.7270/Q2N87BJ7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50166907 (4-nitro-10-azatricyclo[6.3.1.02,7]dodeca-2(7),3,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to nicotinic acetylcholine receptor alpha-7 subunit in rat GH4C1 cells | J Med Chem 48: 3474-7 (2005) Article DOI: 10.1021/jm050069n BindingDB Entry DOI: 10.7270/Q2N87BJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM50015711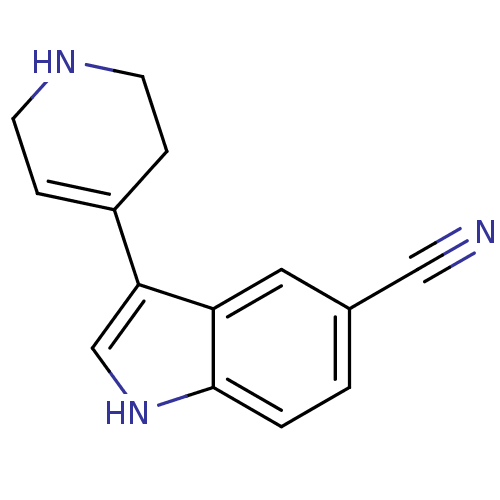 (3-(1,2,3,6-Tetrahydro-pyridin-4-yl)-1H-indole-5-ca...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1B receptor of rat cortex using [3H]-5-HT | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM50015714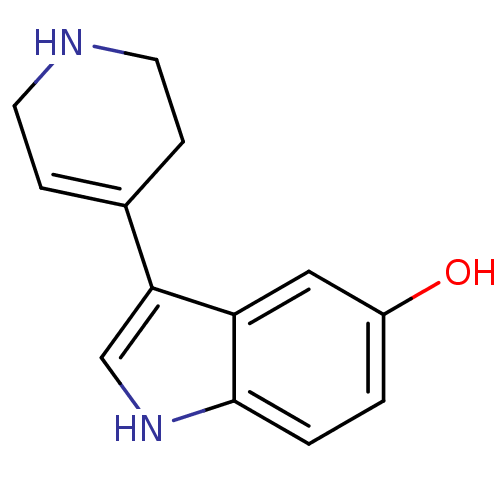 (3-(1,2,3,6-Tetrahydro-pyridin-4-yl)-1H-indol-5-ol ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1B receptor of rat cortex using [3H]-5-HT | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM50015712 (5-Fluoro-3-(1,2,3,6-tetrahydro-pyridin-4-yl)-1H-in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1B receptor of rat cortex using [3H]-5-HT | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM81498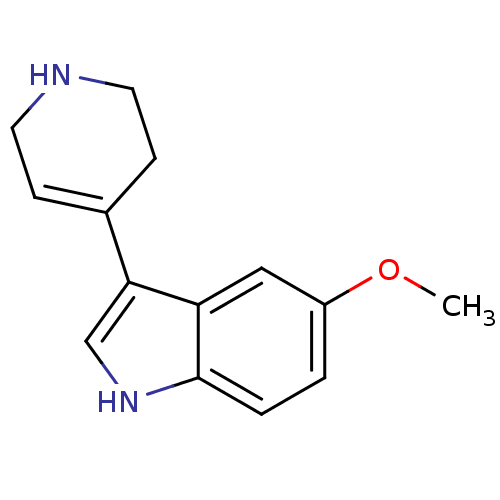 (5-Methoxy-3-(1,2,3,6-tetrahydro-pyridin-4-yl)-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1B receptor of rat cortex using [3H]-5-HT | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50007558 (6-[2-(4-Naphthalen-1-yl-piperazin-1-yl)-ethyl]-3H-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer, Inc. Curated by ChEMBL | Assay Description Binding affinity to 5-hydroxytryptamine 1A receptor in the rat brain using [3H]-8-hydroxy-2-(di-n-propylamine)tetralin as radioligand. | J Med Chem 34: 1860-6 (1991) BindingDB Entry DOI: 10.7270/Q2Q52NK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50007563 (1-Ethyl-5-[2-(4-naphthalen-1-yl-piperazin-1-yl)-et...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer, Inc. Curated by ChEMBL | Assay Description Binding affinity to 5-hydroxytryptamine 1A receptor in the rat brain using [3H]-8-hydroxy-2-(di-n-propylamine)tetralin as radioligand. | J Med Chem 34: 1860-6 (1991) BindingDB Entry DOI: 10.7270/Q2Q52NK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1D receptor of bovine caudate using [3H]-5-HT as the radioligand | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer, Inc. Curated by ChEMBL | Assay Description Compound was evaluated In vitro for its activity by binding to Dopamine receptor D2 in the rat brain using [3H]-NPA as radioligand. | J Med Chem 34: 1860-6 (1991) BindingDB Entry DOI: 10.7270/Q2Q52NK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM50015709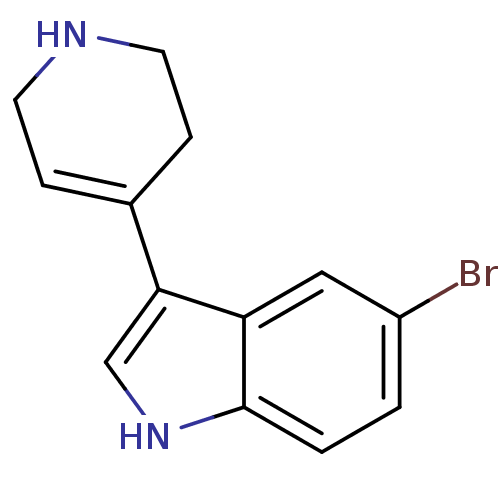 (5-Bromo-3-(1,2,3,6-tetrahydro-pyridin-4-yl)-1H-ind...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1B receptor of rat cortex using [3H]-5-HT | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM50015717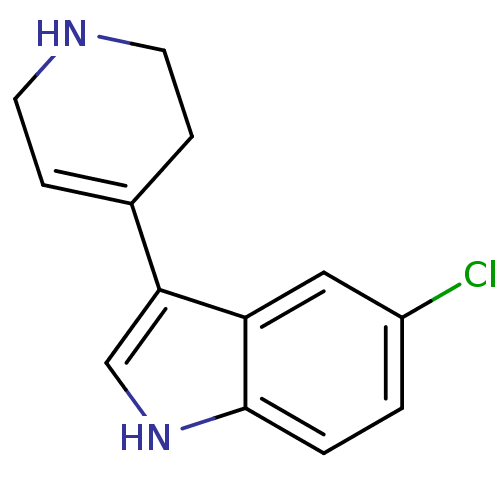 (5-Chloro-3-(1,2,3,6-tetrahydro-pyridin-4-yl)-1H-in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1B receptor of rat cortex using [3H]-5-HT | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM50015718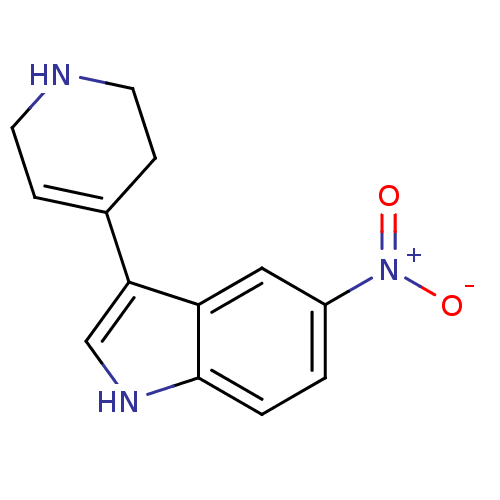 (5-Nitro-3-(1,2,3,6-tetrahydro-pyridin-4-yl)-1H-ind...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1B receptor of rat cortex using [3H]-5-HT | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM31023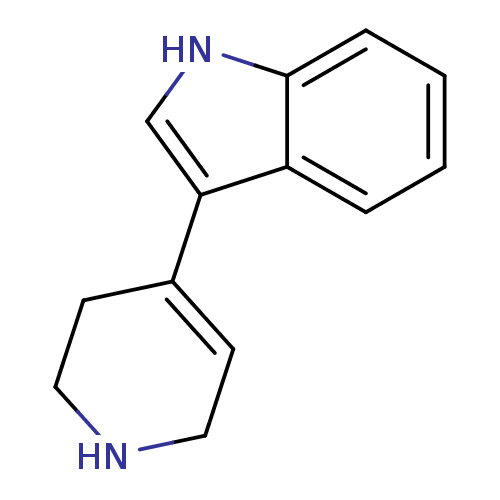 (3-(1,2,3,6-tetrahydropyridin-4-yl)-1H-indole | CHE...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1B receptor of rat cortex using [3H]-5-HT as the radioligand. | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50007559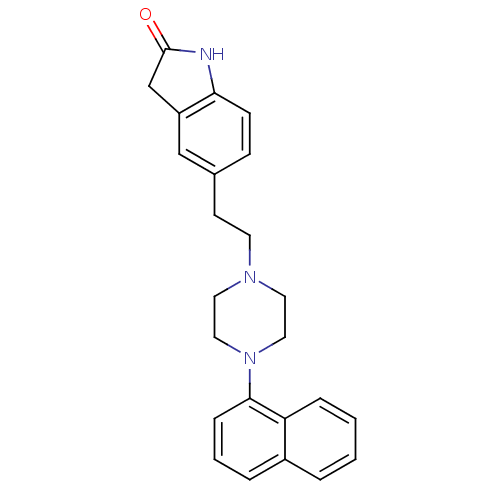 (5-[2-(4-Naphthalen-1-yl-piperazin-1-yl)-ethyl]-1,3...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.87 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer, Inc. Curated by ChEMBL | Assay Description Binding affinity to 5-hydroxytryptamine 1A receptor in the rat brain using [3H]-8-hydroxy-2-(di-n-propylamine)tetralin as radioligand. | J Med Chem 34: 1860-6 (1991) BindingDB Entry DOI: 10.7270/Q2Q52NK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1B receptor of rat cortex using [3H]-5-HT as the radioligand. | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from rat cortex 5-hydroxytryptamine 1A receptor | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50007556 (6-[2-(4-Naphthalen-1-yl-piperazin-1-yl)-ethyl]-3H-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer, Inc. Curated by ChEMBL | Assay Description Binding affinity to 5-hydroxytryptamine 1A receptor in the rat brain using [3H]-8-hydroxy-2-(di-n-propylamine)tetralin as radioligand. | J Med Chem 34: 1860-6 (1991) BindingDB Entry DOI: 10.7270/Q2Q52NK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50007557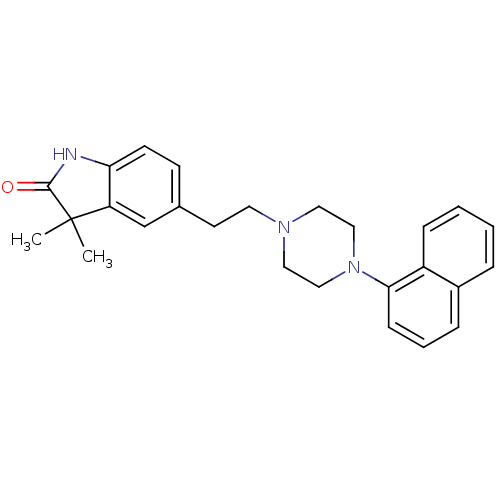 (3,3-Dimethyl-5-[2-(4-naphthalen-1-yl-piperazin-1-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer, Inc. Curated by ChEMBL | Assay Description Binding affinity to 5-hydroxytryptamine 1A receptor in the rat brain using [3H]-8-hydroxy-2-(di-n-propylamine)tetralin as radioligand. | J Med Chem 34: 1860-6 (1991) BindingDB Entry DOI: 10.7270/Q2Q52NK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50007562 (4-{4-[2-(4-Naphthalen-1-yl-piperazin-1-yl)-ethyl]-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer, Inc. Curated by ChEMBL | Assay Description Binding affinity to 5-hydroxytryptamine 1A receptor in the rat brain using [3H]-8-hydroxy-2-(di-n-propylamine)tetralin as radioligand. | J Med Chem 34: 1860-6 (1991) BindingDB Entry DOI: 10.7270/Q2Q52NK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50015709 (5-Bromo-3-(1,2,3,6-tetrahydro-pyridin-4-yl)-1H-ind...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from rat cortex 5-hydroxytryptamine 1A receptor | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM81498 (5-Methoxy-3-(1,2,3,6-tetrahydro-pyridin-4-yl)-1H-i...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from rat cortex 5-hydroxytryptamine 1A receptor | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM84737 (3-(1,2,3,6-Tetrahydro-pyridin-4-yl)-1,4-dihydro-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1B receptor of rat cortex using [3H]5-HT | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM81497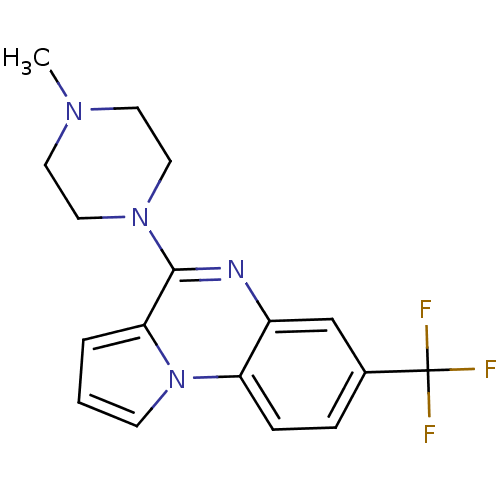 (4-(4-Methyl-piperazin-1-yl)-7-trifluoromethyl-pyrr...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from rat cortex 5-hydroxytryptamine 1A receptor | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM50015710 (4-Piperazin-1-yl-7-trifluoromethyl-pyrrolo[1,2-a]q...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1B receptor of rat cortex using [3H]-5-HT | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50015717 (5-Chloro-3-(1,2,3,6-tetrahydro-pyridin-4-yl)-1H-in...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from rat cortex 5-hydroxytryptamine 1A receptor | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM50007406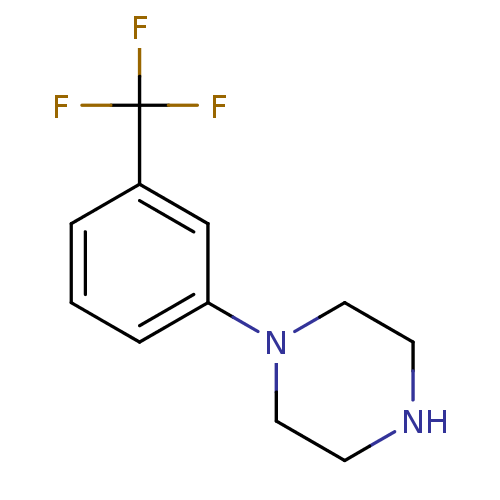 (1-(3-(trifluoromethyl)phenyl)piperazine | 1-(3-Tri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1B receptor of rat cortex using [3H]-5-HT | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50015714 (3-(1,2,3,6-Tetrahydro-pyridin-4-yl)-1H-indol-5-ol ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from rat cortex 5-hydroxytryptamine 1A receptor | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50015711 (3-(1,2,3,6-Tetrahydro-pyridin-4-yl)-1H-indole-5-ca...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from rat cortex 5-hydroxytryptamine 1A receptor | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM81497 (4-(4-Methyl-piperazin-1-yl)-7-trifluoromethyl-pyrr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against 5-hydroxytryptamine 1D receptor of bovine caudate using [3H]-5-HT as the radioligand | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50015718 (5-Nitro-3-(1,2,3,6-tetrahydro-pyridin-4-yl)-1H-ind...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from rat cortex 5-hydroxytryptamine 1A receptor | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50015710 (4-Piperazin-1-yl-7-trifluoromethyl-pyrrolo[1,2-a]q...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from rat cortex 5-hydroxytryptamine 1A receptor | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007559 (5-[2-(4-Naphthalen-1-yl-piperazin-1-yl)-ethyl]-1,3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer, Inc. Curated by ChEMBL | Assay Description Binding affinity to dopamine receptor D2 in the rat brain using [3H]-NPA as radioligand | J Med Chem 34: 1860-6 (1991) BindingDB Entry DOI: 10.7270/Q2Q52NK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM81498 (5-Methoxy-3-(1,2,3,6-tetrahydro-pyridin-4-yl)-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against 5-hydroxytryptamine 1D receptor of bovine caudate using [3H]-5-HT as the radioligand | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007556 (6-[2-(4-Naphthalen-1-yl-piperazin-1-yl)-ethyl]-3H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer, Inc. Curated by ChEMBL | Assay Description Binding affinity to dopamine receptor D2 in the rat brain using [3H]-NPA as radioligand | J Med Chem 34: 1860-6 (1991) BindingDB Entry DOI: 10.7270/Q2Q52NK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50015712 (5-Fluoro-3-(1,2,3,6-tetrahydro-pyridin-4-yl)-1H-in...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from rat cortex 5-hydroxytryptamine 1A receptor | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007562 (4-{4-[2-(4-Naphthalen-1-yl-piperazin-1-yl)-ethyl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer, Inc. Curated by ChEMBL | Assay Description Binding affinity to dopamine receptor D2 in the rat brain using [3H]-NPA as radioligand | J Med Chem 34: 1860-6 (1991) BindingDB Entry DOI: 10.7270/Q2Q52NK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007565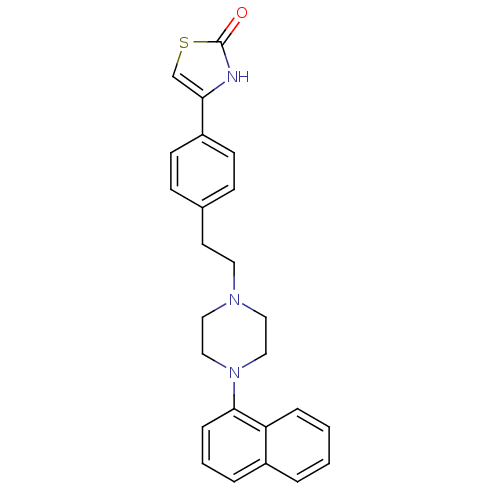 (4-{4-[2-(4-Naphthalen-1-yl-piperazin-1-yl)-ethyl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer, Inc. Curated by ChEMBL | Assay Description Binding affinity to dopamine receptor D2 in the rat brain using [3H]-NPA as radioligand | J Med Chem 34: 1860-6 (1991) BindingDB Entry DOI: 10.7270/Q2Q52NK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description inhibitory activity against 5-hydroxytryptamine 1C receptor of pig choroid plexus using [3H]-mesulergine as the radioligand | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007563 (1-Ethyl-5-[2-(4-naphthalen-1-yl-piperazin-1-yl)-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 81.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer, Inc. Curated by ChEMBL | Assay Description Binding affinity to dopamine receptor D2 in the rat brain using [3H]-NPA as radioligand | J Med Chem 34: 1860-6 (1991) BindingDB Entry DOI: 10.7270/Q2Q52NK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007560 (1-Naphthalen-1-yl-4-[2-(4-[1,2,3]thiadiazol-4-yl-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer, Inc. Curated by ChEMBL | Assay Description Binding affinity to dopamine receptor D2 in the rat brain using [3H]-NPA as radioligand | J Med Chem 34: 1860-6 (1991) BindingDB Entry DOI: 10.7270/Q2Q52NK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007557 (3,3-Dimethyl-5-[2-(4-naphthalen-1-yl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer, Inc. Curated by ChEMBL | Assay Description Binding affinity to dopamine receptor D2 in the rat brain using [3H]-NPA as radioligand | J Med Chem 34: 1860-6 (1991) BindingDB Entry DOI: 10.7270/Q2Q52NK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 81 total ) | Next | Last >> |