

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090760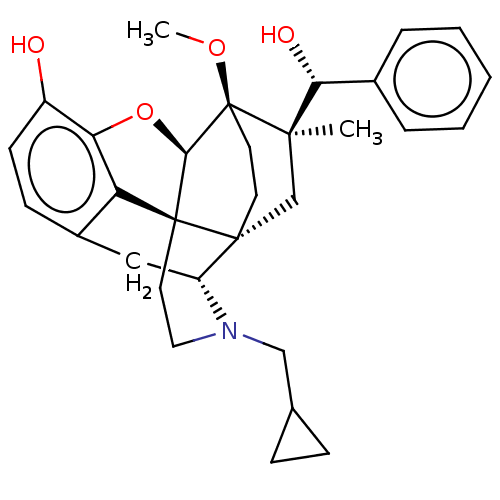 (CHEMBL3581750 | US9259422, 30, R = Ph-BU10119 | US...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090731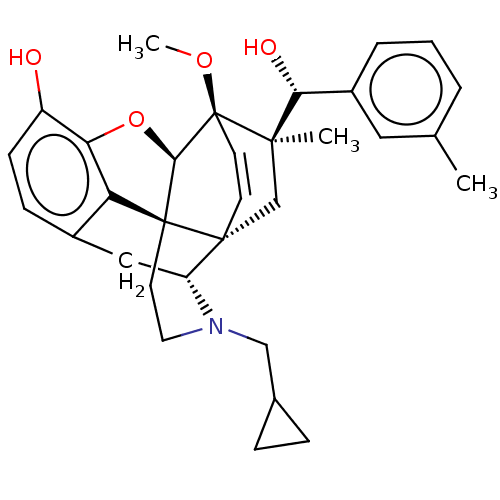 (CHEMBL3581741 | US9259422, 22, R = 3-MePh- BU10112...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50015003 (CHEMBL3262089 | US9259422, 7a, R = Ph-BU127 | US94...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090755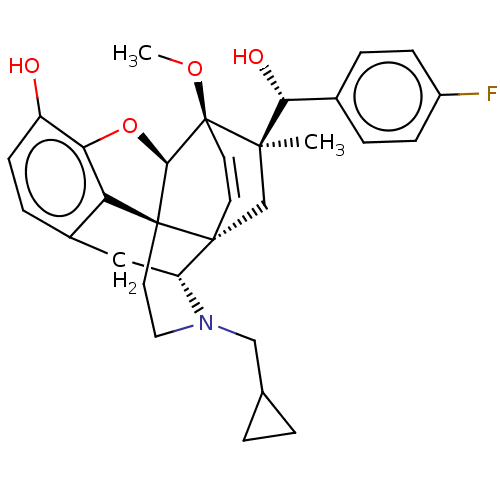 (CHEMBL3581743 | US9259422, 22, R = 4-FPh- BU10120 ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090766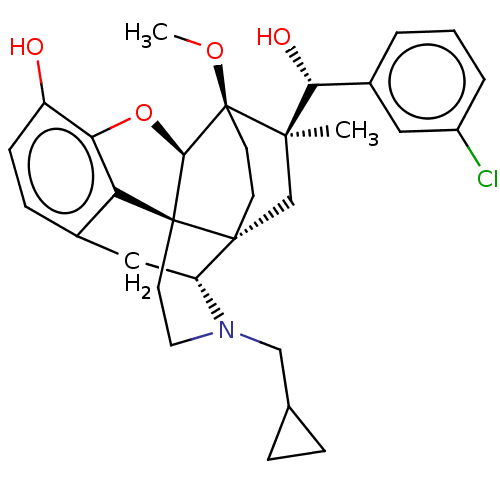 (CHEMBL3581756) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM85134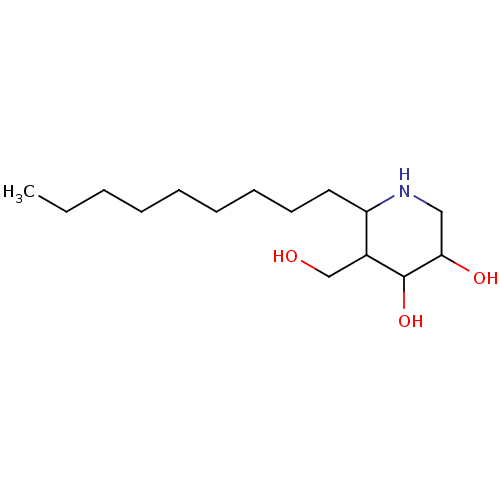 (Isofagomine derivative, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
University of British Columbia | Assay Description Fluorescene-based assay using beta-glucocerebrosidase. | Chembiochem 12: 2151-4 (2011) Article DOI: 10.1002/cbic.201100332 BindingDB Entry DOI: 10.7270/Q2WD3Z3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090782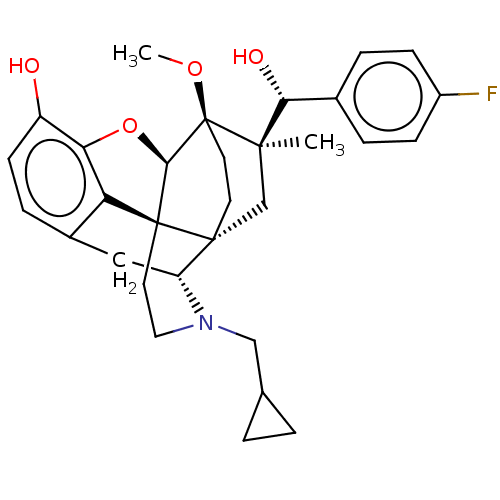 (CHEMBL3581754) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090694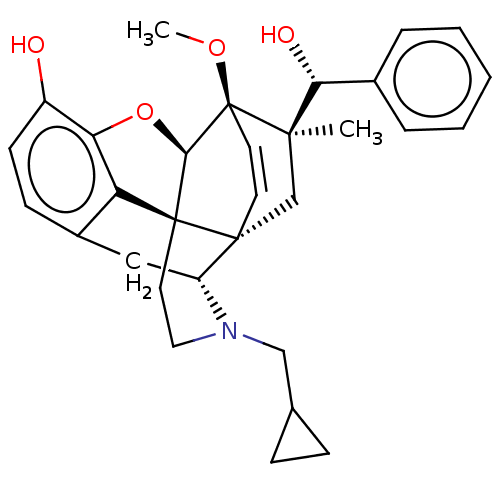 (CHEMBL3581740 | US9259422, 22, R = Ph-BU128 | US94...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090694 (CHEMBL3581740 | US9259422, 22, R = Ph-BU128 | US94...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265728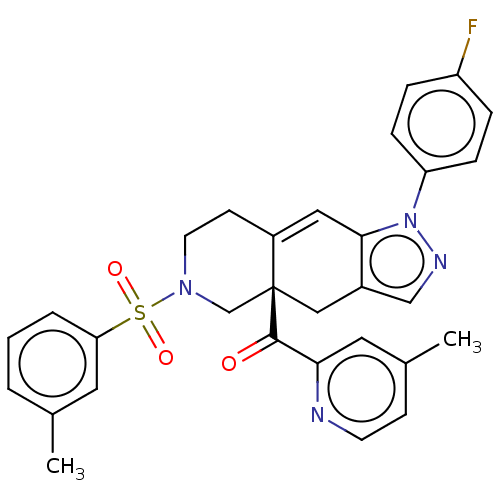 (CHEMBL4081121) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of fluormone GS Red from human glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50354578 (BUPRENORPHINE | US10752592, Compound buprenorphine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090767 (CHEMBL3581757) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of fluormone GS Red from human glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090766 (CHEMBL3581756) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090765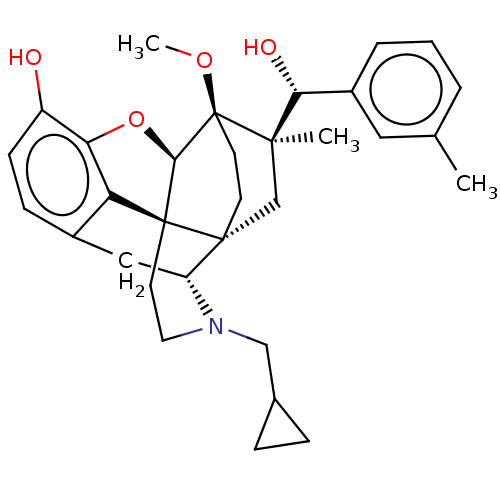 (CHEMBL3581752) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090782 (CHEMBL3581754) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090760 (CHEMBL3581750 | US9259422, 30, R = Ph-BU10119 | US...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265798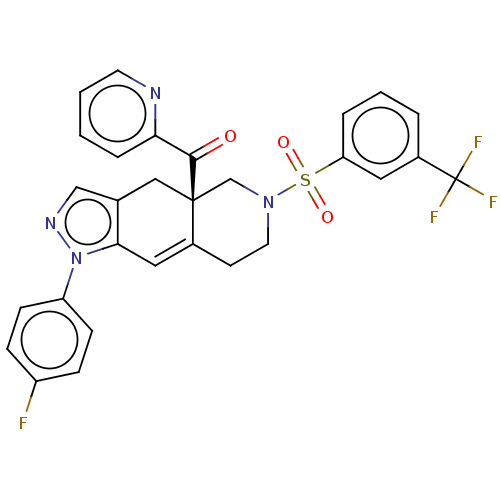 (CHEMBL3736358) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of fluormone GS Red from human glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265798 (CHEMBL3736358) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Binding affinity to human recombinant glucocorticoid receptor by fluorescence polarization assay | Bioorg Med Chem Lett 25: 5720-5 (2015) Article DOI: 10.1016/j.bmcl.2015.10.097 BindingDB Entry DOI: 10.7270/Q2ST7STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090765 (CHEMBL3581752) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50231952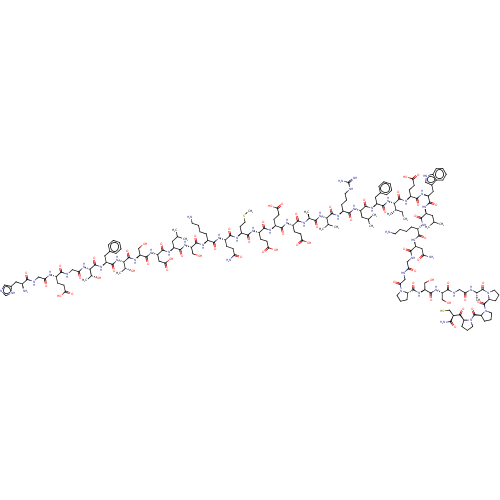 (CHEMBL4081554) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Displacement of [125I]-GLP (7 to 36 residues) from human GLP1R expressed in CHO cell membranes incubated for 30 mins measured after 10 hrs by scintil... | Eur J Med Chem 127: 703-714 (2017) Article DOI: 10.1016/j.ejmech.2016.10.044 BindingDB Entry DOI: 10.7270/Q2T155W1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090768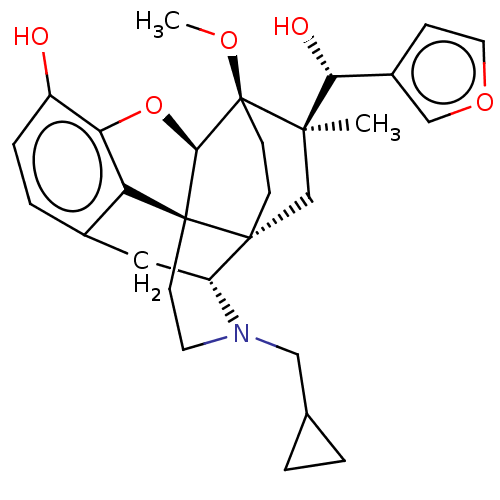 (CHEMBL3581762) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090769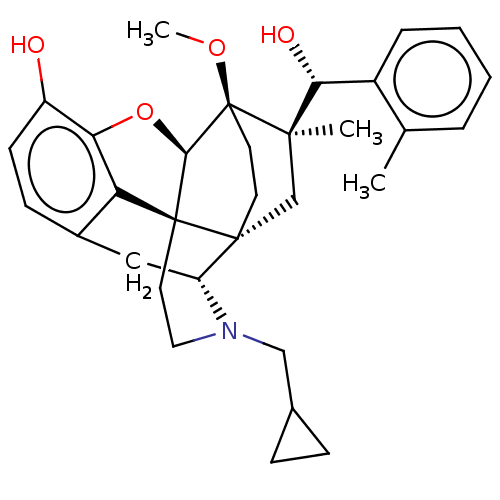 (CHEMBL3581751) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164899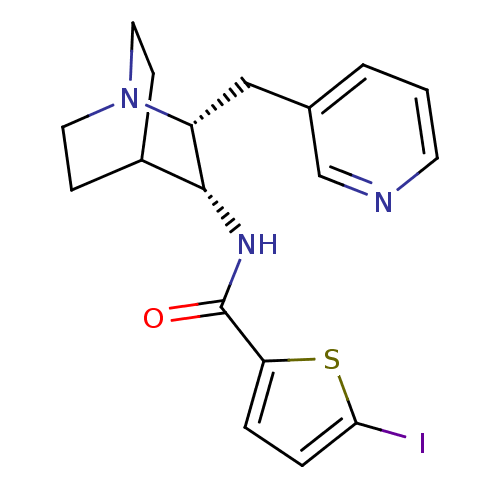 (5-Iodo-thiophene-2-carboxylic acid ((2R,3R)-2-pyri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against [3H]methyllycaconitine binding towards Nicotinic acetylcholine receptor alpha-7 of rat brain hippocampus | Bioorg Med Chem Lett 15: 2073-7 (2005) Article DOI: 10.1016/j.bmcl.2005.02.045 BindingDB Entry DOI: 10.7270/Q2GT5NX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265797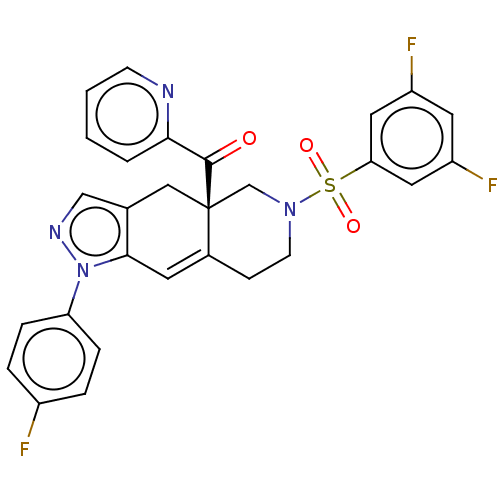 (CHEMBL4088286) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of fluormone GS Red from human glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50499246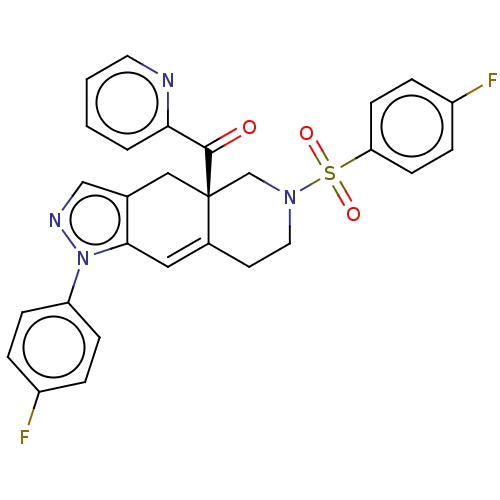 (CHEMBL3735376) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Binding affinity to human recombinant glucocorticoid receptor by fluorescence polarization assay | Bioorg Med Chem Lett 25: 5720-5 (2015) Article DOI: 10.1016/j.bmcl.2015.10.097 BindingDB Entry DOI: 10.7270/Q2ST7STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50354578 (BUPRENORPHINE | US10752592, Compound buprenorphine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090767 (CHEMBL3581757) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090773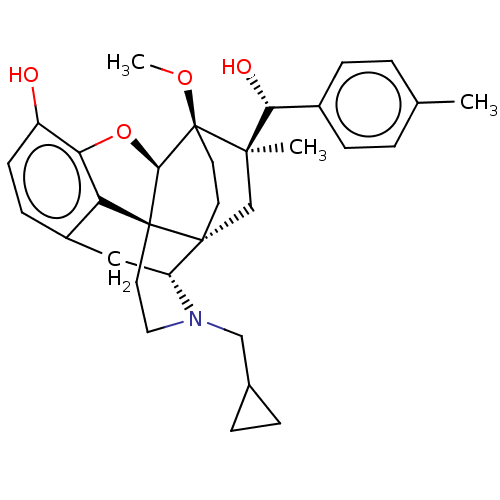 (CHEMBL3581753) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090768 (CHEMBL3581762) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265801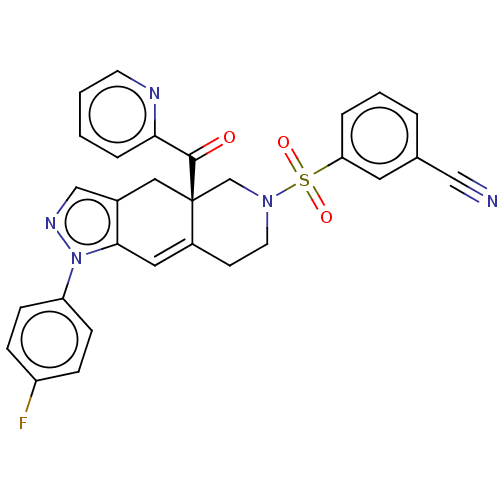 (CHEMBL3735851) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Binding affinity to human recombinant glucocorticoid receptor by fluorescence polarization assay | Bioorg Med Chem Lett 25: 5720-5 (2015) Article DOI: 10.1016/j.bmcl.2015.10.097 BindingDB Entry DOI: 10.7270/Q2ST7STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265804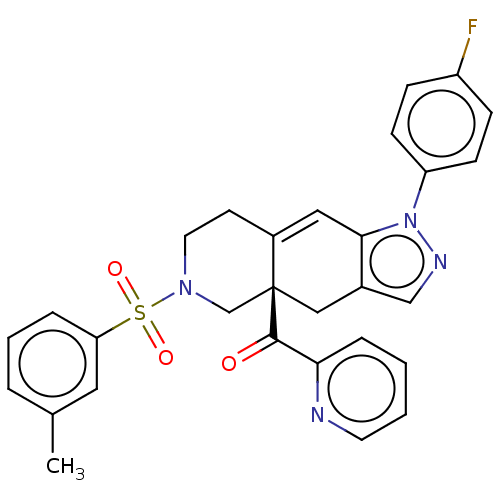 (CHEMBL3735124) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Binding affinity to human recombinant glucocorticoid receptor by fluorescence polarization assay | Bioorg Med Chem Lett 25: 5720-5 (2015) Article DOI: 10.1016/j.bmcl.2015.10.097 BindingDB Entry DOI: 10.7270/Q2ST7STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50499255 (CHEMBL3735008) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Binding affinity to human recombinant glucocorticoid receptor by fluorescence polarization assay | Bioorg Med Chem Lett 25: 5720-5 (2015) Article DOI: 10.1016/j.bmcl.2015.10.097 BindingDB Entry DOI: 10.7270/Q2ST7STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265729 (CHEMBL4105376) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of fluormone GS Red from human glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265796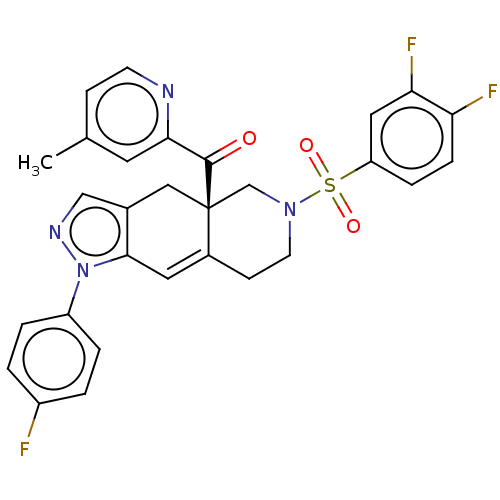 (CHEMBL4067017) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of fluormone GS Red from human glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265801 (CHEMBL3735851) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of fluormone GS Red from human glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265804 (CHEMBL3735124) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of fluormone GS Red from human glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265803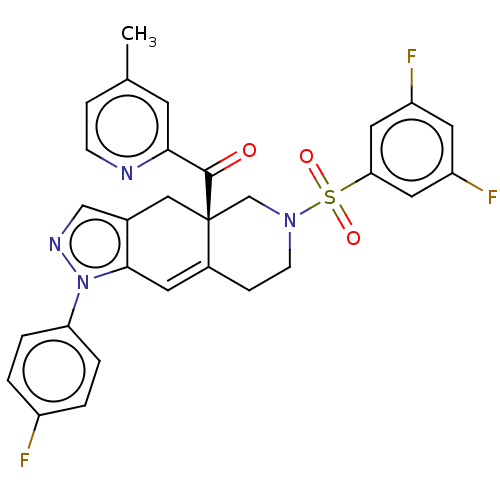 (CHEMBL4077976) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of fluormone GS Red from human glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50128358 (CHEMBL3629347) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle Curated by ChEMBL | Assay Description Inhibition of human recombinant melanocortin 4 receptor expressed in CHO cells | J Med Chem 60: 349-361 (2017) Article DOI: 10.1021/acs.jmedchem.6b01422 BindingDB Entry DOI: 10.7270/Q27M0B6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265730 (CHEMBL4101325) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of fluormone GS Red from human glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090755 (CHEMBL3581743 | US9259422, 22, R = 4-FPh- BU10120 ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090773 (CHEMBL3581753) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50499257 (CHEMBL3736457) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Binding affinity to human recombinant glucocorticoid receptor by fluorescence polarization assay | Bioorg Med Chem Lett 25: 5720-5 (2015) Article DOI: 10.1016/j.bmcl.2015.10.097 BindingDB Entry DOI: 10.7270/Q2ST7STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265674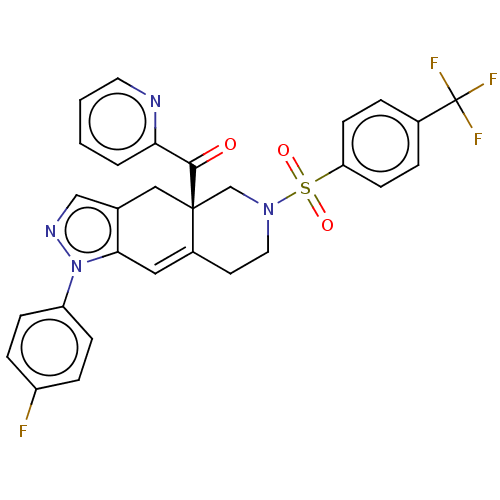 (CHEMBL3734774) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Binding affinity to human recombinant glucocorticoid receptor by fluorescence polarization assay | Bioorg Med Chem Lett 25: 5720-5 (2015) Article DOI: 10.1016/j.bmcl.2015.10.097 BindingDB Entry DOI: 10.7270/Q2ST7STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Ability to inhibit the binding of iodine-125-labelled lysergic acid diethylamide([125I]-LSD) to the S-2A serotonin receptor. | J Med Chem 38: 708-14 (1995) BindingDB Entry DOI: 10.7270/Q2PG1SCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50015003 (CHEMBL3262089 | US9259422, 7a, R = Ph-BU127 | US94...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human recombinant 5-HT7 receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 60: 349-361 (2017) Article DOI: 10.1021/acs.jmedchem.6b01422 BindingDB Entry DOI: 10.7270/Q27M0B6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50499256 (CHEMBL3736390) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Binding affinity to human recombinant glucocorticoid receptor by fluorescence polarization assay | Bioorg Med Chem Lett 25: 5720-5 (2015) Article DOI: 10.1016/j.bmcl.2015.10.097 BindingDB Entry DOI: 10.7270/Q2ST7STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090731 (CHEMBL3581741 | US9259422, 22, R = 3-MePh- BU10112...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265806 (CHEMBL4074627) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of fluormone GS Red from human glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1676 total ) | Next | Last >> |