 Found 42 hits with Last Name = 'hoffner' and Initial = 's'
Found 42 hits with Last Name = 'hoffner' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM50385299
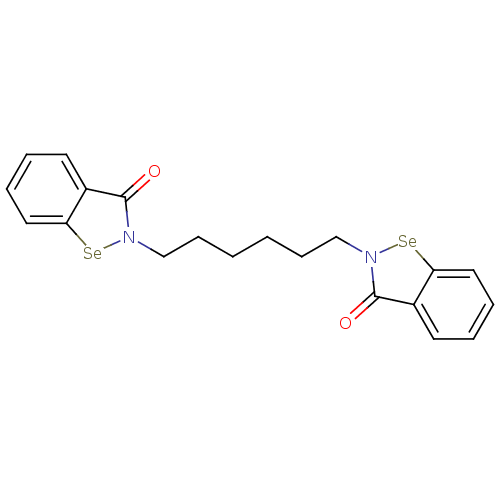
(CHEMBL2035464 | US8592468, EbSe16)Show SMILES O=c1n(CCCCCCn2[se]c3ccccc3c2=O)[se]c2ccccc12 Show InChI InChI=1S/C20H20N2O2Se2/c23-19-15-9-3-5-11-17(15)25-21(19)13-7-1-2-8-14-22-20(24)16-10-4-6-12-18(16)26-22/h3-6,9-12H,1-2,7-8,13-14H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 10 | n/a | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM50385302
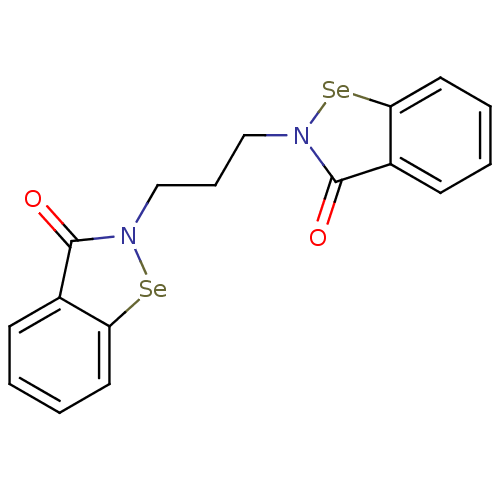
(CHEMBL2035461 | US8592468, EbSe15)Show InChI InChI=1S/C17H14N2O2Se2/c20-16-12-6-1-3-8-14(12)22-18(16)10-5-11-19-17(21)13-7-2-4-9-15(13)23-19/h1-4,6-9H,5,10-11H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 40 | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM50385303
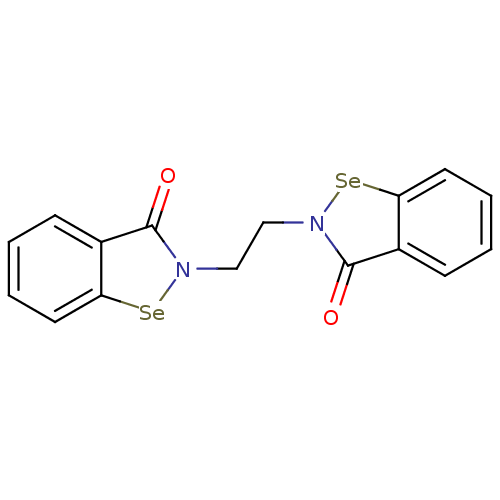
(CHEMBL2035460 | US8592468, EbSe14)Show InChI InChI=1S/C16H12N2O2Se2/c19-15-11-5-1-3-7-13(11)21-17(15)9-10-18-16(20)12-6-2-4-8-14(12)22-18/h1-8H,9-10H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| 50 | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM106948

(US8592468, EbSe12)Show InChI InChI=1S/C12H7N3O3Se/c16-12-8-4-1-2-6-10(8)19-14(12)11-9(15(17)18)5-3-7-13-11/h1-7H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 250 | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM106941
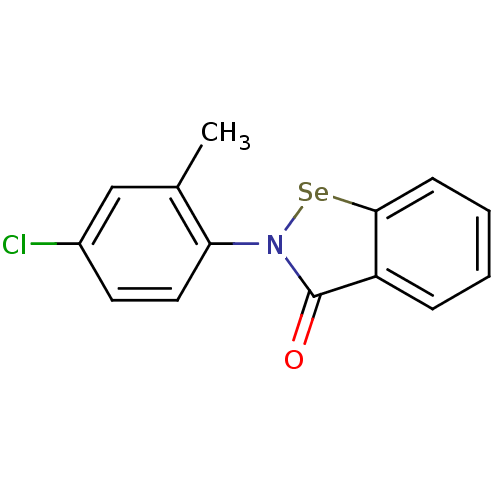
(US8592468, EbSe8)Show InChI InChI=1S/C14H10ClNOSe/c1-9-8-10(15)6-7-12(9)16-14(17)11-4-2-3-5-13(11)18-16/h2-8H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 250 | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM34233

(2-Phenyl-benzo[d]isoselenazol-3-one | 2-Phenyl-ben...)Show InChI InChI=1S/C13H9NOSe/c15-13-11-8-4-5-9-12(11)16-14(13)10-6-2-1-3-7-10/h1-9H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| 300 | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM106940
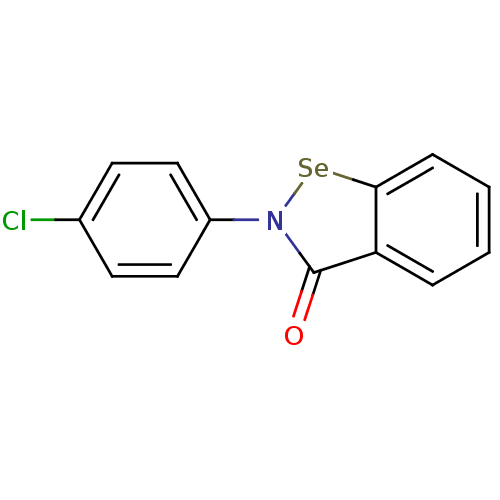
(US8592468, EbSe7)Show InChI InChI=1S/C13H8ClNOSe/c14-9-5-7-10(8-6-9)15-13(16)11-3-1-2-4-12(11)17-15/h1-8H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 550 | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM106944
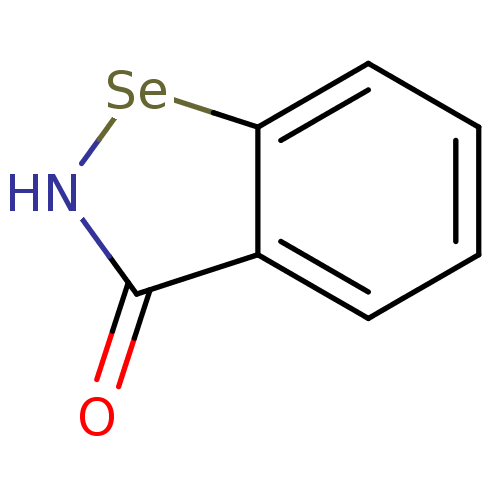
(US8592468, EbSe2)Show InChI InChI=1S/C7H5NOSe/c9-7-5-3-1-2-4-6(5)10-8-7/h1-4H,(H,8,9) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.00E+3 | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM106942
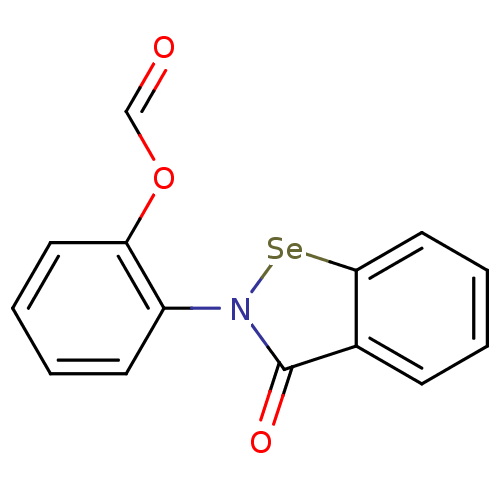
(US8592468, EbSe9)Show InChI InChI=1S/C14H9NO3Se/c16-9-18-12-7-3-2-6-11(12)15-14(17)10-5-1-4-8-13(10)19-15/h1-9H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.20E+3 | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM106949
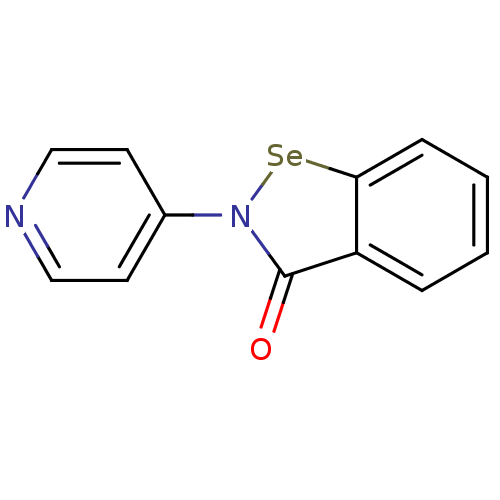
(US8592468, EbSe13)Show InChI InChI=1S/C12H8N2OSe/c15-12-10-3-1-2-4-11(10)16-14(12)9-5-7-13-8-6-9/h1-8H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.50E+3 | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM106943
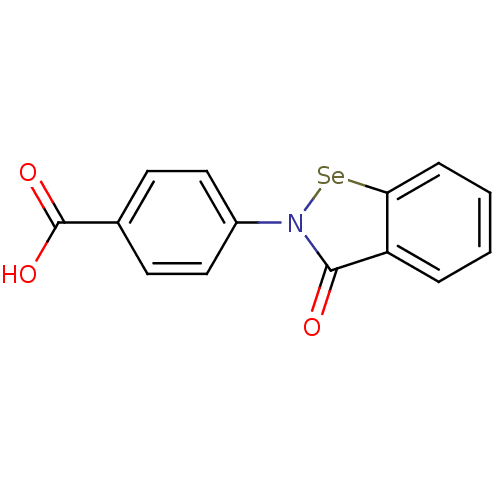
(US8592468, EbSe10 | acs.jmedchem.1c00409_ST.159)Show InChI InChI=1S/C14H9NO3Se/c16-13-11-3-1-2-4-12(11)19-15(13)10-7-5-9(6-8-10)14(17)18/h1-8H,(H,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50018442

(CHEMBL3286436)Show SMILES COc1cc(C#N)c2ccc(=O)n(CCN3CCC(CC3)NCc3cnc(C)c(c3)C#N)c2c1 Show InChI InChI=1S/C26H28N6O2/c1-18-20(14-27)11-19(16-29-18)17-30-22-5-7-31(8-6-22)9-10-32-25-13-23(34-2)12-21(15-28)24(25)3-4-26(32)33/h3-4,11-13,16,22,30H,5-10,17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp electrophysiological analysis |
J Med Chem 57: 4889-905 (2014)
Article DOI: 10.1021/jm500432n
BindingDB Entry DOI: 10.7270/Q2416ZM4 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50018290

(CHEMBL3290338)Show SMILES Cc1nc(CNC2CCN(CCn3c4cc(ccc4ccc3=O)C#N)CC2)ccc1C#N Show InChI InChI=1S/C25H26N6O/c1-18-21(16-27)4-6-23(29-18)17-28-22-8-10-30(11-9-22)12-13-31-24-14-19(15-26)2-3-20(24)5-7-25(31)32/h2-7,14,22,28H,8-13,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp electrophysiological analysis |
J Med Chem 57: 4889-905 (2014)
Article DOI: 10.1021/jm500432n
BindingDB Entry DOI: 10.7270/Q2416ZM4 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50018440

(CHEMBL3290342)Show SMILES COc1ccc2n(CCN3CCC(CC3)NCc3cnc(C)c(c3)C#N)c(=O)ccc2n1 Show InChI InChI=1S/C24H28N6O2/c1-17-19(14-25)13-18(15-26-17)16-27-20-7-9-29(10-8-20)11-12-30-22-4-5-23(32-2)28-21(22)3-6-24(30)31/h3-6,13,15,20,27H,7-12,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp electrophysiological analysis |
J Med Chem 57: 4889-905 (2014)
Article DOI: 10.1021/jm500432n
BindingDB Entry DOI: 10.7270/Q2416ZM4 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50018444
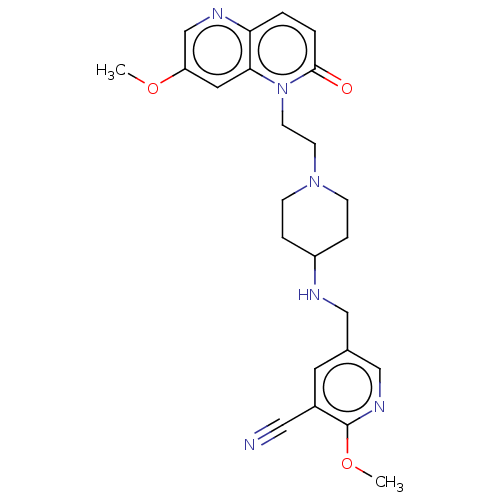
(CHEMBL3290345)Show SMILES COc1cnc2ccc(=O)n(CCN3CCC(CC3)NCc3cnc(OC)c(c3)C#N)c2c1 Show InChI InChI=1S/C24H28N6O3/c1-32-20-12-22-21(27-16-20)3-4-23(31)30(22)10-9-29-7-5-19(6-8-29)26-14-17-11-18(13-25)24(33-2)28-15-17/h3-4,11-12,15-16,19,26H,5-10,14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp electrophysiological analysis |
J Med Chem 57: 4889-905 (2014)
Article DOI: 10.1021/jm500432n
BindingDB Entry DOI: 10.7270/Q2416ZM4 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50018447

(CHEMBL3290347)Show SMILES COc1ccc2ncc(=O)n(CCN3CCC(CC3)NCc3cnc(C)c(c3)C#N)c2n1 Show InChI InChI=1S/C23H27N7O2/c1-16-18(12-24)11-17(13-25-16)14-26-19-5-7-29(8-6-19)9-10-30-22(31)15-27-20-3-4-21(32-2)28-23(20)30/h3-4,11,13,15,19,26H,5-10,14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp electrophysiological analysis |
J Med Chem 57: 4889-905 (2014)
Article DOI: 10.1021/jm500432n
BindingDB Entry DOI: 10.7270/Q2416ZM4 |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM106945

(US8592468, EbSe3)Show InChI InChI=1S/C8H7NOSe/c1-9-8(10)6-4-2-3-5-7(6)11-9/h2-5H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM106946
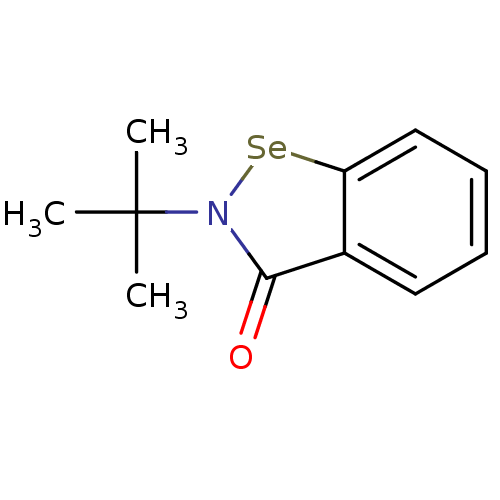
(US8592468, EbSe4)Show InChI InChI=1S/C11H13NOSe/c1-11(2,3)12-10(13)8-6-4-5-7-9(8)14-12/h4-7H,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50018288
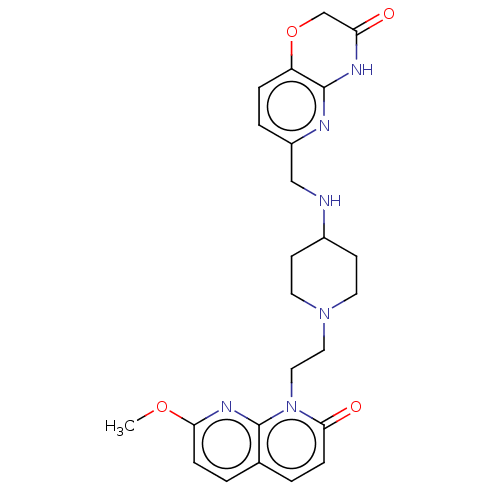
(CHEMBL3290336)Show SMILES COc1ccc2ccc(=O)n(CCN3CCC(CC3)NCc3ccc4OCC(=O)Nc4n3)c2n1 Show InChI InChI=1S/C24H28N6O4/c1-33-21-6-2-16-3-7-22(32)30(24(16)28-21)13-12-29-10-8-17(9-11-29)25-14-18-4-5-19-23(26-18)27-20(31)15-34-19/h2-7,17,25H,8-15H2,1H3,(H,26,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp electrophysiological analysis |
J Med Chem 57: 4889-905 (2014)
Article DOI: 10.1021/jm500432n
BindingDB Entry DOI: 10.7270/Q2416ZM4 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50018407
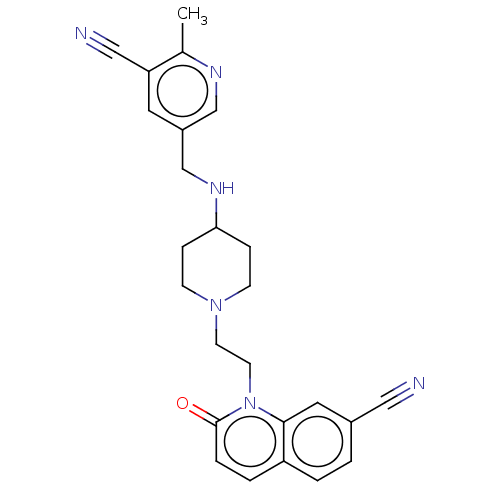
(CHEMBL3290339)Show SMILES Cc1ncc(CNC2CCN(CCn3c4cc(ccc4ccc3=O)C#N)CC2)cc1C#N Show InChI InChI=1S/C25H26N6O/c1-18-22(15-27)12-20(16-28-18)17-29-23-6-8-30(9-7-23)10-11-31-24-13-19(14-26)2-3-21(24)4-5-25(31)32/h2-5,12-13,16,23,29H,6-11,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp electrophysiological analysis |
J Med Chem 57: 4889-905 (2014)
Article DOI: 10.1021/jm500432n
BindingDB Entry DOI: 10.7270/Q2416ZM4 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50018441
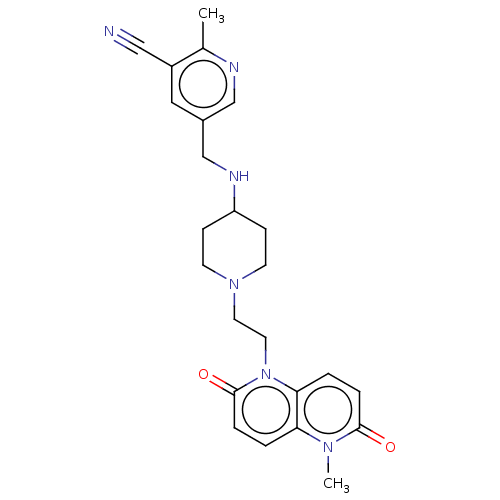
(CHEMBL3290343)Show SMILES Cc1ncc(CNC2CCN(CCn3c4ccc(=O)n(C)c4ccc3=O)CC2)cc1C#N Show InChI InChI=1S/C24H28N6O2/c1-17-19(14-25)13-18(15-26-17)16-27-20-7-9-29(10-8-20)11-12-30-22-4-5-23(31)28(2)21(22)3-6-24(30)32/h3-6,13,15,20,27H,7-12,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp electrophysiological analysis |
J Med Chem 57: 4889-905 (2014)
Article DOI: 10.1021/jm500432n
BindingDB Entry DOI: 10.7270/Q2416ZM4 |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM106947
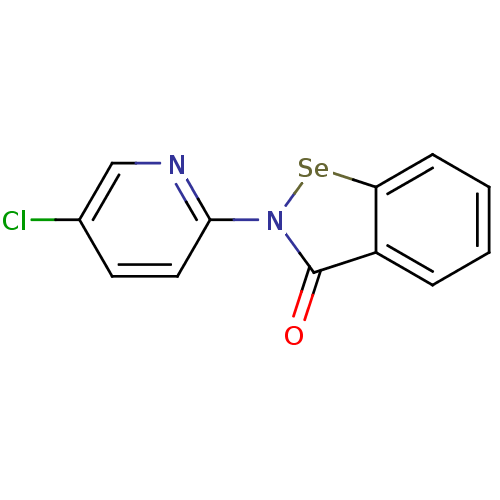
(US8592468, EbSe11)Show InChI InChI=1S/C12H7ClN2OSe/c13-8-5-6-11(14-7-8)15-12(16)9-3-1-2-4-10(9)17-15/h1-7H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50395400
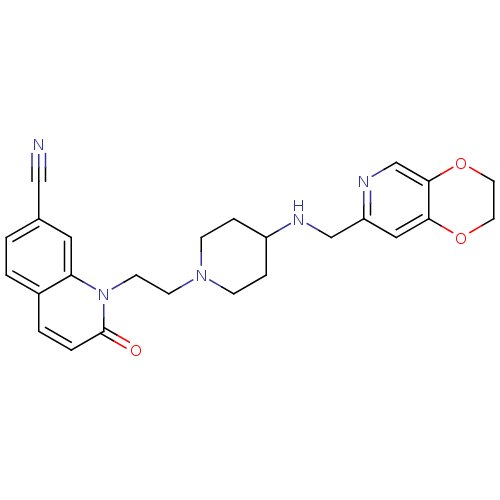
(CHEMBL2165062)Show SMILES O=c1ccc2ccc(cc2n1CCN1CCC(CC1)NCc1cc2OCCOc2cn1)C#N Show InChI InChI=1S/C25H27N5O3/c26-15-18-1-2-19-3-4-25(31)30(22(19)13-18)10-9-29-7-5-20(6-8-29)27-16-21-14-23-24(17-28-21)33-12-11-32-23/h1-4,13-14,17,20,27H,5-12,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp electrophysiological analysis |
J Med Chem 57: 4889-905 (2014)
Article DOI: 10.1021/jm500432n
BindingDB Entry DOI: 10.7270/Q2416ZM4 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50018439
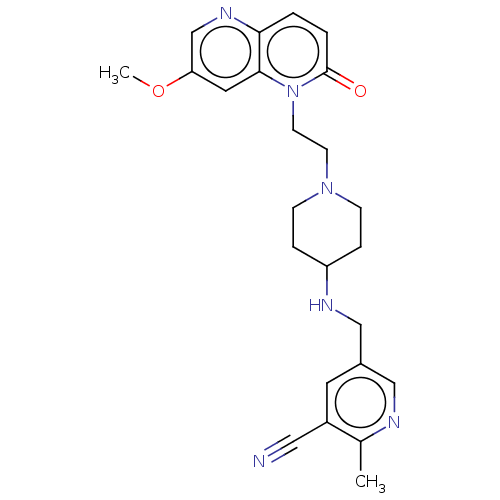
(CHEMBL3290341)Show SMILES COc1cnc2ccc(=O)n(CCN3CCC(CC3)NCc3cnc(C)c(c3)C#N)c2c1 Show InChI InChI=1S/C24H28N6O2/c1-17-19(13-25)11-18(14-26-17)15-27-20-5-7-29(8-6-20)9-10-30-23-12-21(32-2)16-28-22(23)3-4-24(30)31/h3-4,11-12,14,16,20,27H,5-10,15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp electrophysiological analysis |
J Med Chem 57: 4889-905 (2014)
Article DOI: 10.1021/jm500432n
BindingDB Entry DOI: 10.7270/Q2416ZM4 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50018422

(CHEMBL3290340)Show SMILES Cc1ncc(CNC2CCN(CCn3c4cc(F)cnc4ccc3=O)CC2)cc1C#N Show InChI InChI=1S/C23H25FN6O/c1-16-18(12-25)10-17(13-26-16)14-27-20-4-6-29(7-5-20)8-9-30-22-11-19(24)15-28-21(22)2-3-23(30)31/h2-3,10-11,13,15,20,27H,4-9,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp electrophysiological analysis |
J Med Chem 57: 4889-905 (2014)
Article DOI: 10.1021/jm500432n
BindingDB Entry DOI: 10.7270/Q2416ZM4 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50018446

(CHEMBL3290346)Show SMILES COc1cnc2ccc(=O)n(CCN3CCC(CC3)NCc3cnc(C)c(n3)C#N)c2c1 Show InChI InChI=1S/C23H27N7O2/c1-16-21(12-24)28-18(13-25-16)14-26-17-5-7-29(8-6-17)9-10-30-22-11-19(32-2)15-27-20(22)3-4-23(30)31/h3-4,11,13,15,17,26H,5-10,14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp electrophysiological analysis |
J Med Chem 57: 4889-905 (2014)
Article DOI: 10.1021/jm500432n
BindingDB Entry DOI: 10.7270/Q2416ZM4 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50018443

(CHEMBL3290344)Show SMILES COc1cnc2ccc(=O)n(CCN3CCC(CC3)NC(=O)c3cnc(C)c(c3)C#N)c2c1 Show InChI InChI=1S/C24H26N6O3/c1-16-17(13-25)11-18(14-26-16)24(32)28-19-5-7-29(8-6-19)9-10-30-22-12-20(33-2)15-27-21(22)3-4-23(30)31/h3-4,11-12,14-15,19H,5-10H2,1-2H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp electrophysiological analysis |
J Med Chem 57: 4889-905 (2014)
Article DOI: 10.1021/jm500432n
BindingDB Entry DOI: 10.7270/Q2416ZM4 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50018483

(CHEMBL3290351)Show SMILES COc1cnc2ccc(=O)n(CCN3CC[C@@H](NCc4cnc(C)c(c4)C#N)[C@@H](F)C3)c2c1 |r| Show InChI InChI=1S/C24H27FN6O2/c1-16-18(11-26)9-17(12-27-16)13-28-21-5-6-30(15-20(21)25)7-8-31-23-10-19(33-2)14-29-22(23)3-4-24(31)32/h3-4,9-10,12,14,20-21,28H,5-8,13,15H2,1-2H3/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp electrophysiological analysis |
J Med Chem 57: 4889-905 (2014)
Article DOI: 10.1021/jm500432n
BindingDB Entry DOI: 10.7270/Q2416ZM4 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Mycobacterium smegmatis) | BDBM50018407

(CHEMBL3290339)Show SMILES Cc1ncc(CNC2CCN(CCn3c4cc(ccc4ccc3=O)C#N)CC2)cc1C#N Show InChI InChI=1S/C25H26N6O/c1-18-22(15-27)12-20(16-28-18)17-29-23-6-8-30(9-7-23)10-11-31-24-13-19(14-26)2-3-21(24)4-5-25(31)32/h2-5,12-13,16,23,29H,6-11,17H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium smegmatis DNA gyrase-B ATPase activity |
J Med Chem 57: 4889-905 (2014)
Article DOI: 10.1021/jm500432n
BindingDB Entry DOI: 10.7270/Q2416ZM4 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Mycobacterium smegmatis) | BDBM50018290

(CHEMBL3290338)Show SMILES Cc1nc(CNC2CCN(CCn3c4cc(ccc4ccc3=O)C#N)CC2)ccc1C#N Show InChI InChI=1S/C25H26N6O/c1-18-21(16-27)4-6-23(29-18)17-28-22-8-10-30(11-9-22)12-13-31-24-14-19(15-26)2-3-20(24)5-7-25(31)32/h2-7,14,22,28H,8-13,17H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium smegmatis DNA gyrase-B ATPase activity |
J Med Chem 57: 4889-905 (2014)
Article DOI: 10.1021/jm500432n
BindingDB Entry DOI: 10.7270/Q2416ZM4 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Mycobacterium smegmatis) | BDBM50018289
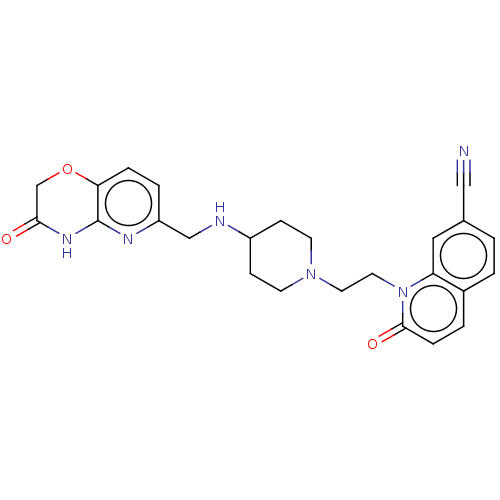
(CHEMBL3290337)Show SMILES O=C1COc2ccc(CNC3CCN(CCn4c5cc(ccc5ccc4=O)C#N)CC3)nc2N1 Show InChI InChI=1S/C25H26N6O3/c26-14-17-1-2-18-3-6-24(33)31(21(18)13-17)12-11-30-9-7-19(8-10-30)27-15-20-4-5-22-25(28-20)29-23(32)16-34-22/h1-6,13,19,27H,7-12,15-16H2,(H,28,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium smegmatis DNA gyrase-B ATPase activity |
J Med Chem 57: 4889-905 (2014)
Article DOI: 10.1021/jm500432n
BindingDB Entry DOI: 10.7270/Q2416ZM4 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Mycobacterium smegmatis) | BDBM50018288

(CHEMBL3290336)Show SMILES COc1ccc2ccc(=O)n(CCN3CCC(CC3)NCc3ccc4OCC(=O)Nc4n3)c2n1 Show InChI InChI=1S/C24H28N6O4/c1-33-21-6-2-16-3-7-22(32)30(24(16)28-21)13-12-29-10-8-17(9-11-29)25-14-18-4-5-19-23(26-18)27-20(31)15-34-19/h2-7,17,25H,8-15H2,1H3,(H,26,27,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium smegmatis DNA gyrase-B ATPase activity |
J Med Chem 57: 4889-905 (2014)
Article DOI: 10.1021/jm500432n
BindingDB Entry DOI: 10.7270/Q2416ZM4 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Mycobacterium smegmatis) | BDBM50395400

(CHEMBL2165062)Show SMILES O=c1ccc2ccc(cc2n1CCN1CCC(CC1)NCc1cc2OCCOc2cn1)C#N Show InChI InChI=1S/C25H27N5O3/c26-15-18-1-2-19-3-4-25(31)30(22(19)13-18)10-9-29-7-5-20(6-8-29)27-16-21-14-23-24(17-28-21)33-12-11-32-23/h1-4,13-14,17,20,27H,5-12,16H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium smegmatis DNA gyrase-B ATPase activity |
J Med Chem 57: 4889-905 (2014)
Article DOI: 10.1021/jm500432n
BindingDB Entry DOI: 10.7270/Q2416ZM4 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50018489

(CHEMBL3290357)Show SMILES CO[C@H]1CN(CCn2c3cc(OC)cnc3ccc2=O)CC[C@H]1NCc1ccc2OCC(=O)Nc2n1 |r| Show InChI InChI=1S/C25H30N6O5/c1-34-17-11-20-18(27-13-17)4-6-24(33)31(20)10-9-30-8-7-19(22(14-30)35-2)26-12-16-3-5-21-25(28-16)29-23(32)15-36-21/h3-6,11,13,19,22,26H,7-10,12,14-15H2,1-2H3,(H,28,29,32)/t19-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp electrophysiological analysis |
J Med Chem 57: 4889-905 (2014)
Article DOI: 10.1021/jm500432n
BindingDB Entry DOI: 10.7270/Q2416ZM4 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50018488

(CHEMBL3290356)Show SMILES COc1cnc2ccc(=O)n(CCN3CC[C@H](NCc4ccc5OCC(=O)Nc5n4)[C@H](F)C3)c2c1 |r| Show InChI InChI=1S/C24H27FN6O4/c1-34-16-10-20-19(27-12-16)3-5-23(33)31(20)9-8-30-7-6-18(17(25)13-30)26-11-15-2-4-21-24(28-15)29-22(32)14-35-21/h2-5,10,12,17-18,26H,6-9,11,13-14H2,1H3,(H,28,29,32)/t17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp electrophysiological analysis |
J Med Chem 57: 4889-905 (2014)
Article DOI: 10.1021/jm500432n
BindingDB Entry DOI: 10.7270/Q2416ZM4 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50018487

(CHEMBL3290355)Show SMILES COc1cnc2ccc(=O)n(CCN3CC[C@@H](NCc4ccc5OCC(=O)Nc5n4)[C@@H](F)C3)c2c1 |r| Show InChI InChI=1S/C24H27FN6O4/c1-34-16-10-20-19(27-12-16)3-5-23(33)31(20)9-8-30-7-6-18(17(25)13-30)26-11-15-2-4-21-24(28-15)29-22(32)14-35-21/h2-5,10,12,17-18,26H,6-9,11,13-14H2,1H3,(H,28,29,32)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp electrophysiological analysis |
J Med Chem 57: 4889-905 (2014)
Article DOI: 10.1021/jm500432n
BindingDB Entry DOI: 10.7270/Q2416ZM4 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50018486
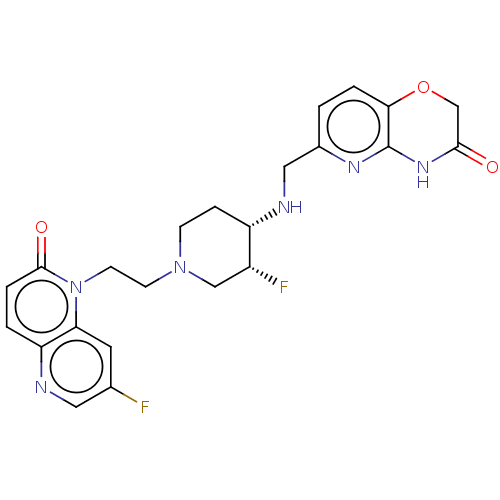
(CHEMBL3290354)Show SMILES F[C@@H]1CN(CCn2c3cc(F)cnc3ccc2=O)CC[C@@H]1NCc1ccc2OCC(=O)Nc2n1 |r| Show InChI InChI=1S/C23H24F2N6O3/c24-14-9-19-18(26-10-14)2-4-22(33)31(19)8-7-30-6-5-17(16(25)12-30)27-11-15-1-3-20-23(28-15)29-21(32)13-34-20/h1-4,9-10,16-17,27H,5-8,11-13H2,(H,28,29,32)/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp electrophysiological analysis |
J Med Chem 57: 4889-905 (2014)
Article DOI: 10.1021/jm500432n
BindingDB Entry DOI: 10.7270/Q2416ZM4 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50018485
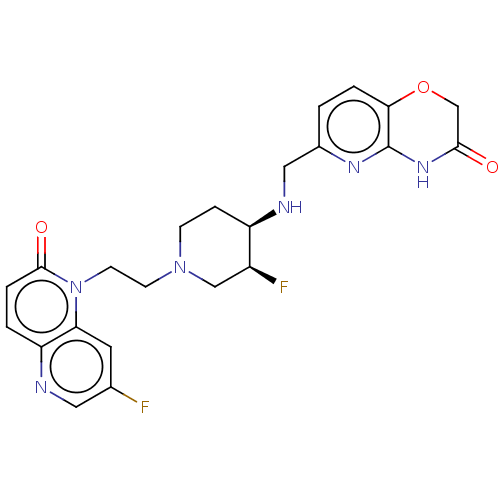
(CHEMBL3290353)Show SMILES F[C@H]1CN(CCn2c3cc(F)cnc3ccc2=O)CC[C@H]1NCc1ccc2OCC(=O)Nc2n1 |r| Show InChI InChI=1S/C23H24F2N6O3/c24-14-9-19-18(26-10-14)2-4-22(33)31(19)8-7-30-6-5-17(16(25)12-30)27-11-15-1-3-20-23(28-15)29-21(32)13-34-20/h1-4,9-10,16-17,27H,5-8,11-13H2,(H,28,29,32)/t16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp electrophysiological analysis |
J Med Chem 57: 4889-905 (2014)
Article DOI: 10.1021/jm500432n
BindingDB Entry DOI: 10.7270/Q2416ZM4 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50018448

(CHEMBL3290348)Show SMILES COc1cnc2ncc(=O)n(CCN3CCC(CC3)NCc3cnc(C)c(c3)C#N)c2c1 Show InChI InChI=1S/C23H27N7O2/c1-16-18(11-24)9-17(12-25-16)13-26-19-3-5-29(6-4-19)7-8-30-21-10-20(32-2)14-27-23(21)28-15-22(30)31/h9-10,12,14-15,19,26H,3-8,13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp electrophysiological analysis |
J Med Chem 57: 4889-905 (2014)
Article DOI: 10.1021/jm500432n
BindingDB Entry DOI: 10.7270/Q2416ZM4 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50018481

(CHEMBL3290349)Show SMILES Cc1ncc(CN[C@@H]2CCN(CCn3c4cc(ccc4ccc3=O)C#N)C[C@@H]2F)cc1C#N |r| Show InChI InChI=1S/C25H25FN6O/c1-17-21(13-28)10-19(14-29-17)15-30-23-6-7-31(16-22(23)26)8-9-32-24-11-18(12-27)2-3-20(24)4-5-25(32)33/h2-5,10-11,14,22-23,30H,6-9,15-16H2,1H3/t22-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp electrophysiological analysis |
J Med Chem 57: 4889-905 (2014)
Article DOI: 10.1021/jm500432n
BindingDB Entry DOI: 10.7270/Q2416ZM4 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50018482

(CHEMBL3290350)Show SMILES Cc1ncc(CN[C@@H]2CCN(CCn3c4cc(F)cnc4ccc3=O)C[C@@H]2F)cc1C#N |r| Show InChI InChI=1S/C23H24F2N6O/c1-15-17(10-26)8-16(11-27-15)12-28-20-4-5-30(14-19(20)25)6-7-31-22-9-18(24)13-29-21(22)2-3-23(31)32/h2-3,8-9,11,13,19-20,28H,4-7,12,14H2,1H3/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp electrophysiological analysis |
J Med Chem 57: 4889-905 (2014)
Article DOI: 10.1021/jm500432n
BindingDB Entry DOI: 10.7270/Q2416ZM4 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50018484

(CHEMBL3290352)Show SMILES CO[C@H]1CN(CCn2c3cc(OC)cnc3ccc2=O)CC[C@H]1NCc1cnc(C)c(c1)C#N |r| Show InChI InChI=1S/C25H30N6O3/c1-17-19(12-26)10-18(13-27-17)14-28-22-6-7-30(16-24(22)34-3)8-9-31-23-11-20(33-2)15-29-21(23)4-5-25(31)32/h4-5,10-11,13,15,22,24,28H,6-9,14,16H2,1-3H3/t22-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.51E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp electrophysiological analysis |
J Med Chem 57: 4889-905 (2014)
Article DOI: 10.1021/jm500432n
BindingDB Entry DOI: 10.7270/Q2416ZM4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data