 Found 99 hits with Last Name = 'hohlweg' and Initial = 'r'
Found 99 hits with Last Name = 'hohlweg' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(1A) dopamine receptor
(RAT) | BDBM82247

(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk
Curated by PDSP Ki Database
| |
Eur J Pharmacol 219: 45-52 (1992)
Article DOI: 10.1016/0014-2999(92)90578-r
BindingDB Entry DOI: 10.7270/Q2H70D9P |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM81778

(CAS_132421 | NNC-756 | NSC_132421)Show InChI InChI=1S/C19H20ClNO2/c1-21-7-5-13-9-17(20)18(22)10-15(13)16(11-21)14-4-2-3-12-6-8-23-19(12)14/h2-4,9-10,16,22H,5-8,11H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk
Curated by PDSP Ki Database
| |
Eur J Pharmacol 219: 45-52 (1992)
Article DOI: 10.1016/0014-2999(92)90578-r
BindingDB Entry DOI: 10.7270/Q2H70D9P |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50010709
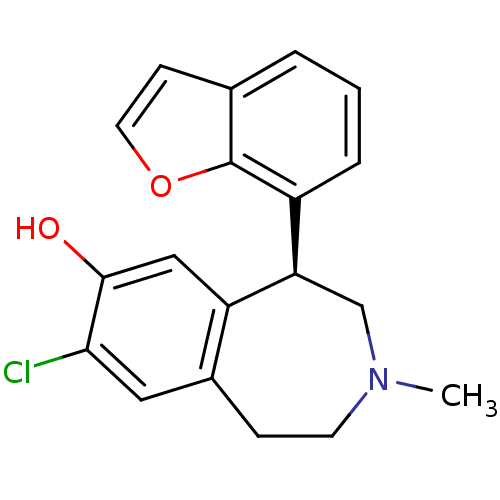
(5-Benzofuran-7-yl-8-chloro-3-methyl-2,3,4,5-tetrah...)Show InChI InChI=1S/C19H18ClNO2/c1-21-7-5-13-9-17(20)18(22)10-15(13)16(11-21)14-4-2-3-12-6-8-23-19(12)14/h2-4,6,8-10,16,22H,5,7,11H2,1H3/t16-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk
Curated by PDSP Ki Database
| |
Eur J Pharmacol 219: 45-52 (1992)
Article DOI: 10.1016/0014-2999(92)90578-r
BindingDB Entry DOI: 10.7270/Q2H70D9P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM126004
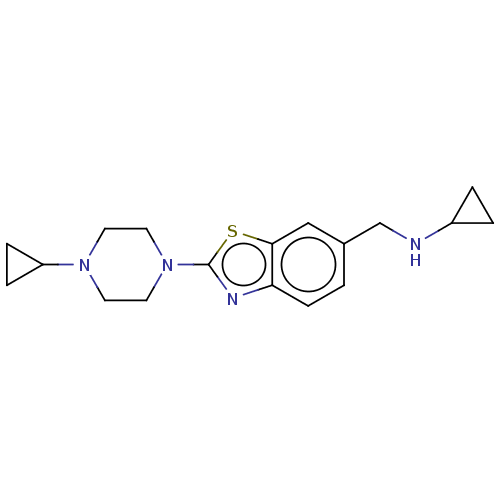
(US8772285, 27)Show InChI InChI=1S/C18H24N4S/c1-6-16-17(11-13(1)12-19-14-2-3-14)23-18(20-16)22-9-7-21(8-10-22)15-4-5-15/h1,6,11,14-15,19H,2-5,7-10,12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
High Point Pharmaceuticals, LLC
US Patent
| Assay Description
The ability of the compounds to bind and interact with the human H3 receptor as agonists, inverse agonists and/or antagonists, is determined by a fun... |
US Patent US8772285 (2014)
BindingDB Entry DOI: 10.7270/Q2P849KF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM126003
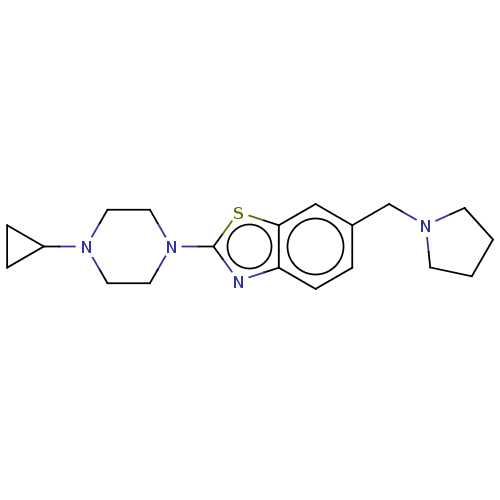
(US8772285, 17)Show InChI InChI=1S/C19H26N4S/c1-2-8-21(7-1)14-15-3-6-17-18(13-15)24-19(20-17)23-11-9-22(10-12-23)16-4-5-16/h3,6,13,16H,1-2,4-5,7-12,14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
High Point Pharmaceuticals, LLC
US Patent
| Assay Description
The ability of the compounds to bind and interact with the human H3 receptor as agonists, inverse agonists and/or antagonists, is determined by a fun... |
US Patent US8772285 (2014)
BindingDB Entry DOI: 10.7270/Q2P849KF |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 2
(Rattus norvegicus) | BDBM81778

(CAS_132421 | NNC-756 | NSC_132421)Show InChI InChI=1S/C19H20ClNO2/c1-21-7-5-13-9-17(20)18(22)10-15(13)16(11-21)14-4-2-3-12-6-8-23-19(12)14/h2-4,9-10,16,22H,5-8,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk
Curated by PDSP Ki Database
| |
Eur J Pharmacol 219: 45-52 (1992)
Article DOI: 10.1016/0014-2999(92)90578-r
BindingDB Entry DOI: 10.7270/Q2H70D9P |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 2
(Rattus norvegicus) | BDBM50010709

(5-Benzofuran-7-yl-8-chloro-3-methyl-2,3,4,5-tetrah...)Show InChI InChI=1S/C19H18ClNO2/c1-21-7-5-13-9-17(20)18(22)10-15(13)16(11-21)14-4-2-3-12-6-8-23-19(12)14/h2-4,6,8-10,16,22H,5,7,11H2,1H3/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk
Curated by PDSP Ki Database
| |
Eur J Pharmacol 219: 45-52 (1992)
Article DOI: 10.1016/0014-2999(92)90578-r
BindingDB Entry DOI: 10.7270/Q2H70D9P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193204
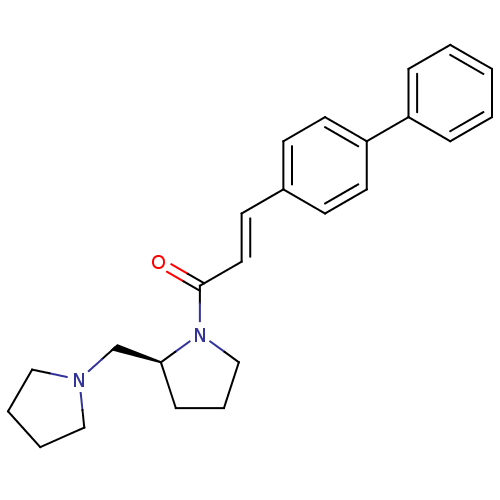
(3-biphenyl-4-yl-1-((S)-2-pyrrolidin-1-ylmethyl-pyr...)Show SMILES O=C(\C=C\c1ccc(cc1)-c1ccccc1)N1CCC[C@H]1CN1CCCC1 Show InChI InChI=1S/C24H28N2O/c27-24(26-18-6-9-23(26)19-25-16-4-5-17-25)15-12-20-10-13-22(14-11-20)21-7-2-1-3-8-21/h1-3,7-8,10-15,23H,4-6,9,16-19H2/b15-12+/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50185383
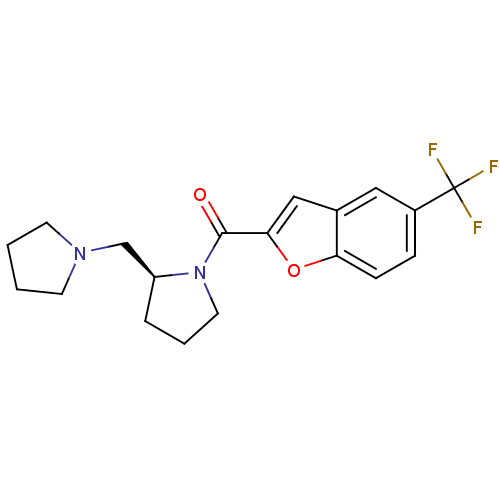
((S)-(2-(pyrrolidin-1-ylmethyl)pyrrolidin-1-yl)(5-(...)Show SMILES FC(F)(F)c1ccc2oc(cc2c1)C(=O)N1CCC[C@H]1CN1CCCC1 Show InChI InChI=1S/C19H21F3N2O2/c20-19(21,22)14-5-6-16-13(10-14)11-17(26-16)18(25)24-9-3-4-15(24)12-23-7-1-2-8-23/h5-6,10-11,15H,1-4,7-9,12H2/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor by [35S]GTPgammaS assay |
Bioorg Med Chem Lett 16: 3162-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.074
BindingDB Entry DOI: 10.7270/Q2T1537P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50185376
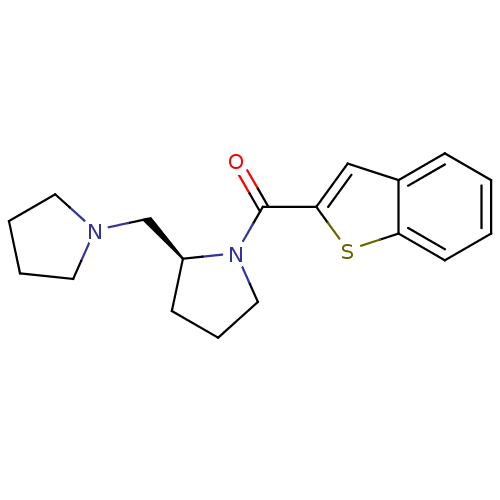
((S)-benzo[b]thiophen-2-yl(2-(pyrrolidin-1-ylmethyl...)Show InChI InChI=1S/C18H22N2OS/c21-18(17-12-14-6-1-2-8-16(14)22-17)20-11-5-7-15(20)13-19-9-3-4-10-19/h1-2,6,8,12,15H,3-5,7,9-11,13H2/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor by [35S]GTPgammaS assay |
Bioorg Med Chem Lett 16: 3162-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.074
BindingDB Entry DOI: 10.7270/Q2T1537P |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM81778

(CAS_132421 | NNC-756 | NSC_132421)Show InChI InChI=1S/C19H20ClNO2/c1-21-7-5-13-9-17(20)18(22)10-15(13)16(11-21)14-4-2-3-12-6-8-23-19(12)14/h2-4,9-10,16,22H,5-8,11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk
Curated by PDSP Ki Database
| |
Eur J Pharmacol 219: 45-52 (1992)
Article DOI: 10.1016/0014-2999(92)90578-r
BindingDB Entry DOI: 10.7270/Q2H70D9P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193199
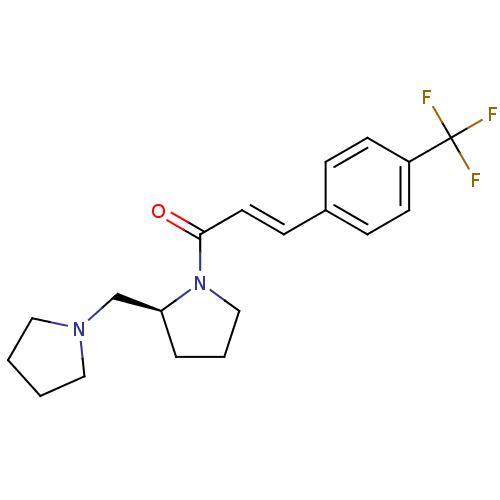
((S)-1-(2-(pyrrolidin-1-ylmethyl)pyrrolidin-1-yl)-3...)Show SMILES FC(F)(F)c1ccc(\C=C\C(=O)N2CCC[C@H]2CN2CCCC2)cc1 |r| Show InChI InChI=1S/C19H23F3N2O/c20-19(21,22)16-8-5-15(6-9-16)7-10-18(25)24-13-3-4-17(24)14-23-11-1-2-12-23/h5-10,17H,1-4,11-14H2/b10-7+/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50185378
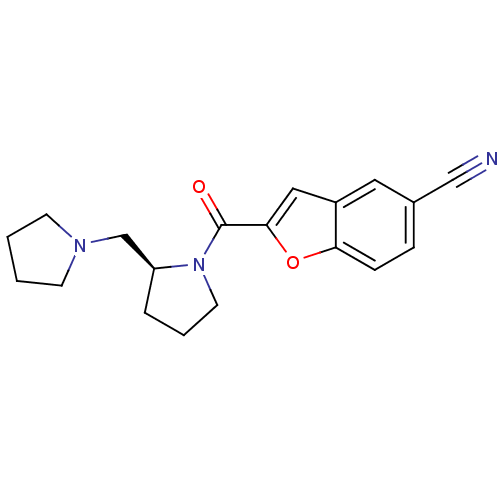
((S)-2-(2-(pyrrolidin-1-ylmethyl)pyrrolidine-1-carb...)Show InChI InChI=1S/C19H21N3O2/c20-12-14-5-6-17-15(10-14)11-18(24-17)19(23)22-9-3-4-16(22)13-21-7-1-2-8-21/h5-6,10-11,16H,1-4,7-9,13H2/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor by [35S]GTPgammaS assay |
Bioorg Med Chem Lett 16: 3162-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.074
BindingDB Entry DOI: 10.7270/Q2T1537P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50185371
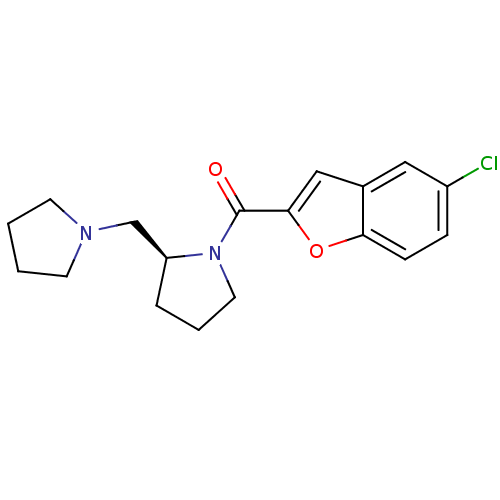
((S)-(5-chlorobenzofuran-2-yl)(2-(pyrrolidin-1-ylme...)Show InChI InChI=1S/C18H21ClN2O2/c19-14-5-6-16-13(10-14)11-17(23-16)18(22)21-9-3-4-15(21)12-20-7-1-2-8-20/h5-6,10-11,15H,1-4,7-9,12H2/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor by [35S]GTPgammaS assay |
Bioorg Med Chem Lett 16: 3162-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.074
BindingDB Entry DOI: 10.7270/Q2T1537P |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM81779
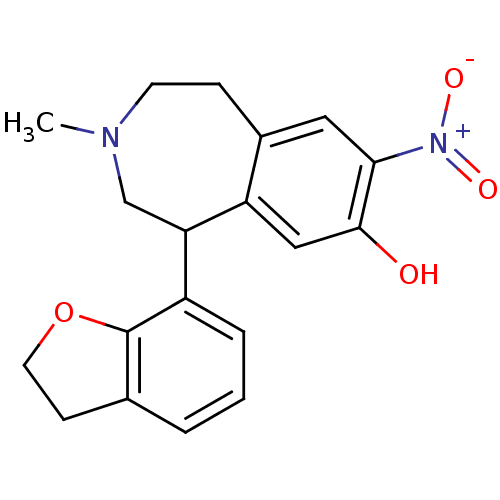
(CAS_164252 | NNC-687 | NSC_164252)Show SMILES CN1CCc2cc(c(O)cc2C(C1)c1cccc2CCOc12)[N+]([O-])=O Show InChI InChI=1S/C19H20N2O4/c1-20-7-5-13-9-17(21(23)24)18(22)10-15(13)16(11-20)14-4-2-3-12-6-8-25-19(12)14/h2-4,9-10,16,22H,5-8,11H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk
Curated by PDSP Ki Database
| |
Eur J Pharmacol 219: 45-52 (1992)
Article DOI: 10.1016/0014-2999(92)90578-r
BindingDB Entry DOI: 10.7270/Q2H70D9P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50185382
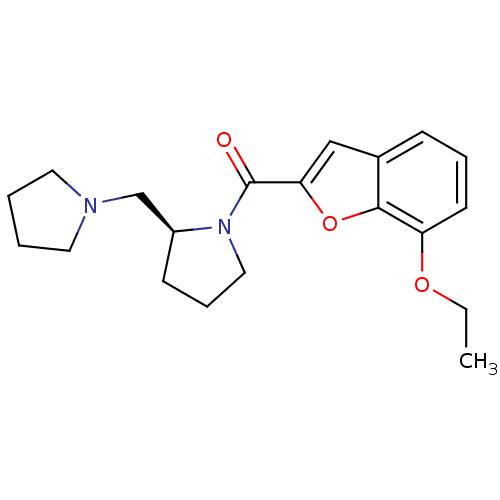
((S)-(7-ethoxybenzofuran-2-yl)(2-(pyrrolidin-1-ylme...)Show InChI InChI=1S/C20H26N2O3/c1-2-24-17-9-5-7-15-13-18(25-19(15)17)20(23)22-12-6-8-16(22)14-21-10-3-4-11-21/h5,7,9,13,16H,2-4,6,8,10-12,14H2,1H3/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor by [35S]GTPgammaS assay |
Bioorg Med Chem Lett 16: 3162-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.074
BindingDB Entry DOI: 10.7270/Q2T1537P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50185370
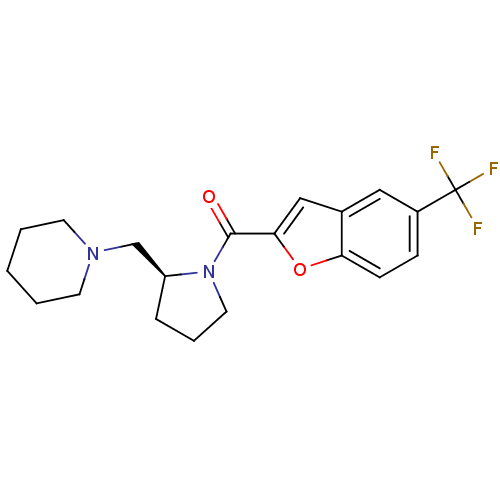
((S)-(2-(piperidin-1-ylmethyl)pyrrolidin-1-yl)(5-(t...)Show SMILES FC(F)(F)c1ccc2oc(cc2c1)C(=O)N1CCC[C@H]1CN1CCCCC1 Show InChI InChI=1S/C20H23F3N2O2/c21-20(22,23)15-6-7-17-14(11-15)12-18(27-17)19(26)25-10-4-5-16(25)13-24-8-2-1-3-9-24/h6-7,11-12,16H,1-5,8-10,13H2/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor by [35S]GTPgammaS assay |
Bioorg Med Chem Lett 16: 3162-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.074
BindingDB Entry DOI: 10.7270/Q2T1537P |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 2
(Rattus norvegicus) | BDBM81779

(CAS_164252 | NNC-687 | NSC_164252)Show SMILES CN1CCc2cc(c(O)cc2C(C1)c1cccc2CCOc12)[N+]([O-])=O Show InChI InChI=1S/C19H20N2O4/c1-20-7-5-13-9-17(21(23)24)18(22)10-15(13)16(11-20)14-4-2-3-12-6-8-25-19(12)14/h2-4,9-10,16,22H,5-8,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk
Curated by PDSP Ki Database
| |
Eur J Pharmacol 219: 45-52 (1992)
Article DOI: 10.1016/0014-2999(92)90578-r
BindingDB Entry DOI: 10.7270/Q2H70D9P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50185377
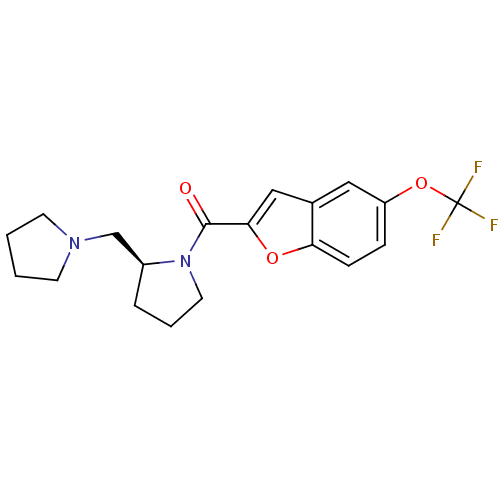
((S)-(2-(pyrrolidin-1-ylmethyl)pyrrolidin-1-yl)(5-(...)Show SMILES FC(F)(F)Oc1ccc2oc(cc2c1)C(=O)N1CCC[C@H]1CN1CCCC1 Show InChI InChI=1S/C19H21F3N2O3/c20-19(21,22)27-15-5-6-16-13(10-15)11-17(26-16)18(25)24-9-3-4-14(24)12-23-7-1-2-8-23/h5-6,10-11,14H,1-4,7-9,12H2/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor by [35S]GTPgammaS assay |
Bioorg Med Chem Lett 16: 3162-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.074
BindingDB Entry DOI: 10.7270/Q2T1537P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193175
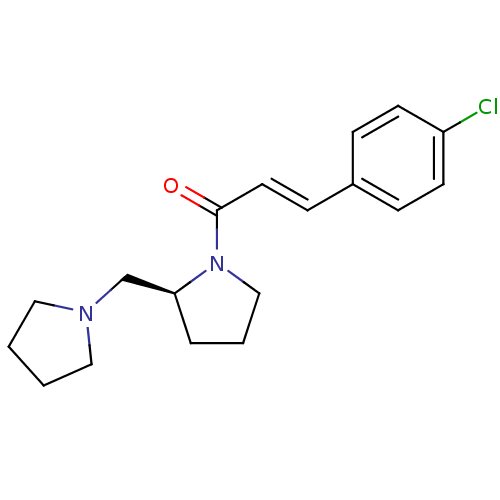
((S)-3-(4-chlorophenyl)-1-(2-(pyrrolidin-1-ylmethyl...)Show InChI InChI=1S/C18H23ClN2O/c19-16-8-5-15(6-9-16)7-10-18(22)21-13-3-4-17(21)14-20-11-1-2-12-20/h5-10,17H,1-4,11-14H2/b10-7+/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50185381
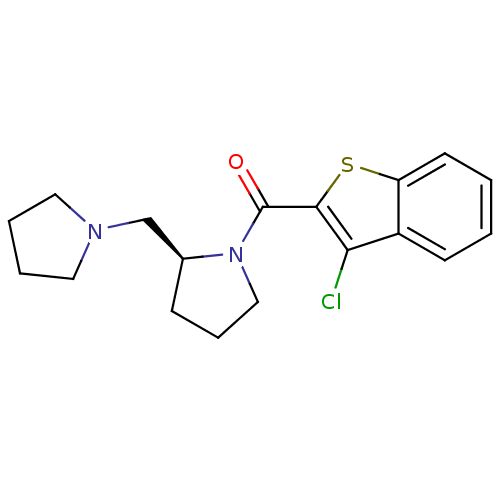
((S)-(3-chlorobenzo[b]thiophen-2-yl)(2-(pyrrolidin-...)Show InChI InChI=1S/C18H21ClN2OS/c19-16-14-7-1-2-8-15(14)23-17(16)18(22)21-11-5-6-13(21)12-20-9-3-4-10-20/h1-2,7-8,13H,3-6,9-12H2/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor by [35S]GTPgammaS assay |
Bioorg Med Chem Lett 16: 3162-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.074
BindingDB Entry DOI: 10.7270/Q2T1537P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193185

((S)-1-(2-(pyrrolidin-1-ylmethyl)pyrrolidin-1-yl)-3...)Show SMILES FC(F)(F)Oc1ccc(\C=C\C(=O)N2CCC[C@H]2CN2CCCC2)cc1 Show InChI InChI=1S/C19H23F3N2O2/c20-19(21,22)26-17-8-5-15(6-9-17)7-10-18(25)24-13-3-4-16(24)14-23-11-1-2-12-23/h5-10,16H,1-4,11-14H2/b10-7+/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 13.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50185372
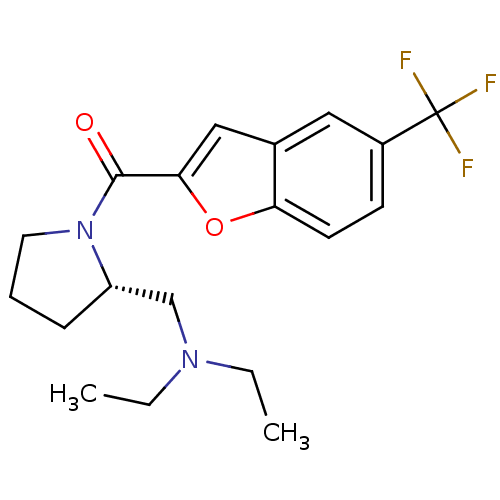
((S)-(2-((diethylamino)methyl)pyrrolidin-1-yl)(5-(t...)Show SMILES CCN(CC)C[C@@H]1CCCN1C(=O)c1cc2cc(ccc2o1)C(F)(F)F Show InChI InChI=1S/C19H23F3N2O2/c1-3-23(4-2)12-15-6-5-9-24(15)18(25)17-11-13-10-14(19(20,21)22)7-8-16(13)26-17/h7-8,10-11,15H,3-6,9,12H2,1-2H3/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor by [35S]GTPgammaS assay |
Bioorg Med Chem Lett 16: 3162-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.074
BindingDB Entry DOI: 10.7270/Q2T1537P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193189

(CHEMBL218834 | [4-(3-Aza-bicyclo[3.2.2]nonane-3-ca...)Show SMILES CC(C)N1CCN(CC1)C(=O)N1CCC(CC1)C(=O)N1CC2CCC(CC2)C1 |(-10.27,-12.49,;-8.93,-13.25,;-8.92,-14.79,;-7.6,-12.47,;-7.61,-10.93,;-6.29,-10.15,;-4.94,-10.9,;-4.93,-12.44,;-6.26,-13.23,;-3.62,-10.12,;-3.64,-8.58,;-2.28,-10.87,;-2.27,-12.41,;-.93,-13.16,;.4,-12.38,;.38,-10.84,;-.96,-10.08,;1.74,-13.14,;1.75,-14.68,;3.07,-12.37,;4.64,-12.83,;5.99,-11.86,;7.33,-12.56,;6.6,-11.37,;4.98,-10.51,;5.01,-9.04,;6.04,-10.28,;4.29,-11.83,)| Show InChI InChI=1S/C22H38N4O2/c1-17(2)23-11-13-25(14-12-23)22(28)24-9-7-20(8-10-24)21(27)26-15-18-3-4-19(16-26)6-5-18/h17-20H,3-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193209
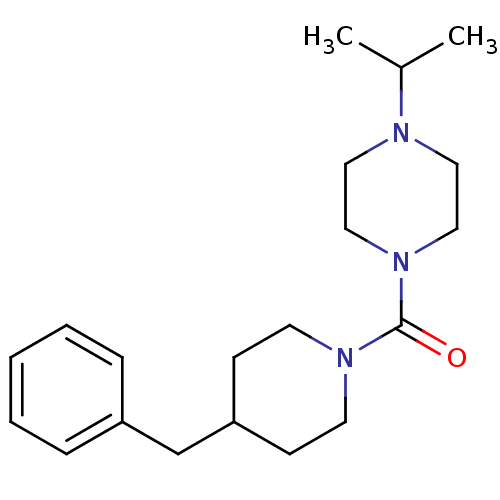
((4-benzylpiperidin-1-yl)(4-isopropylpiperazin-1-yl...)Show InChI InChI=1S/C20H31N3O/c1-17(2)21-12-14-23(15-13-21)20(24)22-10-8-19(9-11-22)16-18-6-4-3-5-7-18/h3-7,17,19H,8-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193171
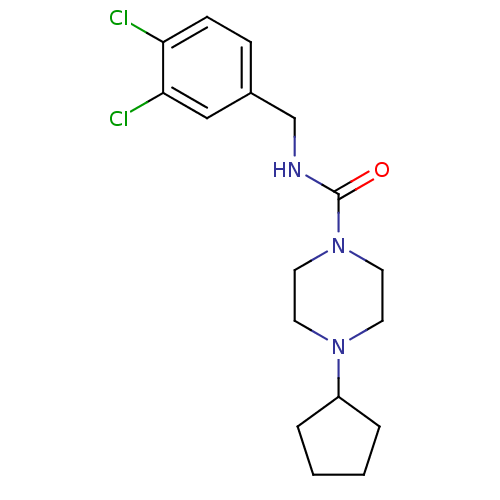
(CHEMBL218984 | N-(3,4-dichlorobenzyl)-4-cyclopenty...)Show InChI InChI=1S/C17H23Cl2N3O/c18-15-6-5-13(11-16(15)19)12-20-17(23)22-9-7-21(8-10-22)14-3-1-2-4-14/h5-6,11,14H,1-4,7-10,12H2,(H,20,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50185380
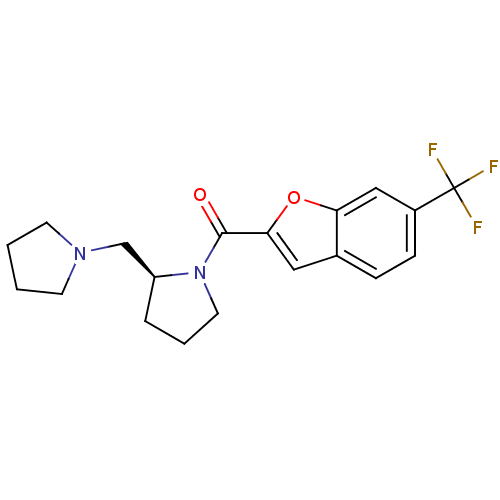
((S)-(2-(pyrrolidin-1-ylmethyl)pyrrolidin-1-yl)(6-(...)Show SMILES FC(F)(F)c1ccc2cc(oc2c1)C(=O)N1CCC[C@H]1CN1CCCC1 Show InChI InChI=1S/C19H21F3N2O2/c20-19(21,22)14-6-5-13-10-17(26-16(13)11-14)18(25)24-9-3-4-15(24)12-23-7-1-2-8-23/h5-6,10-11,15H,1-4,7-9,12H2/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor by [35S]GTPgammaS assay |
Bioorg Med Chem Lett 16: 3162-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.074
BindingDB Entry DOI: 10.7270/Q2T1537P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193208
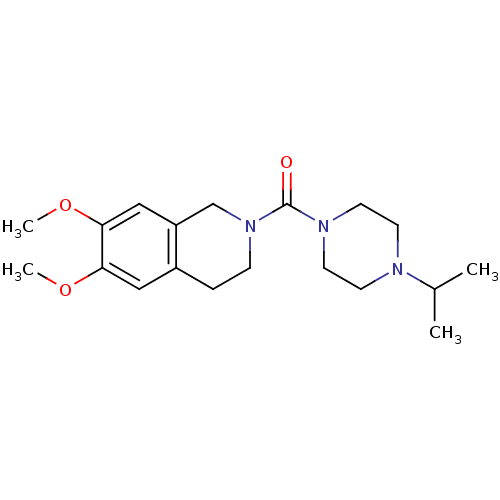
((6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)(4-...)Show InChI InChI=1S/C19H29N3O3/c1-14(2)20-7-9-21(10-8-20)19(23)22-6-5-15-11-17(24-3)18(25-4)12-16(15)13-22/h11-12,14H,5-10,13H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193180
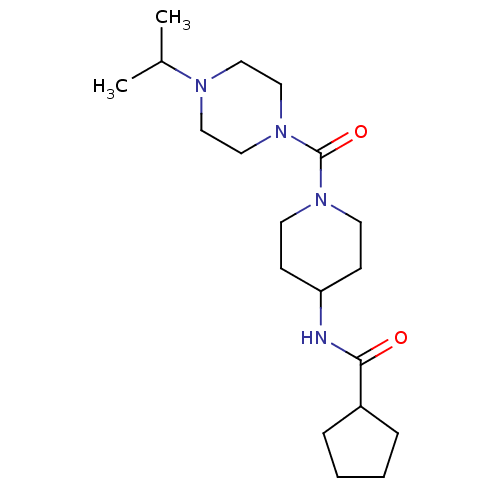
(CHEMBL220407 | N-(1-(1-isopropylpiperazine-4-carbo...)Show SMILES CC(C)N1CCN(CC1)C(=O)N1CCC(CC1)NC(=O)C1CCCC1 Show InChI InChI=1S/C19H34N4O2/c1-15(2)21-11-13-23(14-12-21)19(25)22-9-7-17(8-10-22)20-18(24)16-5-3-4-6-16/h15-17H,3-14H2,1-2H3,(H,20,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50004822
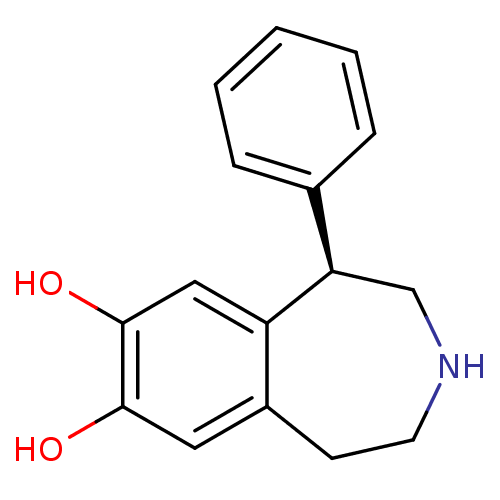
((R)-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]azepine...)Show InChI InChI=1S/C16H17NO2/c18-15-8-12-6-7-17-10-14(13(12)9-16(15)19)11-4-2-1-3-5-11/h1-5,8-9,14,17-19H,6-7,10H2/t14-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk
Curated by PDSP Ki Database
| |
Eur J Pharmacol 219: 45-52 (1992)
Article DOI: 10.1016/0014-2999(92)90578-r
BindingDB Entry DOI: 10.7270/Q2H70D9P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193203
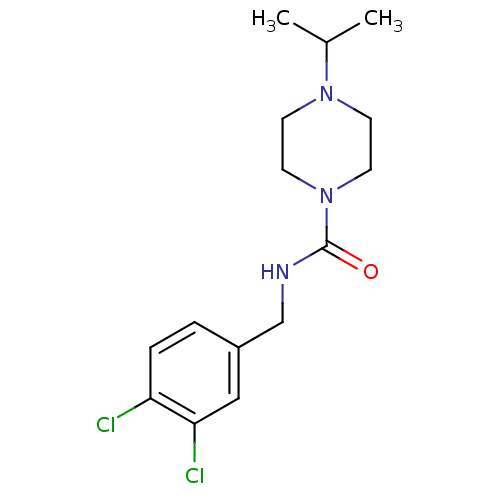
(CHEMBL374023 | N-(3,4-dichlorobenzyl)-4-isopropylp...)Show InChI InChI=1S/C15H21Cl2N3O/c1-11(2)19-5-7-20(8-6-19)15(21)18-10-12-3-4-13(16)14(17)9-12/h3-4,9,11H,5-8,10H2,1-2H3,(H,18,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50185375
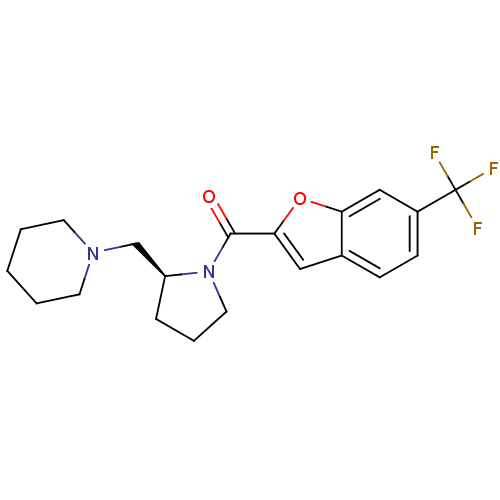
((S)-(2-(piperidin-1-ylmethyl)pyrrolidin-1-yl)(6-(t...)Show SMILES FC(F)(F)c1ccc2cc(oc2c1)C(=O)N1CCC[C@H]1CN1CCCCC1 Show InChI InChI=1S/C20H23F3N2O2/c21-20(22,23)15-7-6-14-11-18(27-17(14)12-15)19(26)25-10-4-5-16(25)13-24-8-2-1-3-9-24/h6-7,11-12,16H,1-5,8-10,13H2/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor by [35S]GTPgammaS assay |
Bioorg Med Chem Lett 16: 3162-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.074
BindingDB Entry DOI: 10.7270/Q2T1537P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193210
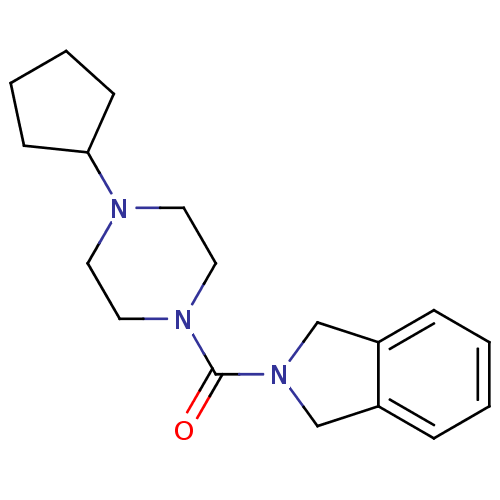
((4-cyclopentylpiperazin-1-yl)(isoindolin-2-yl)meth...)Show InChI InChI=1S/C18H25N3O/c22-18(21-13-15-5-1-2-6-16(15)14-21)20-11-9-19(10-12-20)17-7-3-4-8-17/h1-2,5-6,17H,3-4,7-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193179
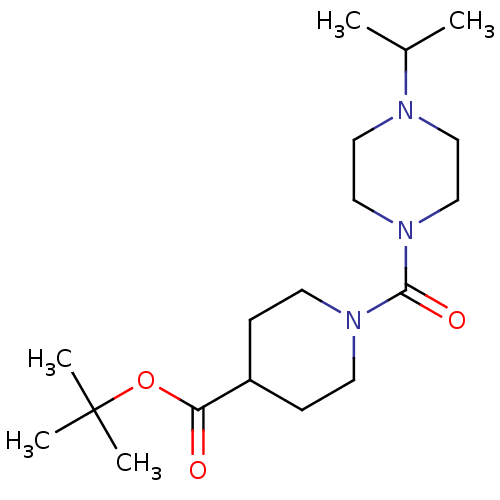
(CHEMBL218410 | tert-butyl 1-(1-isopropylpiperazine...)Show SMILES CC(C)N1CCN(CC1)C(=O)N1CCC(CC1)C(=O)OC(C)(C)C Show InChI InChI=1S/C18H33N3O3/c1-14(2)19-10-12-21(13-11-19)17(23)20-8-6-15(7-9-20)16(22)24-18(3,4)5/h14-15H,6-13H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193197
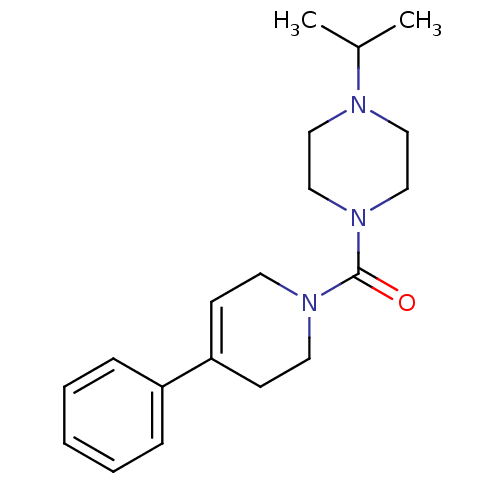
((4-isopropylpiperazin-1-yl)(4-phenyl-5,6-dihydropy...)Show SMILES CC(C)N1CCN(CC1)C(=O)N1CCC(=CC1)c1ccccc1 |c:15| Show InChI InChI=1S/C19H27N3O/c1-16(2)20-12-14-22(15-13-20)19(23)21-10-8-18(9-11-21)17-6-4-3-5-7-17/h3-8,16H,9-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193201
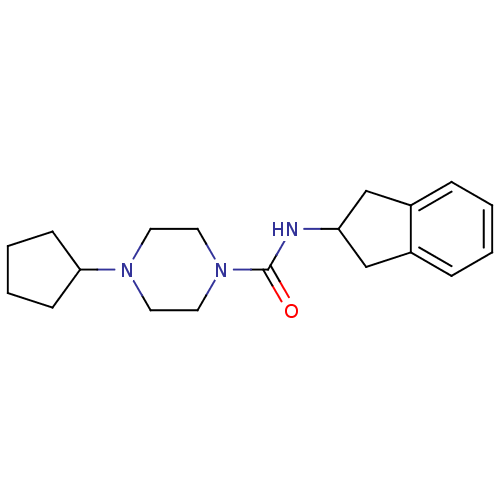
(4-cyclopentyl-N-(2,3-dihydro-1H-inden-2-yl)piperaz...)Show InChI InChI=1S/C19H27N3O/c23-19(20-17-13-15-5-1-2-6-16(15)14-17)22-11-9-21(10-12-22)18-7-3-4-8-18/h1-2,5-6,17-18H,3-4,7-14H2,(H,20,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193181

(CHEMBL219368 | N-(2,3-dihydro-1H-inden-2-yl)-4-iso...)Show InChI InChI=1S/C17H25N3O/c1-13(2)19-7-9-20(10-8-19)17(21)18-16-11-14-5-3-4-6-15(14)12-16/h3-6,13,16H,7-12H2,1-2H3,(H,18,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193178
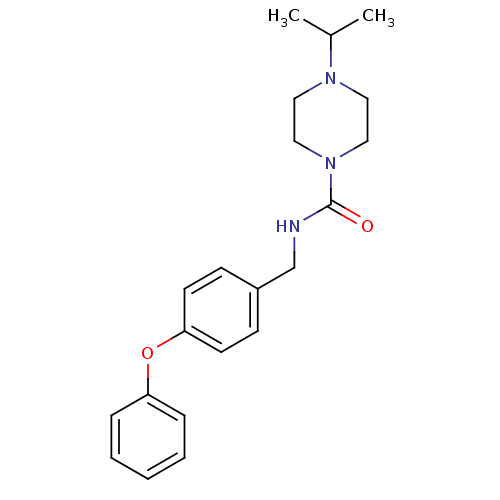
(CHEMBL441708 | N-(4-phenoxybenzyl)-4-isopropylpipe...)Show InChI InChI=1S/C21H27N3O2/c1-17(2)23-12-14-24(15-13-23)21(25)22-16-18-8-10-20(11-9-18)26-19-6-4-3-5-7-19/h3-11,17H,12-16H2,1-2H3,(H,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193196
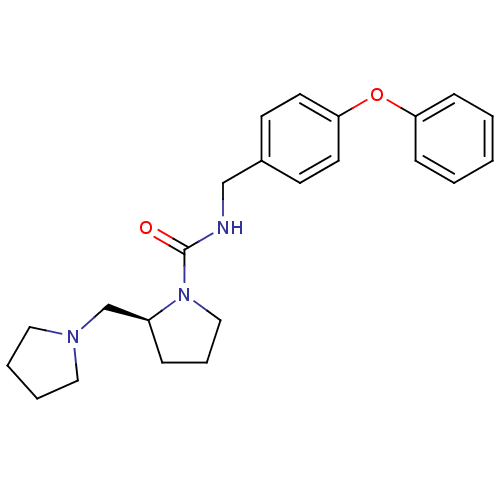
((S)-N-(4-phenoxybenzyl)-2-(pyrrolidin-1-ylmethyl)p...)Show SMILES O=C(NCc1ccc(Oc2ccccc2)cc1)N1CCC[C@H]1CN1CCCC1 Show InChI InChI=1S/C23H29N3O2/c27-23(26-16-6-7-20(26)18-25-14-4-5-15-25)24-17-19-10-12-22(13-11-19)28-21-8-2-1-3-9-21/h1-3,8-13,20H,4-7,14-18H2,(H,24,27)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM82247

(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk
Curated by PDSP Ki Database
| |
Eur J Pharmacol 219: 45-52 (1992)
Article DOI: 10.1016/0014-2999(92)90578-r
BindingDB Entry DOI: 10.7270/Q2H70D9P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193211
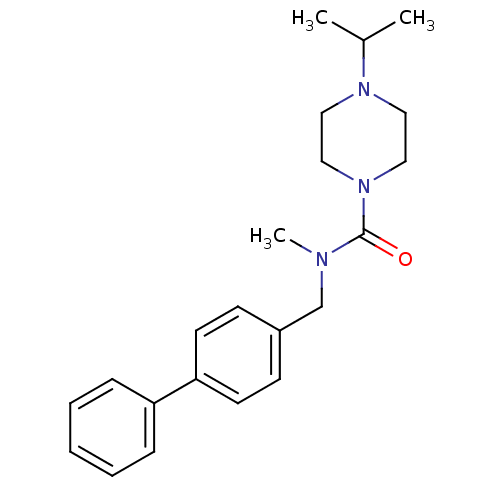
(4-isopropyl-piperazine-1-carboxylic acid biphenyl-...)Show SMILES CC(C)N1CCN(CC1)C(=O)N(C)Cc1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C22H29N3O/c1-18(2)24-13-15-25(16-14-24)22(26)23(3)17-19-9-11-21(12-10-19)20-7-5-4-6-8-20/h4-12,18H,13-17H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193187

(CHEMBL218860 | N-(4-phenoxybenzyl)-4-cyclopentylpi...)Show SMILES O=C(NCc1ccc(Oc2ccccc2)cc1)N1CCN(CC1)C1CCCC1 Show InChI InChI=1S/C23H29N3O2/c27-23(26-16-14-25(15-17-26)20-6-4-5-7-20)24-18-19-10-12-22(13-11-19)28-21-8-2-1-3-9-21/h1-3,8-13,20H,4-7,14-18H2,(H,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 2
(Rattus norvegicus) | BDBM82247

(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk
Curated by PDSP Ki Database
| |
Eur J Pharmacol 219: 45-52 (1992)
Article DOI: 10.1016/0014-2999(92)90578-r
BindingDB Entry DOI: 10.7270/Q2H70D9P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193193
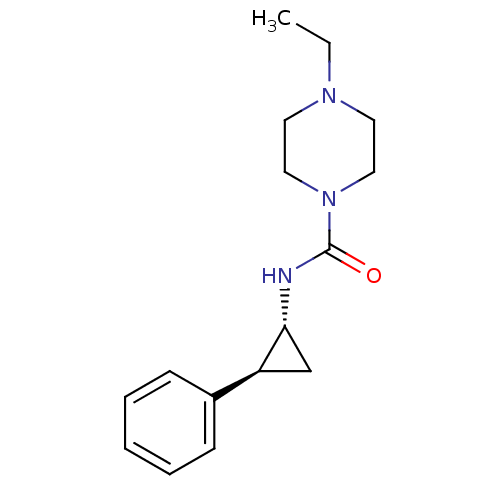
(4-ethyl-N-((1R,2S)-2-phenylcyclopropyl)piperazine-...)Show InChI InChI=1S/C16H23N3O/c1-2-18-8-10-19(11-9-18)16(20)17-15-12-14(15)13-6-4-3-5-7-13/h3-7,14-15H,2,8-12H2,1H3,(H,17,20)/t14-,15+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50019293
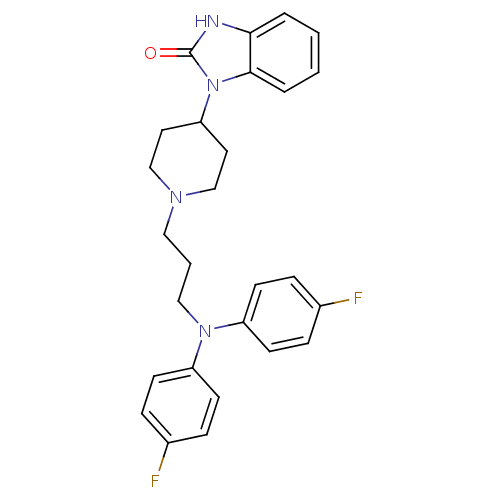
(1-(1-{3-[Bis-(4-fluoro-phenyl)-amino]-propyl}-pipe...)Show SMILES Fc1ccc(cc1)N(CCCN1CCC(CC1)n1c2ccccc2[nH]c1=O)c1ccc(F)cc1 Show InChI InChI=1S/C27H28F2N4O/c28-20-6-10-22(11-7-20)32(23-12-8-21(29)9-13-23)17-3-16-31-18-14-24(15-19-31)33-26-5-2-1-4-25(26)30-27(33)34/h1-2,4-13,24H,3,14-19H2,(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Displacement of [3H]Astemizole from hERG expressed in HEK293 cells at 10 uM |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193176
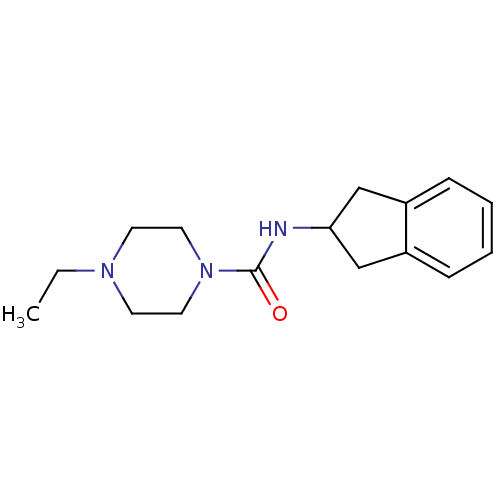
(CHEMBL373540 | N-(2,3-dihydro-1H-inden-2-yl)-4-eth...)Show InChI InChI=1S/C16H23N3O/c1-2-18-7-9-19(10-8-18)16(20)17-15-11-13-5-3-4-6-14(13)12-15/h3-6,15H,2,7-12H2,1H3,(H,17,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193198
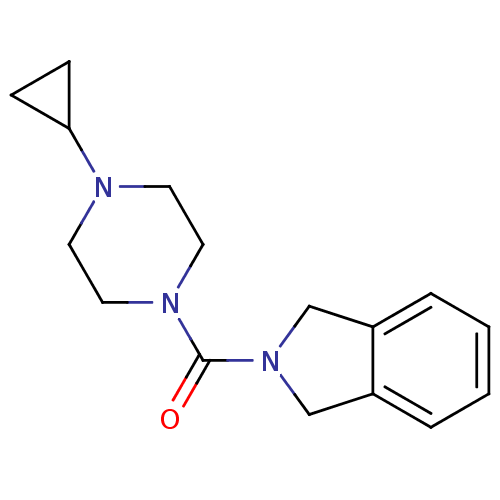
((4-cyclopropylpiperazin-1-yl)(isoindolin-2-yl)meth...)Show InChI InChI=1S/C16H21N3O/c20-16(18-9-7-17(8-10-18)15-5-6-15)19-11-13-3-1-2-4-14(13)12-19/h1-4,15H,5-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50185374

((S)-(2-((diethylamino)methyl)pyrrolidin-1-yl)(6-(t...)Show SMILES CCN(CC)C[C@@H]1CCCN1C(=O)c1cc2ccc(cc2o1)C(F)(F)F Show InChI InChI=1S/C19H23F3N2O2/c1-3-23(4-2)12-15-6-5-9-24(15)18(25)17-10-13-7-8-14(19(20,21)22)11-16(13)26-17/h7-8,10-11,15H,3-6,9,12H2,1-2H3/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor by [35S]GTPgammaS assay |
Bioorg Med Chem Lett 16: 3162-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.074
BindingDB Entry DOI: 10.7270/Q2T1537P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193172
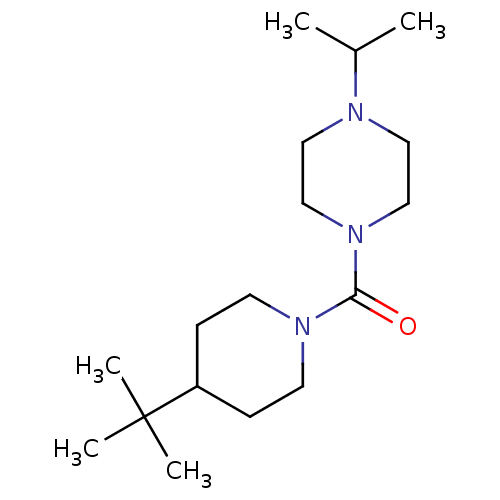
((4-tert-butylpiperidin-1-yl)(4-isopropylpiperazin-...)Show InChI InChI=1S/C17H33N3O/c1-14(2)18-10-12-20(13-11-18)16(21)19-8-6-15(7-9-19)17(3,4)5/h14-15H,6-13H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193205
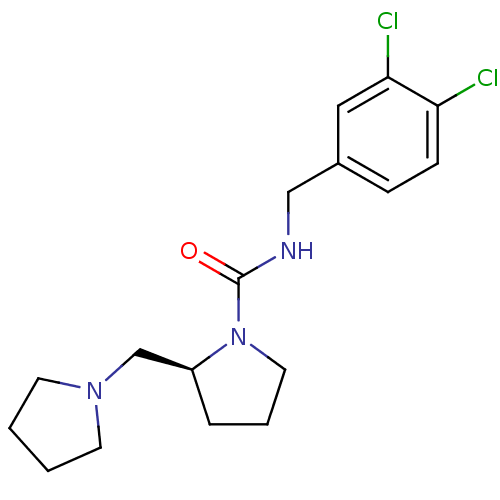
((S)-N-(3,4-dichlorobenzyl)-2-(pyrrolidin-1-ylmethy...)Show InChI InChI=1S/C17H23Cl2N3O/c18-15-6-5-13(10-16(15)19)11-20-17(23)22-9-3-4-14(22)12-21-7-1-2-8-21/h5-6,10,14H,1-4,7-9,11-12H2,(H,20,23)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data