

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase B-raf (Mus musculus) | BDBM5446 (CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Aljouf University Curated by ChEMBL | Assay Description Inhibition of mouse full-length GST-tagged BRAF V600E mutant using recombinant human full length N-terminal His-tagged MEK1 as substrate preincubated... | Eur J Med Chem 146: 260-273 (2018) Article DOI: 10.1016/j.ejmech.2018.01.042 BindingDB Entry DOI: 10.7270/Q25X2CKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5446 (CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Aljouf University Curated by ChEMBL | Assay Description Inhibition of His6-tagged EGFR (unknown origin) cytoplasmic domain expressed in baculovirus infected Sf9 insect cells after 10 mins followed by addit... | Eur J Med Chem 146: 260-273 (2018) Article DOI: 10.1016/j.ejmech.2018.01.042 BindingDB Entry DOI: 10.7270/Q25X2CKD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase B-raf (Mus musculus) | BDBM50457924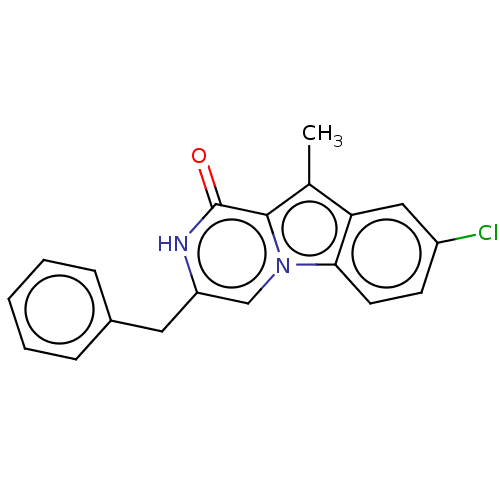 (CHEMBL4208224) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Aljouf University Curated by ChEMBL | Assay Description Inhibition of mouse full-length GST-tagged BRAF V600E mutant using recombinant human full length N-terminal His-tagged MEK1 as substrate preincubated... | Eur J Med Chem 146: 260-273 (2018) Article DOI: 10.1016/j.ejmech.2018.01.042 BindingDB Entry DOI: 10.7270/Q25X2CKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50457923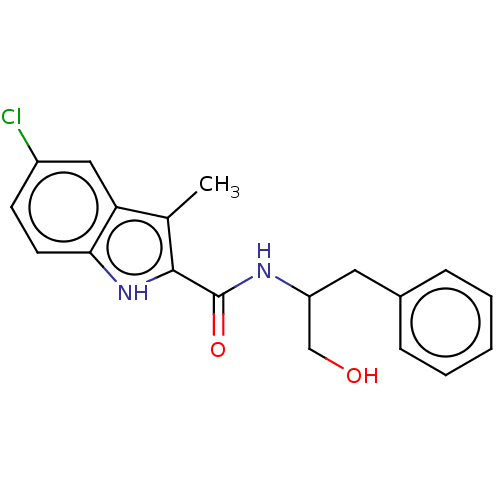 (CHEMBL4209588) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Aljouf University Curated by ChEMBL | Assay Description Inhibition of His6-tagged EGFR (unknown origin) cytoplasmic domain expressed in baculovirus infected Sf9 insect cells after 10 mins followed by addit... | Eur J Med Chem 146: 260-273 (2018) Article DOI: 10.1016/j.ejmech.2018.01.042 BindingDB Entry DOI: 10.7270/Q25X2CKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50457926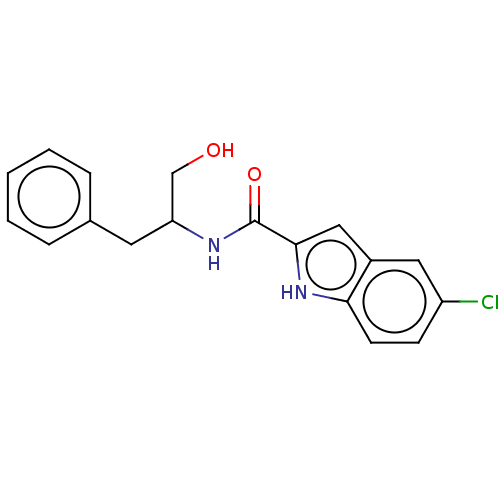 (CHEMBL4214496) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Aljouf University Curated by ChEMBL | Assay Description Inhibition of His6-tagged EGFR (unknown origin) cytoplasmic domain expressed in baculovirus infected Sf9 insect cells after 10 mins followed by addit... | Eur J Med Chem 146: 260-273 (2018) Article DOI: 10.1016/j.ejmech.2018.01.042 BindingDB Entry DOI: 10.7270/Q25X2CKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University Curated by ChEMBL | Assay Description Inhibition of recombinant human COX2 using arachidonic acid as substrate assessed as decrease in PGF2 production preincubated for 15 mins followed by... | Eur J Med Chem 127: 972-985 (2017) Article DOI: 10.1016/j.ejmech.2016.11.006 BindingDB Entry DOI: 10.7270/Q2NC63G3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50457925 (CHEMBL4207316) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Aljouf University Curated by ChEMBL | Assay Description Inhibition of His6-tagged EGFR (unknown origin) cytoplasmic domain expressed in baculovirus infected Sf9 insect cells after 10 mins followed by addit... | Eur J Med Chem 146: 260-273 (2018) Article DOI: 10.1016/j.ejmech.2018.01.042 BindingDB Entry DOI: 10.7270/Q25X2CKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Mus musculus) | BDBM50457928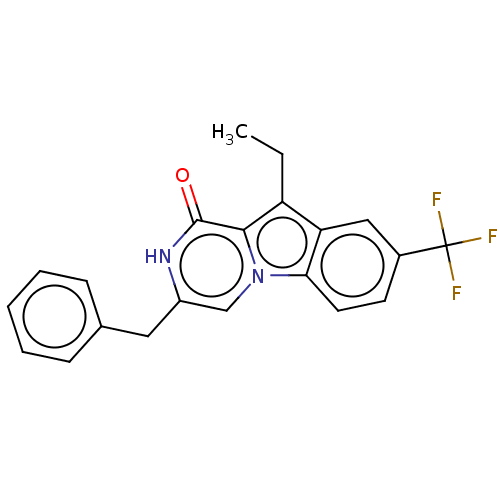 (CHEMBL4213146) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Aljouf University Curated by ChEMBL | Assay Description Inhibition of mouse full-length GST-tagged BRAF V600E mutant using recombinant human full length N-terminal His-tagged MEK1 as substrate preincubated... | Eur J Med Chem 146: 260-273 (2018) Article DOI: 10.1016/j.ejmech.2018.01.042 BindingDB Entry DOI: 10.7270/Q25X2CKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Mus musculus) | BDBM50457927 (CHEMBL4208745) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aljouf University Curated by ChEMBL | Assay Description Inhibition of mouse full-length GST-tagged BRAF V600E mutant using recombinant human full length N-terminal His-tagged MEK1 as substrate preincubated... | Eur J Med Chem 146: 260-273 (2018) Article DOI: 10.1016/j.ejmech.2018.01.042 BindingDB Entry DOI: 10.7270/Q25X2CKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Mus musculus) | BDBM50457925 (CHEMBL4207316) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aljouf University Curated by ChEMBL | Assay Description Inhibition of mouse full-length GST-tagged BRAF V600E mutant using recombinant human full length N-terminal His-tagged MEK1 as substrate preincubated... | Eur J Med Chem 146: 260-273 (2018) Article DOI: 10.1016/j.ejmech.2018.01.042 BindingDB Entry DOI: 10.7270/Q25X2CKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50235313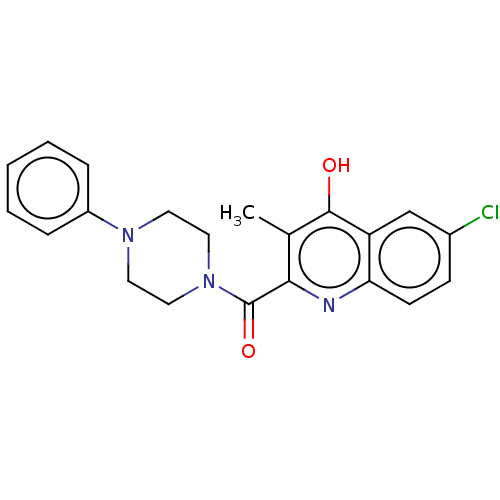 (CHEMBL4074825) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University Curated by ChEMBL | Assay Description Inhibition of recombinant human COX2 using arachidonic acid as substrate assessed as decrease in PGF2 production preincubated for 15 mins followed by... | Eur J Med Chem 127: 972-985 (2017) Article DOI: 10.1016/j.ejmech.2016.11.006 BindingDB Entry DOI: 10.7270/Q2NC63G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50235315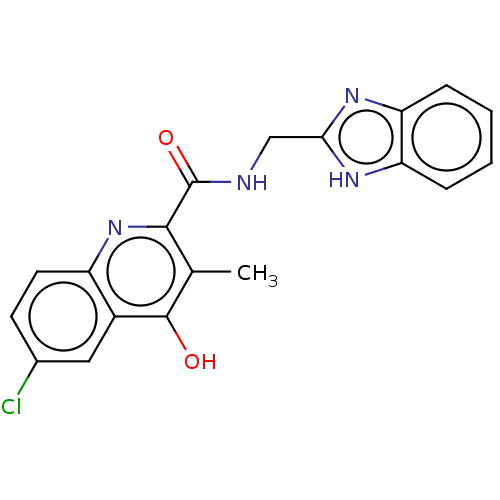 (CHEMBL4069774) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University Curated by ChEMBL | Assay Description Inhibitory activity was determined against almonds Beta-glucosidase | Eur J Med Chem 127: 972-985 (2017) Article DOI: 10.1016/j.ejmech.2016.11.006 BindingDB Entry DOI: 10.7270/Q2NC63G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50235320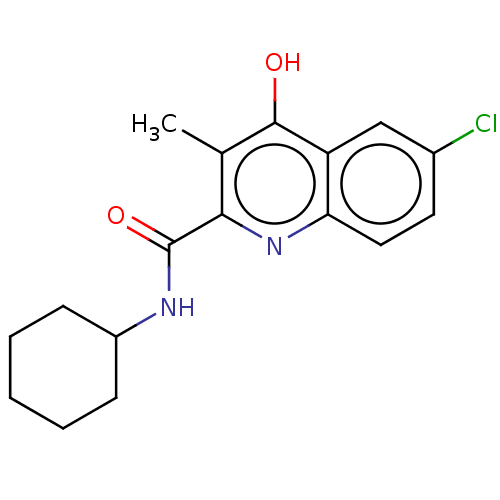 (CHEMBL4087573) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University Curated by ChEMBL | Assay Description Inhibitory activity was determined against Escherichia coli beta galactosidase | Eur J Med Chem 127: 972-985 (2017) Article DOI: 10.1016/j.ejmech.2016.11.006 BindingDB Entry DOI: 10.7270/Q2NC63G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50235317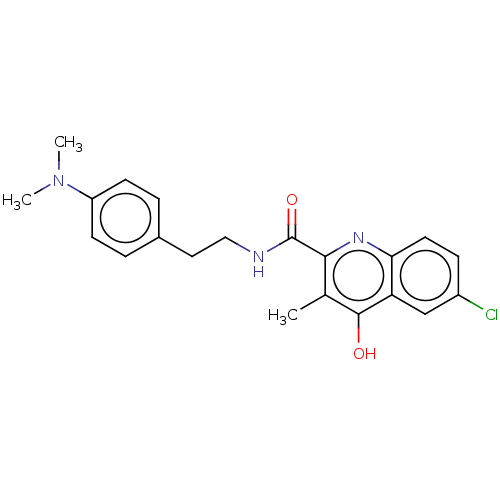 (CHEMBL4080473) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University Curated by ChEMBL | Assay Description Inhibition of recombinant human COX2 using arachidonic acid as substrate assessed as decrease in PGF2 production preincubated for 15 mins followed by... | Eur J Med Chem 127: 972-985 (2017) Article DOI: 10.1016/j.ejmech.2016.11.006 BindingDB Entry DOI: 10.7270/Q2NC63G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50235314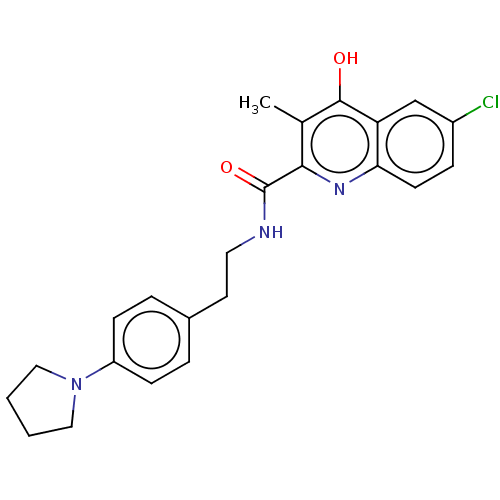 (CHEMBL4103022) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University Curated by ChEMBL | Assay Description Inhibition of recombinant human COX2 using arachidonic acid as substrate assessed as decrease in PGF2 production preincubated for 15 mins followed by... | Eur J Med Chem 127: 972-985 (2017) Article DOI: 10.1016/j.ejmech.2016.11.006 BindingDB Entry DOI: 10.7270/Q2NC63G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50457924 (CHEMBL4208224) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aljouf University Curated by ChEMBL | Assay Description Inhibition of His6-tagged EGFR (unknown origin) cytoplasmic domain expressed in baculovirus infected Sf9 insect cells after 10 mins followed by addit... | Eur J Med Chem 146: 260-273 (2018) Article DOI: 10.1016/j.ejmech.2018.01.042 BindingDB Entry DOI: 10.7270/Q25X2CKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50235309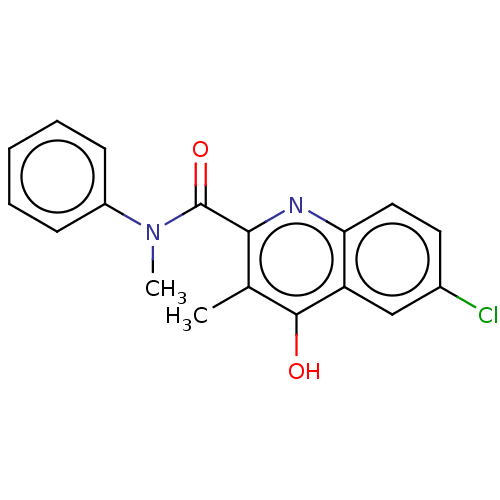 (CHEMBL4096972) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University Curated by ChEMBL | Assay Description Inhibition of recombinant human COX2 using arachidonic acid as substrate assessed as decrease in PGF2 production preincubated for 15 mins followed by... | Eur J Med Chem 127: 972-985 (2017) Article DOI: 10.1016/j.ejmech.2016.11.006 BindingDB Entry DOI: 10.7270/Q2NC63G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50235311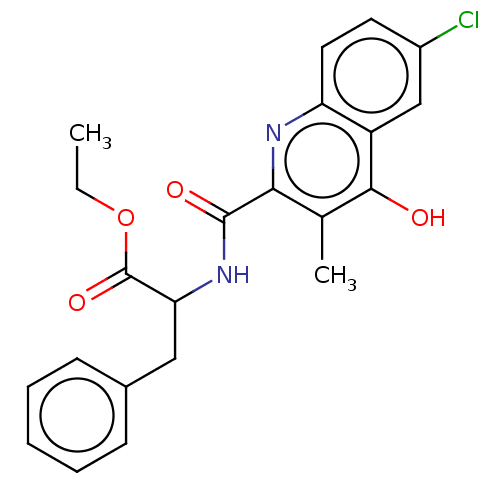 (CHEMBL4095272) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University Curated by ChEMBL | Assay Description Inhibition of recombinant human COX2 using arachidonic acid as substrate assessed as decrease in PGF2 production preincubated for 15 mins followed by... | Eur J Med Chem 127: 972-985 (2017) Article DOI: 10.1016/j.ejmech.2016.11.006 BindingDB Entry DOI: 10.7270/Q2NC63G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50235308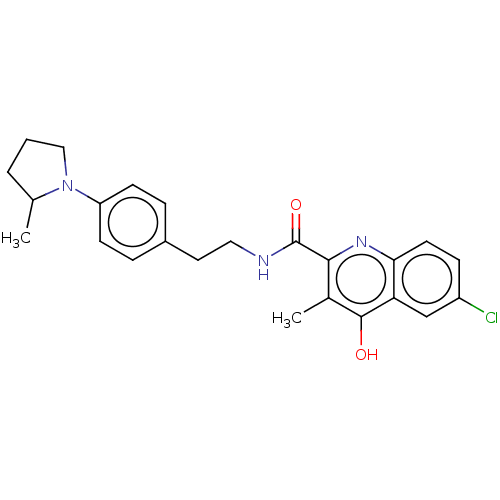 (CHEMBL4089272) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University Curated by ChEMBL | Assay Description Inhibition of recombinant human COX2 using arachidonic acid as substrate assessed as decrease in PGF2 production preincubated for 15 mins followed by... | Eur J Med Chem 127: 972-985 (2017) Article DOI: 10.1016/j.ejmech.2016.11.006 BindingDB Entry DOI: 10.7270/Q2NC63G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50235310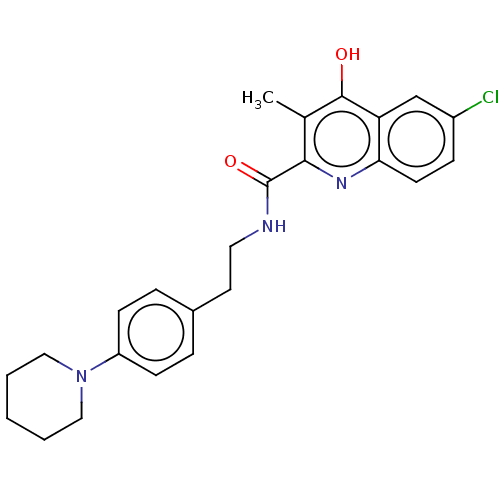 (CHEMBL4099420) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University Curated by ChEMBL | Assay Description Inhibition of recombinant human COX2 using arachidonic acid as substrate assessed as decrease in PGF2 production preincubated for 15 mins followed by... | Eur J Med Chem 127: 972-985 (2017) Article DOI: 10.1016/j.ejmech.2016.11.006 BindingDB Entry DOI: 10.7270/Q2NC63G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50457927 (CHEMBL4208745) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aljouf University Curated by ChEMBL | Assay Description Inhibition of His6-tagged EGFR (unknown origin) cytoplasmic domain expressed in baculovirus infected Sf9 insect cells after 10 mins followed by addit... | Eur J Med Chem 146: 260-273 (2018) Article DOI: 10.1016/j.ejmech.2018.01.042 BindingDB Entry DOI: 10.7270/Q25X2CKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50235307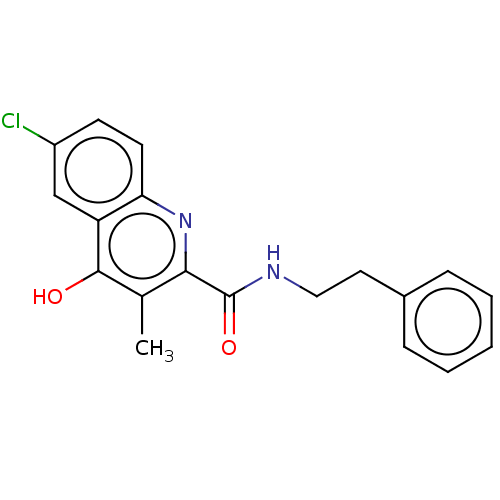 (CHEMBL4061105) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University Curated by ChEMBL | Assay Description Inhibition of recombinant human COX2 using arachidonic acid as substrate assessed as decrease in PGF2 production preincubated for 15 mins followed by... | Eur J Med Chem 127: 972-985 (2017) Article DOI: 10.1016/j.ejmech.2016.11.006 BindingDB Entry DOI: 10.7270/Q2NC63G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50235321 (CHEMBL4066211) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University Curated by ChEMBL | Assay Description Inhibitory activity was determined against Escherichia coli beta galactosidase | Eur J Med Chem 127: 972-985 (2017) Article DOI: 10.1016/j.ejmech.2016.11.006 BindingDB Entry DOI: 10.7270/Q2NC63G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50235319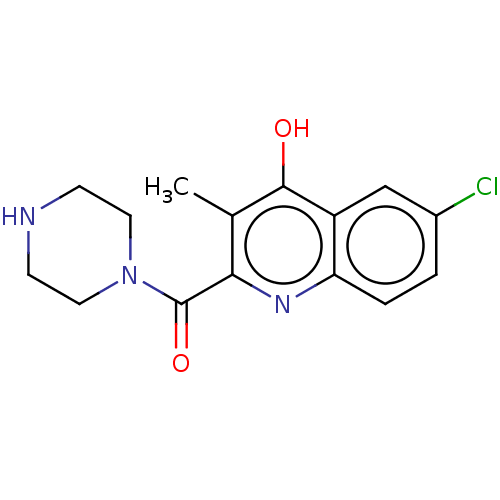 (CHEMBL4066899) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University Curated by ChEMBL | Assay Description Inhibition of recombinant human COX2 using arachidonic acid as substrate assessed as decrease in PGF2 production preincubated for 15 mins followed by... | Eur J Med Chem 127: 972-985 (2017) Article DOI: 10.1016/j.ejmech.2016.11.006 BindingDB Entry DOI: 10.7270/Q2NC63G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50235316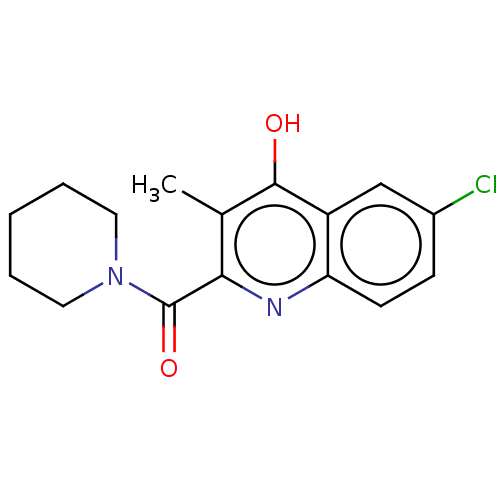 (CHEMBL4104705) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University Curated by ChEMBL | Assay Description Inhibition of recombinant human COX2 using arachidonic acid as substrate assessed as decrease in PGF2 production preincubated for 15 mins followed by... | Eur J Med Chem 127: 972-985 (2017) Article DOI: 10.1016/j.ejmech.2016.11.006 BindingDB Entry DOI: 10.7270/Q2NC63G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50235318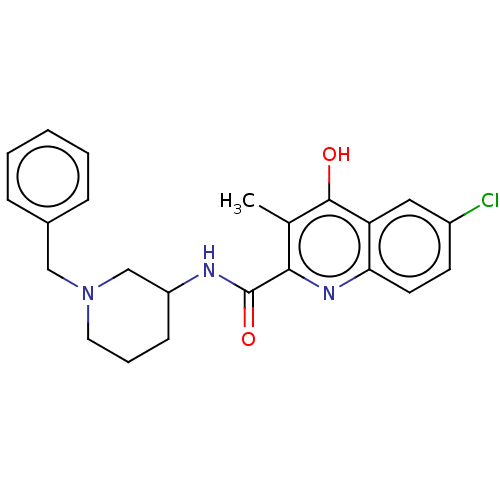 (CHEMBL4076205) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University Curated by ChEMBL | Assay Description Inhibition of recombinant human COX2 using arachidonic acid as substrate assessed as decrease in PGF2 production preincubated for 15 mins followed by... | Eur J Med Chem 127: 972-985 (2017) Article DOI: 10.1016/j.ejmech.2016.11.006 BindingDB Entry DOI: 10.7270/Q2NC63G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Mus musculus) | BDBM50457923 (CHEMBL4209588) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aljouf University Curated by ChEMBL | Assay Description Inhibition of mouse full-length GST-tagged BRAF V600E mutant using recombinant human full length N-terminal His-tagged MEK1 as substrate preincubated... | Eur J Med Chem 146: 260-273 (2018) Article DOI: 10.1016/j.ejmech.2018.01.042 BindingDB Entry DOI: 10.7270/Q25X2CKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50457928 (CHEMBL4213146) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aljouf University Curated by ChEMBL | Assay Description Inhibition of His6-tagged EGFR (unknown origin) cytoplasmic domain expressed in baculovirus infected Sf9 insect cells after 10 mins followed by addit... | Eur J Med Chem 146: 260-273 (2018) Article DOI: 10.1016/j.ejmech.2018.01.042 BindingDB Entry DOI: 10.7270/Q25X2CKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Mus musculus) | BDBM50457926 (CHEMBL4214496) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aljouf University Curated by ChEMBL | Assay Description Inhibition of mouse full-length GST-tagged BRAF V600E mutant using recombinant human full length N-terminal His-tagged MEK1 as substrate preincubated... | Eur J Med Chem 146: 260-273 (2018) Article DOI: 10.1016/j.ejmech.2018.01.042 BindingDB Entry DOI: 10.7270/Q25X2CKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 using arachidonic acid as substrate assessed as decrease in PGF2 production preincubated for 15 mins followed by substrate a... | Eur J Med Chem 127: 972-985 (2017) Article DOI: 10.1016/j.ejmech.2016.11.006 BindingDB Entry DOI: 10.7270/Q2NC63G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50235314 (CHEMBL4103022) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 using arachidonic acid as substrate assessed as decrease in PGF2 production preincubated for 15 mins followed by substrate a... | Eur J Med Chem 127: 972-985 (2017) Article DOI: 10.1016/j.ejmech.2016.11.006 BindingDB Entry DOI: 10.7270/Q2NC63G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50235320 (CHEMBL4087573) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 using arachidonic acid as substrate assessed as decrease in PGF2 production preincubated for 15 mins followed by substrate a... | Eur J Med Chem 127: 972-985 (2017) Article DOI: 10.1016/j.ejmech.2016.11.006 BindingDB Entry DOI: 10.7270/Q2NC63G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50235315 (CHEMBL4069774) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 using arachidonic acid as substrate assessed as decrease in PGF2 production preincubated for 15 mins followed by substrate a... | Eur J Med Chem 127: 972-985 (2017) Article DOI: 10.1016/j.ejmech.2016.11.006 BindingDB Entry DOI: 10.7270/Q2NC63G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50235317 (CHEMBL4080473) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 using arachidonic acid as substrate assessed as decrease in PGF2 production preincubated for 15 mins followed by substrate a... | Eur J Med Chem 127: 972-985 (2017) Article DOI: 10.1016/j.ejmech.2016.11.006 BindingDB Entry DOI: 10.7270/Q2NC63G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50235309 (CHEMBL4096972) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University Curated by ChEMBL | Assay Description Inhibitory activity was determined against almonds Beta-glucosidase | Eur J Med Chem 127: 972-985 (2017) Article DOI: 10.1016/j.ejmech.2016.11.006 BindingDB Entry DOI: 10.7270/Q2NC63G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50235313 (CHEMBL4074825) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 using arachidonic acid as substrate assessed as decrease in PGF2 production preincubated for 15 mins followed by substrate a... | Eur J Med Chem 127: 972-985 (2017) Article DOI: 10.1016/j.ejmech.2016.11.006 BindingDB Entry DOI: 10.7270/Q2NC63G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50235311 (CHEMBL4095272) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 using arachidonic acid as substrate assessed as decrease in PGF2 production preincubated for 15 mins followed by substrate a... | Eur J Med Chem 127: 972-985 (2017) Article DOI: 10.1016/j.ejmech.2016.11.006 BindingDB Entry DOI: 10.7270/Q2NC63G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50235308 (CHEMBL4089272) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University Curated by ChEMBL | Assay Description Inhibitory activity was determined against bovine liver beta galactosidase | Eur J Med Chem 127: 972-985 (2017) Article DOI: 10.1016/j.ejmech.2016.11.006 BindingDB Entry DOI: 10.7270/Q2NC63G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50235310 (CHEMBL4099420) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 using arachidonic acid as substrate assessed as decrease in PGF2 production preincubated for 15 mins followed by substrate a... | Eur J Med Chem 127: 972-985 (2017) Article DOI: 10.1016/j.ejmech.2016.11.006 BindingDB Entry DOI: 10.7270/Q2NC63G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50235319 (CHEMBL4066899) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University Curated by ChEMBL | Assay Description Inhibitory activity was determined against almonds Beta-glucosidase | Eur J Med Chem 127: 972-985 (2017) Article DOI: 10.1016/j.ejmech.2016.11.006 BindingDB Entry DOI: 10.7270/Q2NC63G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50235307 (CHEMBL4061105) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 using arachidonic acid as substrate assessed as decrease in PGF2 production preincubated for 15 mins followed by substrate a... | Eur J Med Chem 127: 972-985 (2017) Article DOI: 10.1016/j.ejmech.2016.11.006 BindingDB Entry DOI: 10.7270/Q2NC63G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50235321 (CHEMBL4066211) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 using arachidonic acid as substrate assessed as decrease in PGF2 production preincubated for 15 mins followed by substrate a... | Eur J Med Chem 127: 972-985 (2017) Article DOI: 10.1016/j.ejmech.2016.11.006 BindingDB Entry DOI: 10.7270/Q2NC63G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50235316 (CHEMBL4104705) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University Curated by ChEMBL | Assay Description Inhibitory activity was determined against Escherichia coli Alpha-galactosidase | Eur J Med Chem 127: 972-985 (2017) Article DOI: 10.1016/j.ejmech.2016.11.006 BindingDB Entry DOI: 10.7270/Q2NC63G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50235318 (CHEMBL4076205) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 using arachidonic acid as substrate assessed as decrease in PGF2 production preincubated for 15 mins followed by substrate a... | Eur J Med Chem 127: 972-985 (2017) Article DOI: 10.1016/j.ejmech.2016.11.006 BindingDB Entry DOI: 10.7270/Q2NC63G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||