

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50441091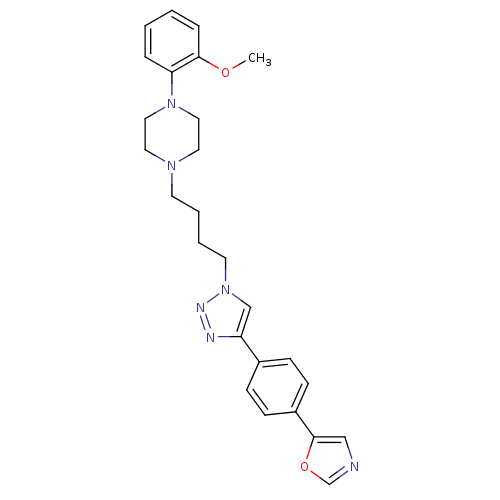 (CHEMBL2430438) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from cloned human dopamine D2 receptor | Bioorg Med Chem Lett 23: 5586-91 (2013) Article DOI: 10.1016/j.bmcl.2013.08.047 BindingDB Entry DOI: 10.7270/Q2KH0PR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50441092 (CHEMBL2430439) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from cloned human dopamine D2 receptor | Bioorg Med Chem Lett 23: 5586-91 (2013) Article DOI: 10.1016/j.bmcl.2013.08.047 BindingDB Entry DOI: 10.7270/Q2KH0PR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50441093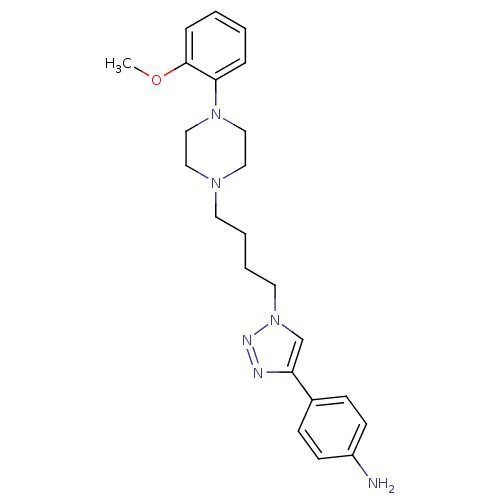 (CHEMBL2430440) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 199 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from cloned human dopamine D2 receptor | Bioorg Med Chem Lett 23: 5586-91 (2013) Article DOI: 10.1016/j.bmcl.2013.08.047 BindingDB Entry DOI: 10.7270/Q2KH0PR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50441094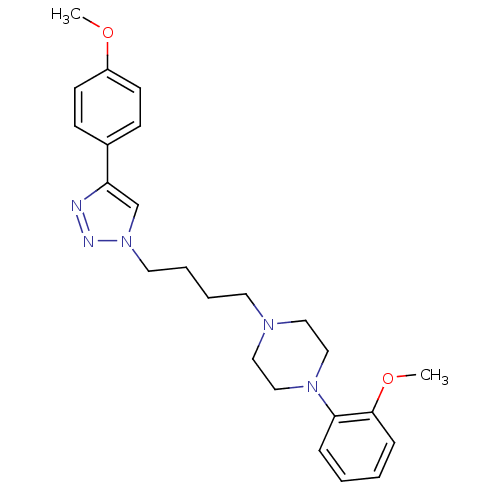 (CHEMBL2430441) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 212 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from cloned human dopamine D2 receptor | Bioorg Med Chem Lett 23: 5586-91 (2013) Article DOI: 10.1016/j.bmcl.2013.08.047 BindingDB Entry DOI: 10.7270/Q2KH0PR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50441095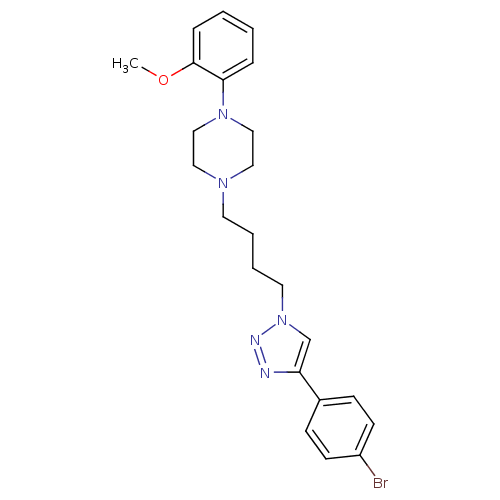 (CHEMBL2430442) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 227 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from cloned human dopamine D2 receptor | Bioorg Med Chem Lett 23: 5586-91 (2013) Article DOI: 10.1016/j.bmcl.2013.08.047 BindingDB Entry DOI: 10.7270/Q2KH0PR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50441096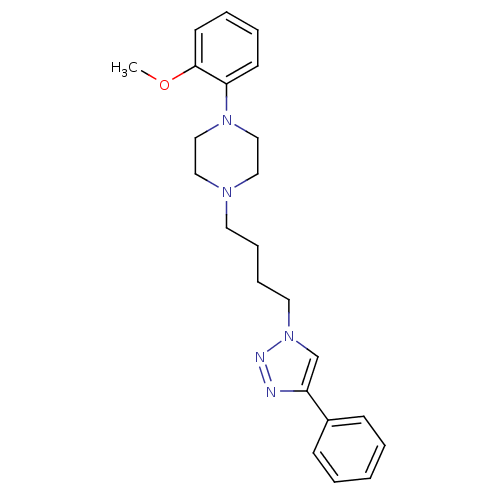 (CHEMBL2430443) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 228 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from cloned human dopamine D2 receptor | Bioorg Med Chem Lett 23: 5586-91 (2013) Article DOI: 10.1016/j.bmcl.2013.08.047 BindingDB Entry DOI: 10.7270/Q2KH0PR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50259706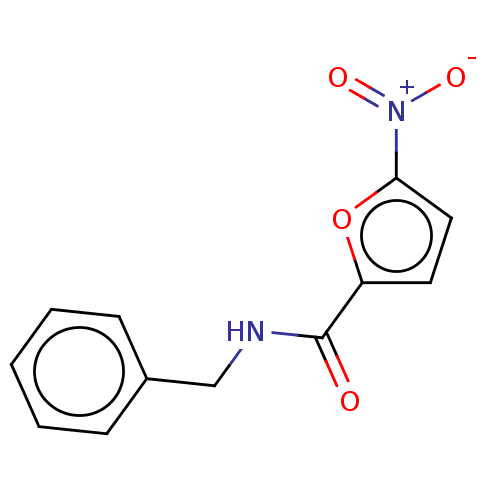 (CHEMBL2313314) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral Curated by ChEMBL | Assay Description Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method | Eur J Med Chem 125: 1088-1097 (2017) Article DOI: 10.1016/j.ejmech.2016.10.055 BindingDB Entry DOI: 10.7270/Q2TM7DKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50259707 (CHEMBL4091464) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral Curated by ChEMBL | Assay Description Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method | Eur J Med Chem 125: 1088-1097 (2017) Article DOI: 10.1016/j.ejmech.2016.10.055 BindingDB Entry DOI: 10.7270/Q2TM7DKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50259704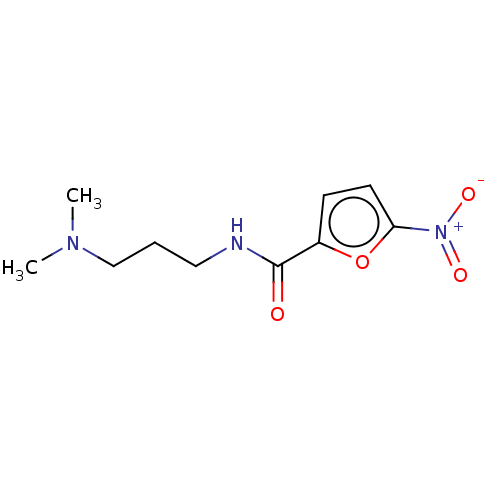 (CHEMBL4066587) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.18E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral Curated by ChEMBL | Assay Description Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method | Eur J Med Chem 125: 1088-1097 (2017) Article DOI: 10.1016/j.ejmech.2016.10.055 BindingDB Entry DOI: 10.7270/Q2TM7DKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50259705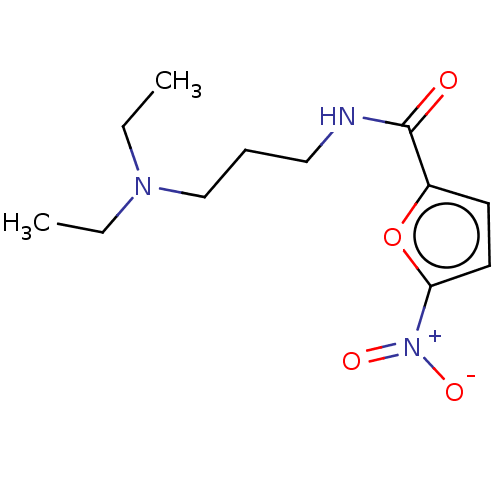 (CHEMBL4088638) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.28E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral Curated by ChEMBL | Assay Description Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method | Eur J Med Chem 125: 1088-1097 (2017) Article DOI: 10.1016/j.ejmech.2016.10.055 BindingDB Entry DOI: 10.7270/Q2TM7DKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50259703 (CHEMBL4084206) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral Curated by ChEMBL | Assay Description Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method | Eur J Med Chem 125: 1088-1097 (2017) Article DOI: 10.1016/j.ejmech.2016.10.055 BindingDB Entry DOI: 10.7270/Q2TM7DKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50259702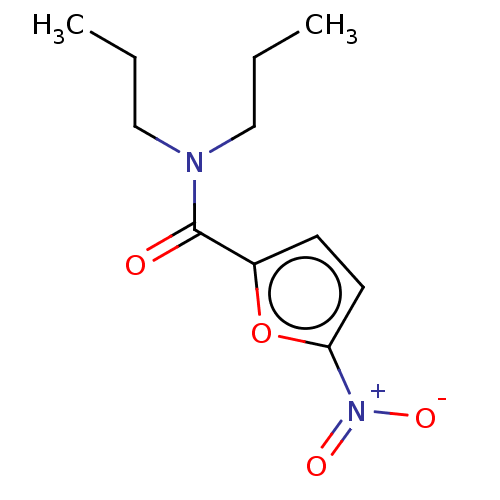 (CHEMBL4079787) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.83E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral Curated by ChEMBL | Assay Description Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method | Eur J Med Chem 125: 1088-1097 (2017) Article DOI: 10.1016/j.ejmech.2016.10.055 BindingDB Entry DOI: 10.7270/Q2TM7DKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50259710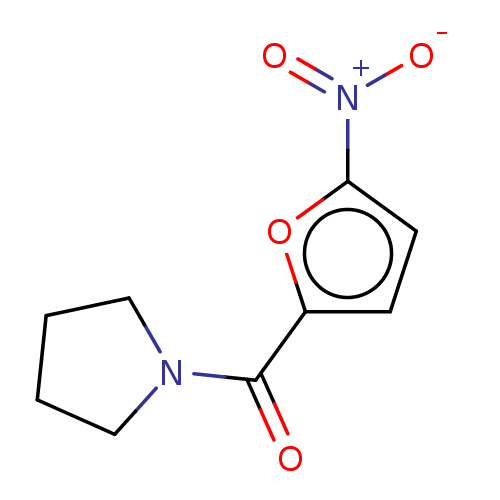 (CHEMBL4071417) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.87E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral Curated by ChEMBL | Assay Description Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method | Eur J Med Chem 125: 1088-1097 (2017) Article DOI: 10.1016/j.ejmech.2016.10.055 BindingDB Entry DOI: 10.7270/Q2TM7DKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50259708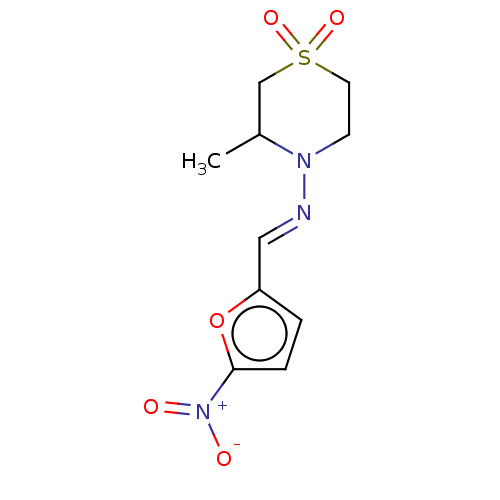 (BAY-2502 | BAY-A2502 | BAYER-2502 | CHEBI:7566 | D...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral Curated by ChEMBL | Assay Description Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method | Eur J Med Chem 125: 1088-1097 (2017) Article DOI: 10.1016/j.ejmech.2016.10.055 BindingDB Entry DOI: 10.7270/Q2TM7DKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50259698 (CHEMBL4092991) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.82E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral Curated by ChEMBL | Assay Description Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method | Eur J Med Chem 125: 1088-1097 (2017) Article DOI: 10.1016/j.ejmech.2016.10.055 BindingDB Entry DOI: 10.7270/Q2TM7DKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50259709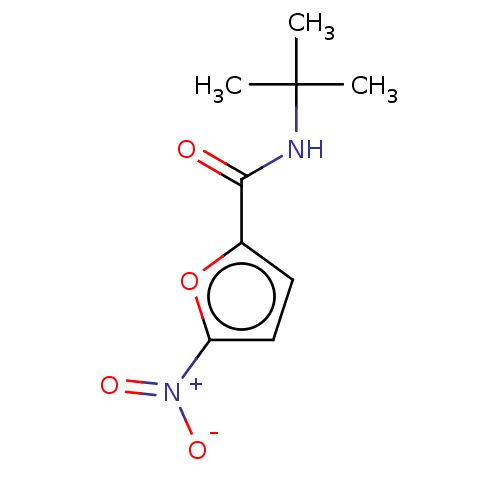 (CHEMBL4061646) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.13E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral Curated by ChEMBL | Assay Description Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method | Eur J Med Chem 125: 1088-1097 (2017) Article DOI: 10.1016/j.ejmech.2016.10.055 BindingDB Entry DOI: 10.7270/Q2TM7DKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50259700 (CHEMBL4064356) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.15E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral Curated by ChEMBL | Assay Description Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method | Eur J Med Chem 125: 1088-1097 (2017) Article DOI: 10.1016/j.ejmech.2016.10.055 BindingDB Entry DOI: 10.7270/Q2TM7DKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50259699 (CHEMBL4079946) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral Curated by ChEMBL | Assay Description Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method | Eur J Med Chem 125: 1088-1097 (2017) Article DOI: 10.1016/j.ejmech.2016.10.055 BindingDB Entry DOI: 10.7270/Q2TM7DKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50259711 (CHEMBL4092070) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.14E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral Curated by ChEMBL | Assay Description Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method | Eur J Med Chem 125: 1088-1097 (2017) Article DOI: 10.1016/j.ejmech.2016.10.055 BindingDB Entry DOI: 10.7270/Q2TM7DKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50259701 (CHEMBL4103302) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.18E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral Curated by ChEMBL | Assay Description Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method | Eur J Med Chem 125: 1088-1097 (2017) Article DOI: 10.1016/j.ejmech.2016.10.055 BindingDB Entry DOI: 10.7270/Q2TM7DKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||