

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50069294 ((S)-3-[4-(carbohydrazonamidol)-phenyl]-N-cyclopent...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1683-6 (1999) BindingDB Entry DOI: 10.7270/Q2BG2N5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069294 ((S)-3-[4-(carbohydrazonamidol)-phenyl]-N-cyclopent...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070780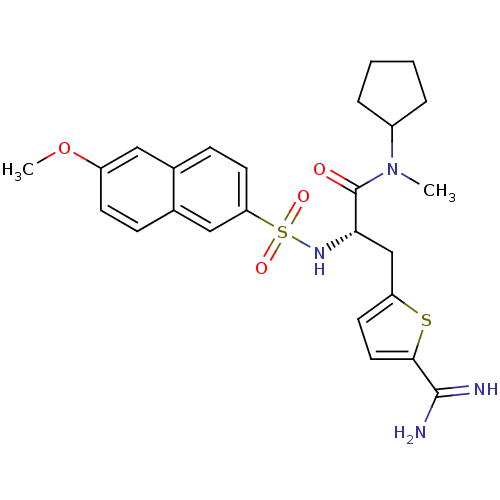 ((S)-3-(5-Carbamimidoyl-thiophen-2-yl)-N-cyclopenty...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1683-6 (1999) BindingDB Entry DOI: 10.7270/Q2BG2N5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070785 ((S)-3-(5-Carbamimidoyl-thiophen-2-yl)-N-cyclopenty...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1683-6 (1999) BindingDB Entry DOI: 10.7270/Q2BG2N5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070782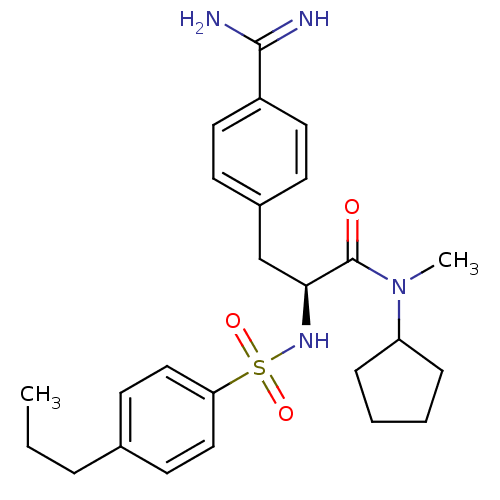 ((S)-3-(4-Carbamimidoyl-phenyl)-N-cyclopentyl-N-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1683-6 (1999) BindingDB Entry DOI: 10.7270/Q2BG2N5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Synaptic vesicular amine transporter (Bos taurus) | BDBM50017712 ((-)-reserpine | (3beta,16beta,17alpha,18beta,20alp...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wichita State University Curated by ChEMBL | Assay Description Inhibition of dopamine uptake at VMAT in bovine chromaffin granule ghosts | J Med Chem 51: 760-8 (2008) Article DOI: 10.1021/jm070875p BindingDB Entry DOI: 10.7270/Q2M909J6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50069294 ((S)-3-[4-(carbohydrazonamidol)-phenyl]-N-cyclopent...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against bovine thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50374634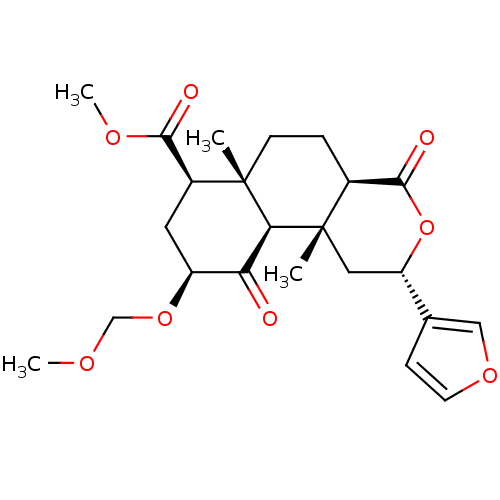 (CHEMBL258098) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cell membrane after 2 hrs by scintillation counting analysis | Medchemcomm 2: 1217-1222 (2011) Article DOI: 10.1039/c1md00192b BindingDB Entry DOI: 10.7270/Q22F7RF9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50216132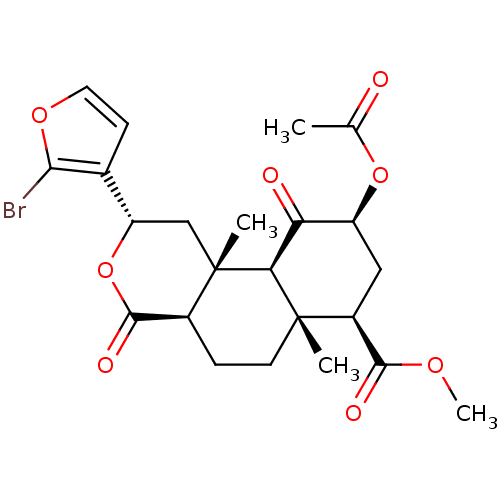 ((2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 9-acetoxy-2-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human kappa opioid receptor expressed in CHO cells | J Med Chem 50: 3596-603 (2007) Article DOI: 10.1021/jm070393d BindingDB Entry DOI: 10.7270/Q2ZK5HH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070784 ((S)-3-(5-Carbamimidoyl-thiophen-2-yl)-N-cyclopenty...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1683-6 (1999) BindingDB Entry DOI: 10.7270/Q2BG2N5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50374645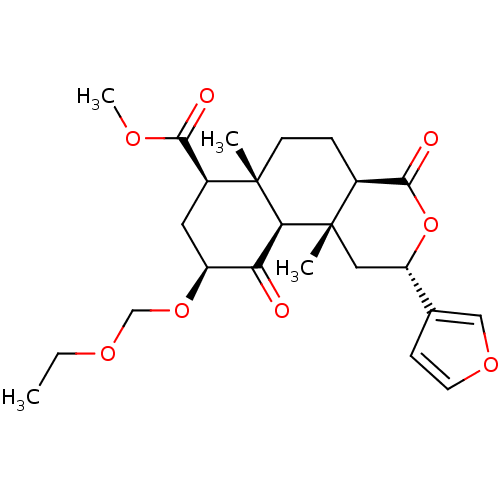 (CHEMBL272939) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cell membrane after 2 hrs by scintillation counting analysis | Medchemcomm 2: 1217-1222 (2011) Article DOI: 10.1039/c1md00192b BindingDB Entry DOI: 10.7270/Q22F7RF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 2 (Homo sapiens (Human)) | BDBM81801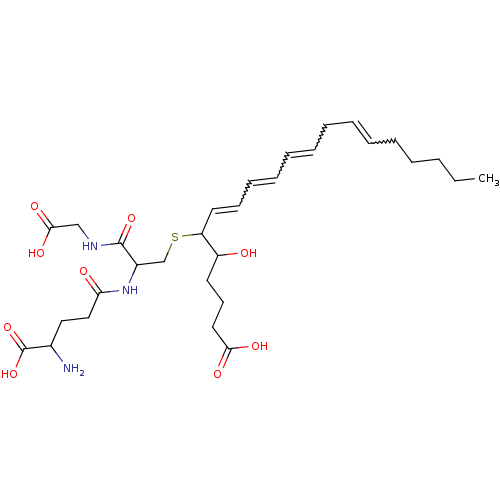 (CAS_5283121 | LTC4 | NSC_5283121) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | Article PubMed | 3.35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by PDSP Ki Database | J Biol Chem 275: 30531-6 (2000) Article DOI: 10.1074/jbc.M003490200 BindingDB Entry DOI: 10.7270/Q2BP01BH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 2 (Homo sapiens (Human)) | BDBM50292408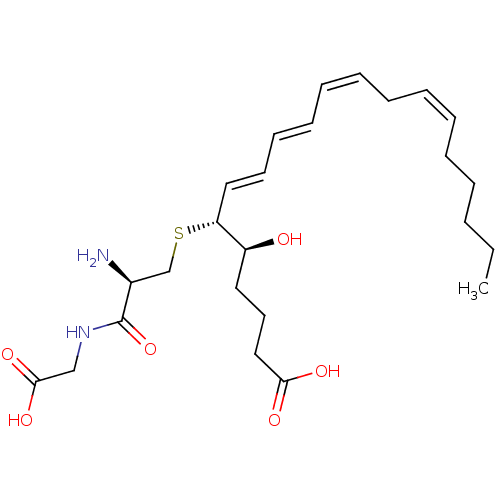 ((R-(R*,S*-(E,E,Z,Z)))-N-(S-(1-(4-Carboxy-1-hydroxy...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by PDSP Ki Database | J Biol Chem 275: 30531-6 (2000) Article DOI: 10.1074/jbc.M003490200 BindingDB Entry DOI: 10.7270/Q2BP01BH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50371092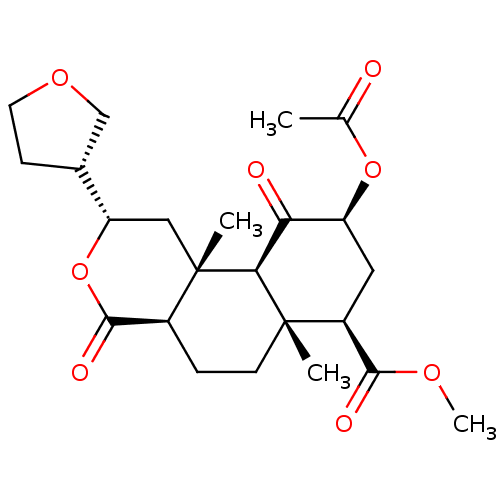 (CHEMBL427280) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human kappa opioid receptor expressed in CHO cells | J Med Chem 50: 3596-603 (2007) Article DOI: 10.1021/jm070393d BindingDB Entry DOI: 10.7270/Q2ZK5HH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50069292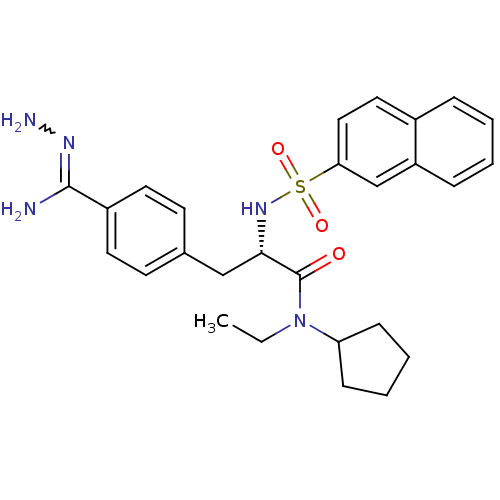 (CHEMBL156082 | N-ethyl-N-cyclopentyl-3-(4-hydrazon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against bovine thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Synaptic vesicular amine transporter (Bos taurus) | BDBM50017701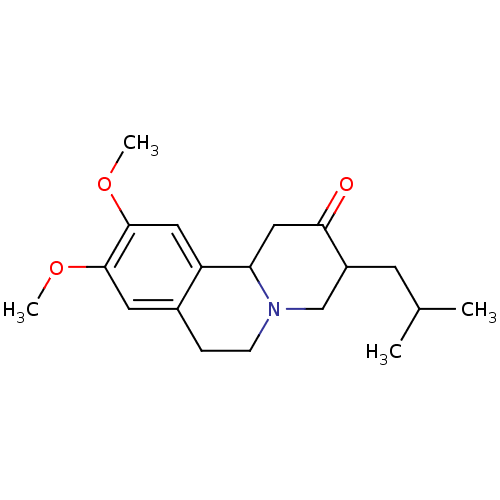 (3-Isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wichita State University Curated by ChEMBL | Assay Description Inhibition of dopamine uptake at VMAT in bovine chromaffin granule ghosts | J Med Chem 51: 760-8 (2008) Article DOI: 10.1021/jm070875p BindingDB Entry DOI: 10.7270/Q2M909J6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50496725 (CHEMBL3219933) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cell membrane after 2 hrs by scintillation counting analysis | Medchemcomm 2: 1217-1222 (2011) Article DOI: 10.1039/c1md00192b BindingDB Entry DOI: 10.7270/Q22F7RF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50496728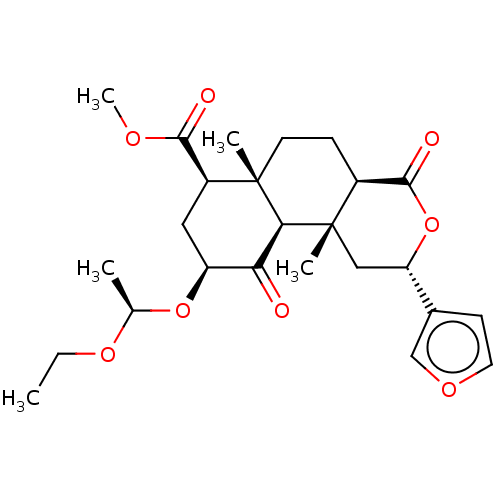 (CHEMBL3219937) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cell membrane after 2 hrs by scintillation counting analysis | Medchemcomm 2: 1217-1222 (2011) Article DOI: 10.1039/c1md00192b BindingDB Entry DOI: 10.7270/Q22F7RF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070783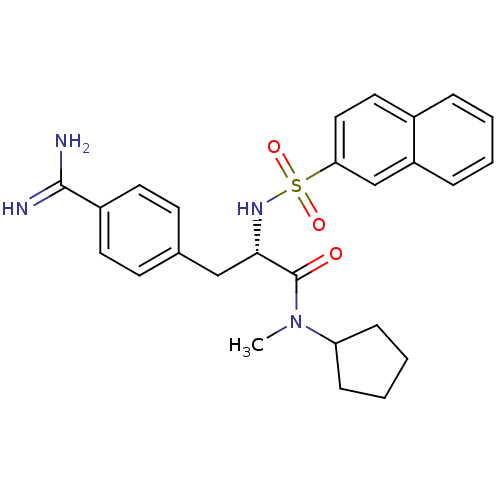 ((S)-3-(4-Carbamimidoyl-phenyl)-N-cyclopentyl-N-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1683-6 (1999) BindingDB Entry DOI: 10.7270/Q2BG2N5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50159168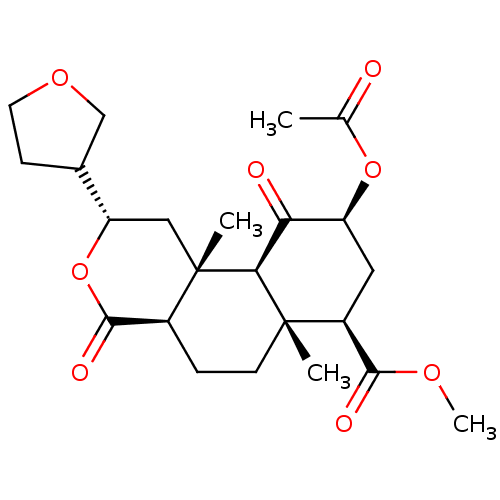 ((2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 9-acetoxy-6a,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human kappa opioid receptor expressed in CHO cells | J Med Chem 50: 3596-603 (2007) Article DOI: 10.1021/jm070393d BindingDB Entry DOI: 10.7270/Q2ZK5HH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50216133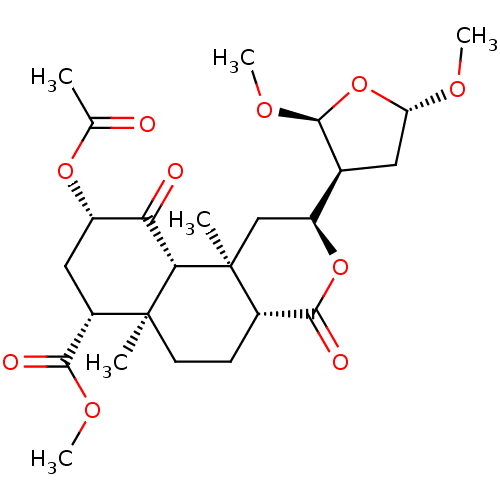 ((2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 9-acetoxy-2-((...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human kappa opioid receptor expressed in CHO cells | J Med Chem 50: 3596-603 (2007) Article DOI: 10.1021/jm070393d BindingDB Entry DOI: 10.7270/Q2ZK5HH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50027234 (CHEMBL2113278) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human kappa opioid receptor expressed in CHO cells | J Med Chem 50: 3596-603 (2007) Article DOI: 10.1021/jm070393d BindingDB Entry DOI: 10.7270/Q2ZK5HH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50159172 ((3S,4aR,4bS,6S,8R,8aR,10aR)-6-Formyloxy-3-furan-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cell membrane after 2 hrs by scintillation counting analysis | Medchemcomm 2: 1217-1222 (2011) Article DOI: 10.1039/c1md00192b BindingDB Entry DOI: 10.7270/Q22F7RF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50069293 (CHEMBL440188 | N-methyl-N-n-butyl-3-(4-hydrazonofo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against bovine thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50374645 (CHEMBL272939) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cell membrane after 2 hrs by liquid scintillation counting analysis | Medchemcomm 2: 1217-1222 (2011) Article DOI: 10.1039/c1md00192b BindingDB Entry DOI: 10.7270/Q22F7RF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50216142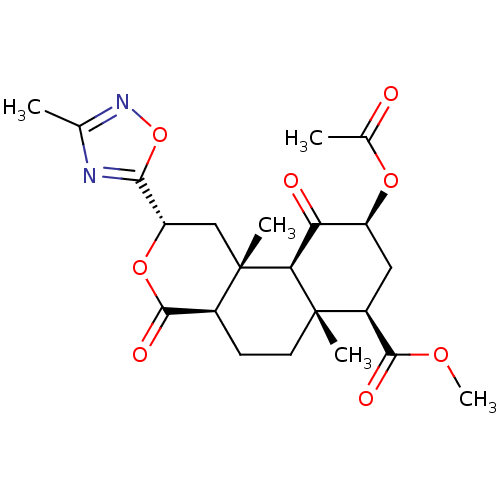 ((2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 9-acetoxy-6a,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human kappa opioid receptor expressed in CHO cells | J Med Chem 50: 3596-603 (2007) Article DOI: 10.1021/jm070393d BindingDB Entry DOI: 10.7270/Q2ZK5HH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50496723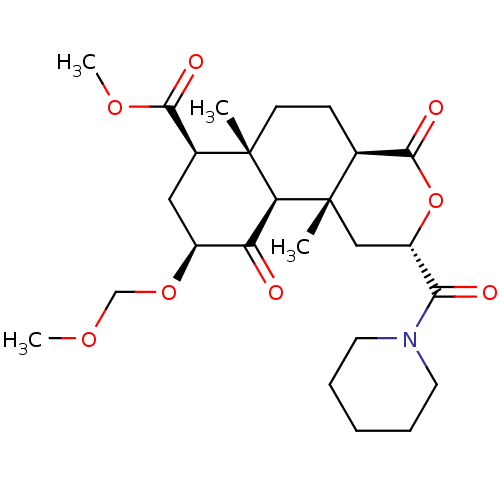 (CHEMBL3219943) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cell membrane after 2 hrs by scintillation counting analysis | Medchemcomm 2: 1217-1222 (2011) Article DOI: 10.1039/c1md00192b BindingDB Entry DOI: 10.7270/Q22F7RF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50496722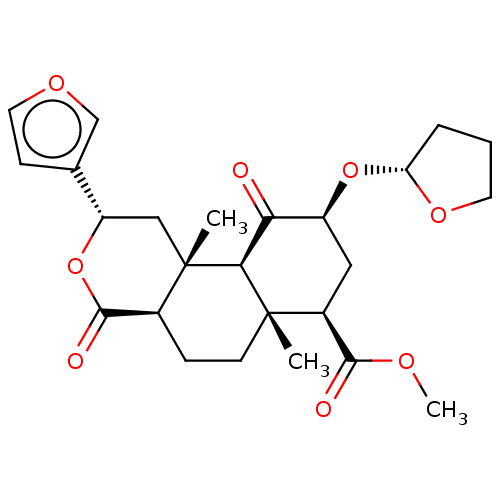 (CHEMBL3219935) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cell membrane after 2 hrs by scintillation counting analysis | Medchemcomm 2: 1217-1222 (2011) Article DOI: 10.1039/c1md00192b BindingDB Entry DOI: 10.7270/Q22F7RF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50496720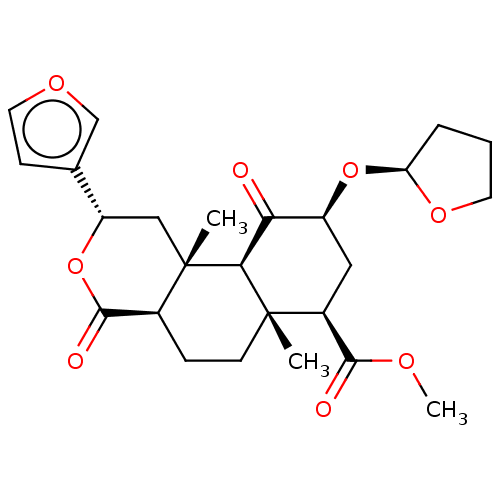 (CHEMBL3219936) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cell membrane after 2 hrs by scintillation counting analysis | Medchemcomm 2: 1217-1222 (2011) Article DOI: 10.1039/c1md00192b BindingDB Entry DOI: 10.7270/Q22F7RF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070781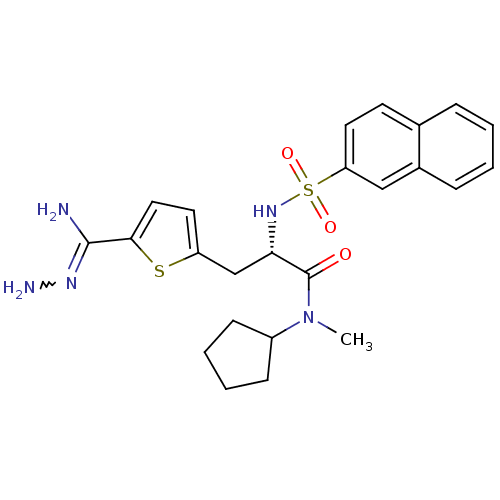 (1N-cyclopentyl-1N-methyl-3-[5-amino(aminoimino)met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1683-6 (1999) BindingDB Entry DOI: 10.7270/Q2BG2N5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50069296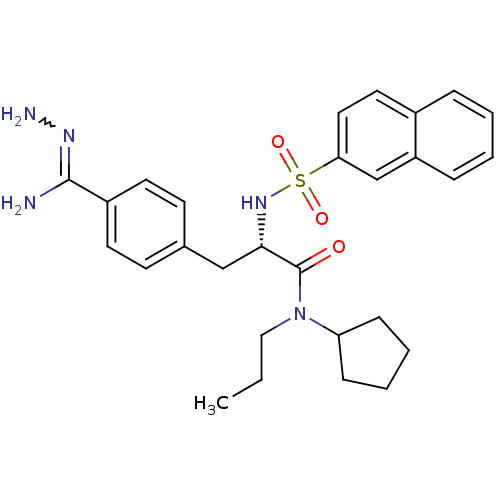 (CHEMBL347371 | N-(n-propyl)-N-cyclopentyl-3-(4-hyd...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against bovine thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50216136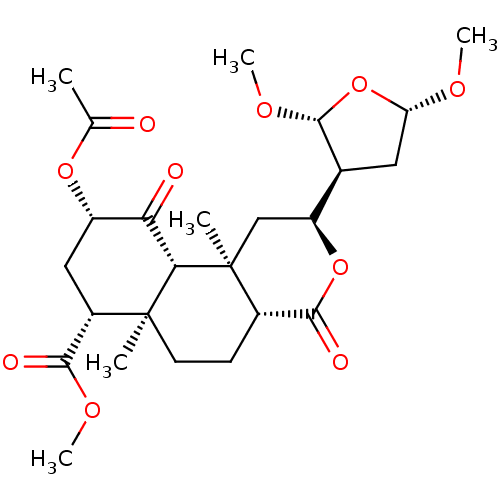 ((2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 9-acetoxy-2-((...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human kappa opioid receptor expressed in CHO cells | J Med Chem 50: 3596-603 (2007) Article DOI: 10.1021/jm070393d BindingDB Entry DOI: 10.7270/Q2ZK5HH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50070780 ((S)-3-(5-Carbamimidoyl-thiophen-2-yl)-N-cyclopenty...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 134 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against bovine trypsin | Bioorg Med Chem Lett 8: 1683-6 (1999) BindingDB Entry DOI: 10.7270/Q2BG2N5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50496731 (CHEMBL3219944) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cell membrane after 2 hrs by scintillation counting analysis | Medchemcomm 2: 1217-1222 (2011) Article DOI: 10.1039/c1md00192b BindingDB Entry DOI: 10.7270/Q22F7RF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50070785 ((S)-3-(5-Carbamimidoyl-thiophen-2-yl)-N-cyclopenty...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against bovine trypsin | Bioorg Med Chem Lett 8: 1683-6 (1999) BindingDB Entry DOI: 10.7270/Q2BG2N5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50069298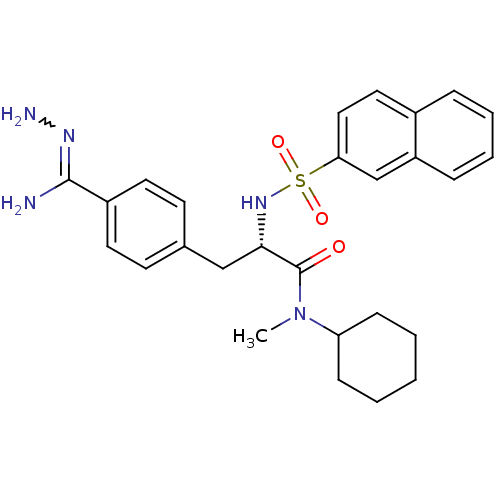 (CHEMBL434678 | N-methyl-N-cyclohexyl-3-(4-hydrazon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against bovine thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50070784 ((S)-3-(5-Carbamimidoyl-thiophen-2-yl)-N-cyclopenty...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 153 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against bovine trypsin | Bioorg Med Chem Lett 8: 1683-6 (1999) BindingDB Entry DOI: 10.7270/Q2BG2N5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50496721 (CHEMBL3219941) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 171 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cell membrane after 2 hrs by scintillation counting analysis | Medchemcomm 2: 1217-1222 (2011) Article DOI: 10.1039/c1md00192b BindingDB Entry DOI: 10.7270/Q22F7RF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50216134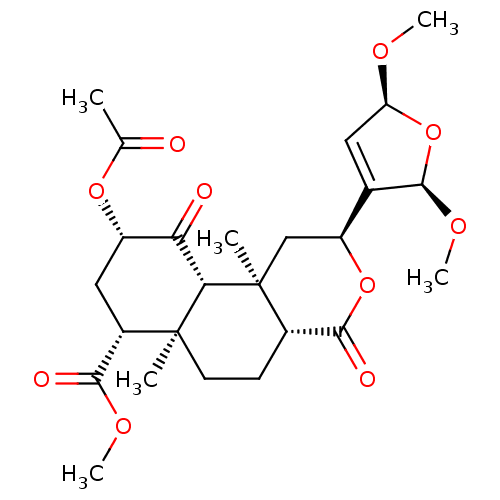 ((4aR,6aR,7R,9S,10aS,10bR)-methyl 9-acetoxy-2-((2R,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human kappa opioid receptor expressed in CHO cells | J Med Chem 50: 3596-603 (2007) Article DOI: 10.1021/jm070393d BindingDB Entry DOI: 10.7270/Q2ZK5HH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50069299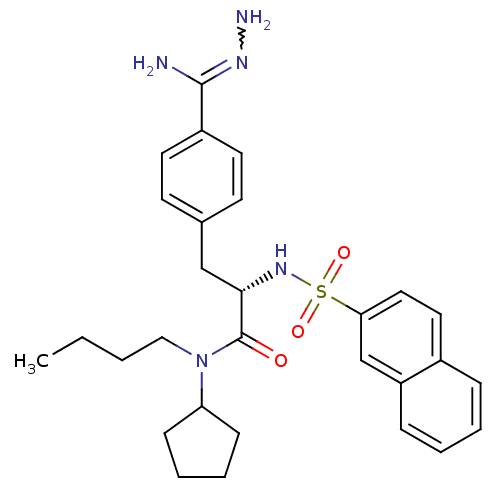 (CHEMBL155317 | N-(n-butyl)-N-cyclopentyl-3-(4-hydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 231 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against bovine thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50069295 (CHEMBL348175 | N-methyl-N-cyclopropyl-3-(4-hydrazo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 247 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against bovine thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50189136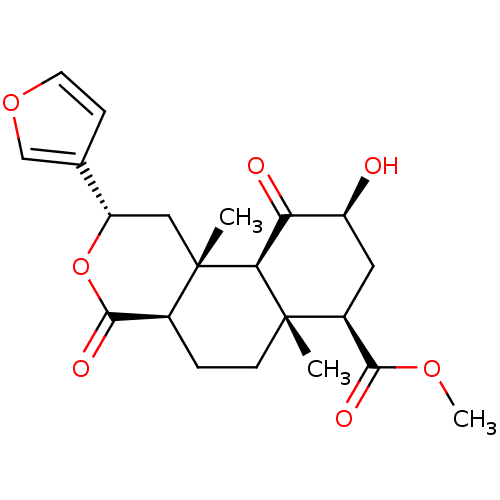 (CHEMBL424698 | Salvinorin B) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human kappa opioid receptor expressed in CHO cells | J Med Chem 50: 3596-603 (2007) Article DOI: 10.1021/jm070393d BindingDB Entry DOI: 10.7270/Q2ZK5HH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50496729 (CHEMBL3219934) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cell membrane after 2 hrs by scintillation counting analysis | Medchemcomm 2: 1217-1222 (2011) Article DOI: 10.1039/c1md00192b BindingDB Entry DOI: 10.7270/Q22F7RF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50216143 ((2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 9-acetoxy-2-(4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human kappa opioid receptor expressed in CHO cells | J Med Chem 50: 3596-603 (2007) Article DOI: 10.1021/jm070393d BindingDB Entry DOI: 10.7270/Q2ZK5HH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50069300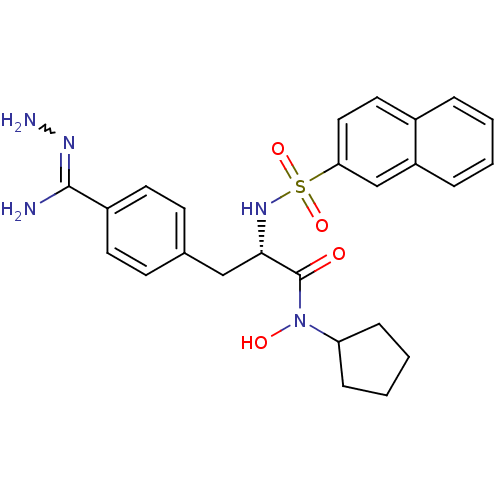 (CHEMBL350901 | N-hydroxy-N-cyclopentyl-3-(4-hydraz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 367 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against bovine thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50374634 (CHEMBL258098) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cell membrane after 2 hrs by liquid scintillation counting analysis | Medchemcomm 2: 1217-1222 (2011) Article DOI: 10.1039/c1md00192b BindingDB Entry DOI: 10.7270/Q22F7RF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50027232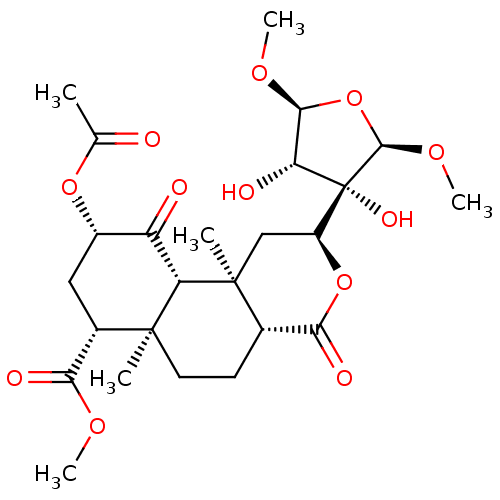 (Salvinicin A) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human kappa opioid receptor expressed in CHO cells | J Med Chem 50: 3596-603 (2007) Article DOI: 10.1021/jm070393d BindingDB Entry DOI: 10.7270/Q2ZK5HH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Synaptic vesicular amine transporter (Bos taurus) | BDBM50374536 (CHEMBL272202) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wichita State University Curated by ChEMBL | Assay Description Inhibition of dopamine uptake at VMAT in bovine chromaffin granule ghosts | J Med Chem 51: 760-8 (2008) Article DOI: 10.1021/jm070875p BindingDB Entry DOI: 10.7270/Q2M909J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50216139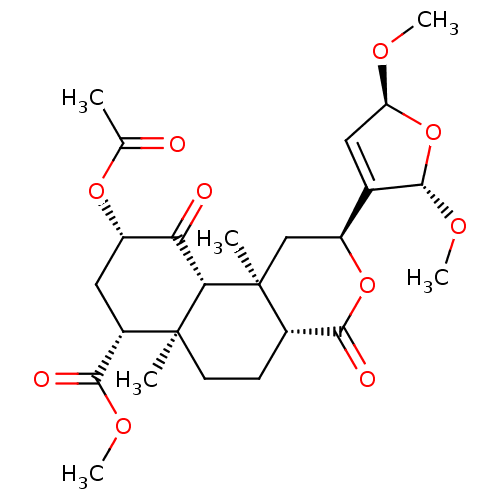 ((4aR,6aR,7R,9S,10aS,10bR)-methyl 9-acetoxy-2-((2S,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human kappa opioid receptor expressed in CHO cells | J Med Chem 50: 3596-603 (2007) Article DOI: 10.1021/jm070393d BindingDB Entry DOI: 10.7270/Q2ZK5HH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50216142 ((2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 9-acetoxy-6a,1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | J Med Chem 50: 3596-603 (2007) Article DOI: 10.1021/jm070393d BindingDB Entry DOI: 10.7270/Q2ZK5HH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 890 total ) | Next | Last >> |