

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase Fer (Homo sapiens (Human)) | BDBM50501949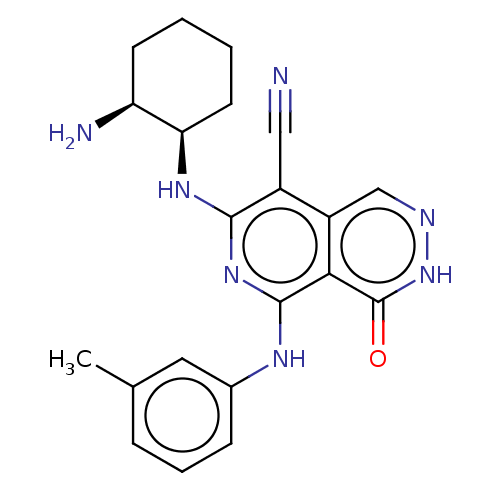 (CHEMBL4461851) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His-tagged human FER (SH2 domain to C-terminal) expressed in Escherichia coli assessed as decrease in FL-Peptide... | ACS Med Chem Lett 10: 737-742 (2019) Article DOI: 10.1021/acsmedchemlett.8b00631 BindingDB Entry DOI: 10.7270/Q2WS8XG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50394470 (CHEMBL2160099) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR3 receptor in human eosinophils assessed as inhibition of CCL11-induced degranulation after 4 hrs by ELISA | Bioorg Med Chem Lett 22: 6876-81 (2012) Article DOI: 10.1016/j.bmcl.2012.09.035 BindingDB Entry DOI: 10.7270/Q2QC04MN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297172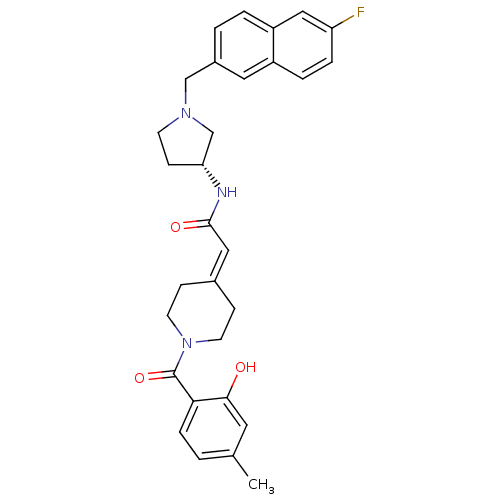 (CHEMBL560275 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297171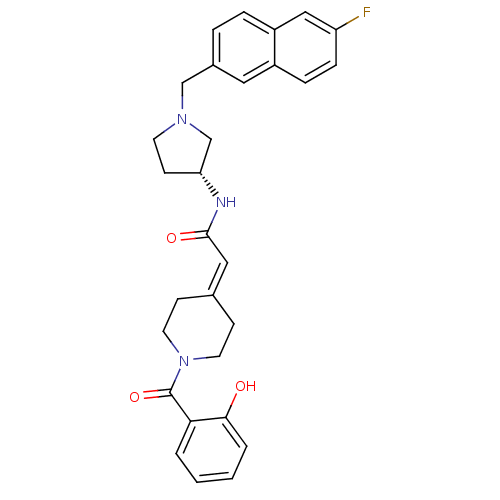 (CHEMBL551735 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells by functional inhibition curve analysis | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297181 (2-[1-(1,3-Benzodioxol-5-ylcarbonyl)piperidin-4-yli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50394470 (CHEMBL2160099) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR3 receptor | Bioorg Med Chem Lett 22: 6876-81 (2012) Article DOI: 10.1016/j.bmcl.2012.09.035 BindingDB Entry DOI: 10.7270/Q2QC04MN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297183 (CHEMBL551738 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50394487 (CHEMBL2160111) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR3 receptor | Bioorg Med Chem Lett 22: 6876-81 (2012) Article DOI: 10.1016/j.bmcl.2012.09.035 BindingDB Entry DOI: 10.7270/Q2QC04MN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fer (Homo sapiens (Human)) | BDBM50501965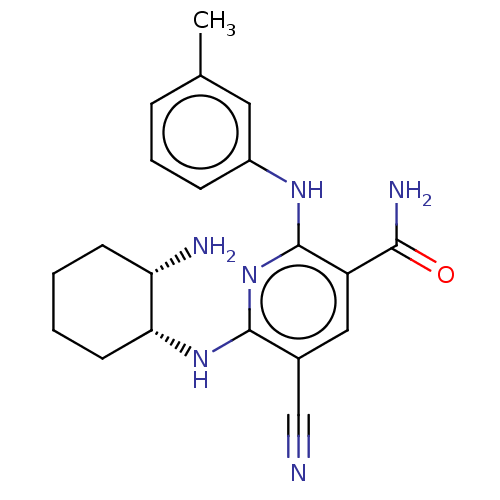 (CHEMBL4457164) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His-tagged human FER (SH2 domain to C-terminal) expressed in Escherichia coli assessed as decrease in FL-Peptide... | ACS Med Chem Lett 10: 737-742 (2019) Article DOI: 10.1021/acsmedchemlett.8b00631 BindingDB Entry DOI: 10.7270/Q2WS8XG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fer (Homo sapiens (Human)) | BDBM50501961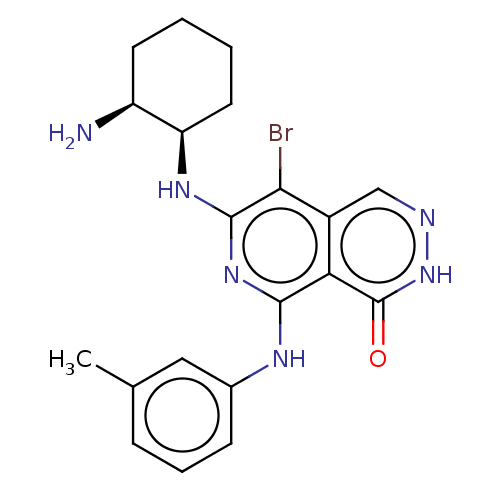 (CHEMBL4456804) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His-tagged human FER (SH2 domain to C-terminal) expressed in Escherichia coli assessed as decrease in FL-Peptide... | ACS Med Chem Lett 10: 737-742 (2019) Article DOI: 10.1021/acsmedchemlett.8b00631 BindingDB Entry DOI: 10.7270/Q2WS8XG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297177 (CHEMBL561535 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297182 (2-[1-(3,4-Dimethoxybenzoyl)piperidin-4-ylidene]-N-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297190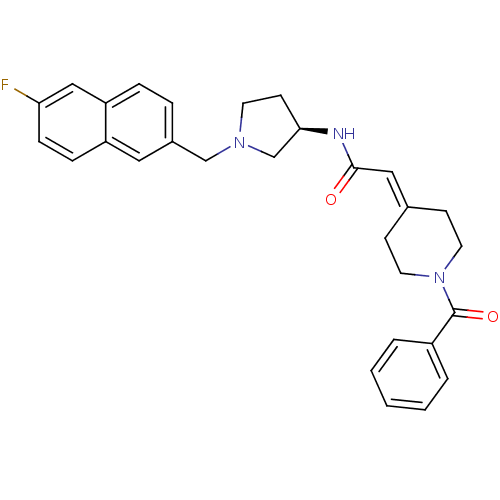 (2-(1-Benzoylpiperidin-4-ylidene)-N-{(3R)-1-[(6-flu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fer (Homo sapiens (Human)) | BDBM50501963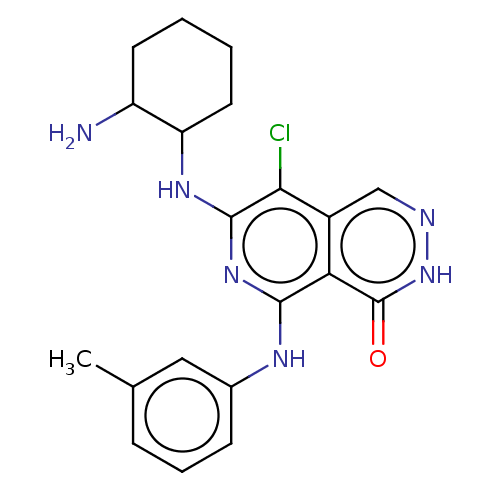 (CHEMBL4524587) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His-tagged human FER (SH2 domain to C-terminal) expressed in Escherichia coli assessed as decrease in FL-Peptide... | ACS Med Chem Lett 10: 737-742 (2019) Article DOI: 10.1021/acsmedchemlett.8b00631 BindingDB Entry DOI: 10.7270/Q2WS8XG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297188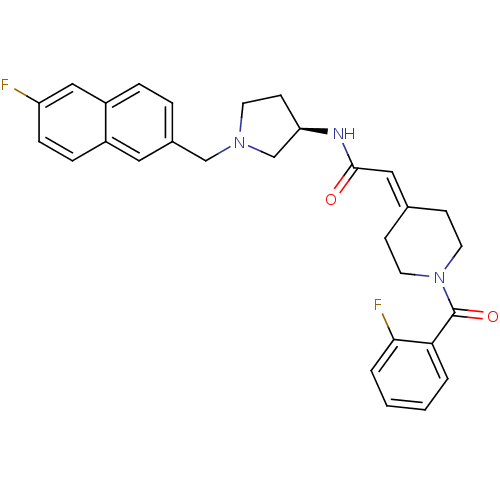 (2-[1-(2-Fluorobenzoyl)piperidin-4-ylidene]-N-{(3R)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50387669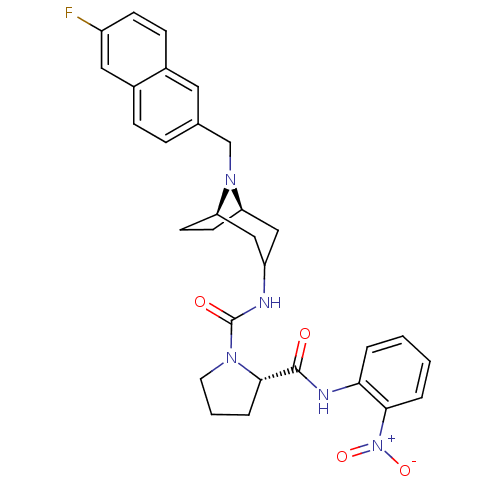 (CHEMBL2058071) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 | Bioorg Med Chem Lett 22: 4951-4 (2012) Article DOI: 10.1016/j.bmcl.2012.06.042 BindingDB Entry DOI: 10.7270/Q2057H06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297180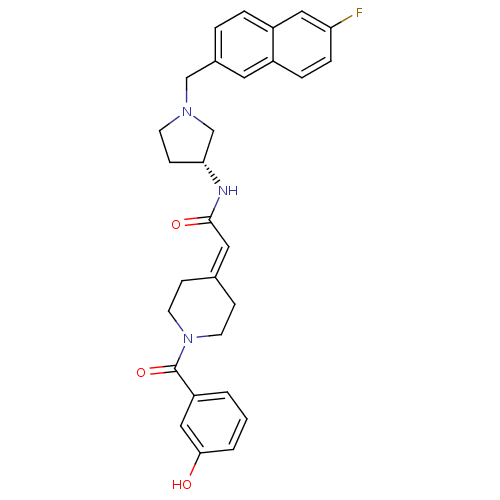 (CHEMBL556916 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297179 (CHEMBL562923 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297185 (CHEMBL556227 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297184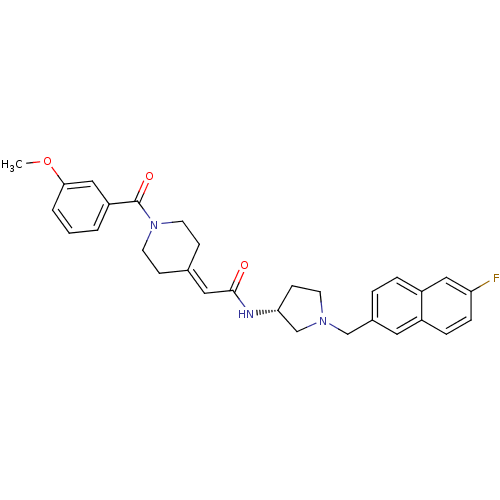 (CHEMBL557118 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297174 (2-[1-(4-Fluoro-2-hydroxybenzoyl)piperidin-4-yliden...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297173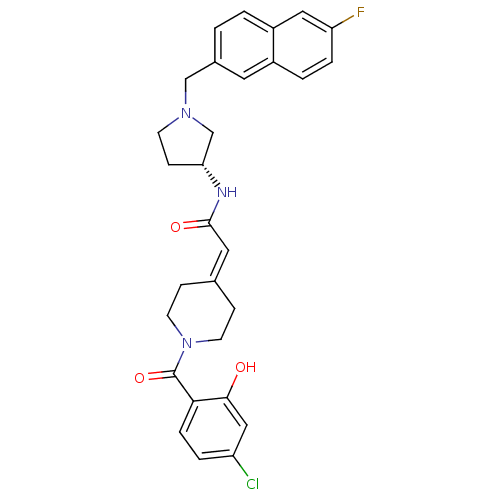 (2-[1-(4-Chloro-2-hydroxybenzoyl)piperidin-4-yliden...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50387649 (CHEMBL2057751) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 | Bioorg Med Chem Lett 22: 4951-4 (2012) Article DOI: 10.1016/j.bmcl.2012.06.042 BindingDB Entry DOI: 10.7270/Q2057H06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297171 (CHEMBL551735 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50394482 (CHEMBL2159867) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR3 receptor | Bioorg Med Chem Lett 22: 6876-81 (2012) Article DOI: 10.1016/j.bmcl.2012.09.035 BindingDB Entry DOI: 10.7270/Q2QC04MN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fer (Homo sapiens (Human)) | BDBM50501959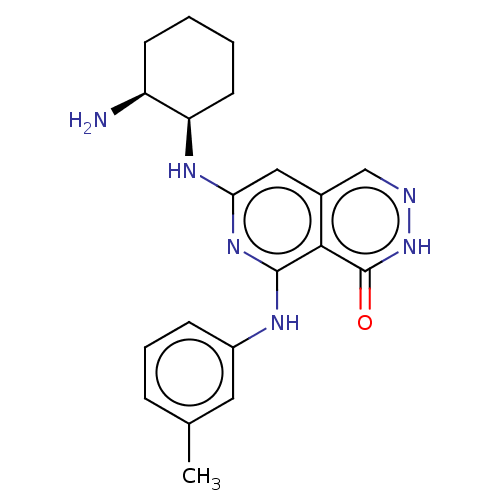 (CHEMBL4557212) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His-tagged human FER (SH2 domain to C-terminal) expressed in Escherichia coli assessed as decrease in FL-Peptide... | ACS Med Chem Lett 10: 737-742 (2019) Article DOI: 10.1021/acsmedchemlett.8b00631 BindingDB Entry DOI: 10.7270/Q2WS8XG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50264210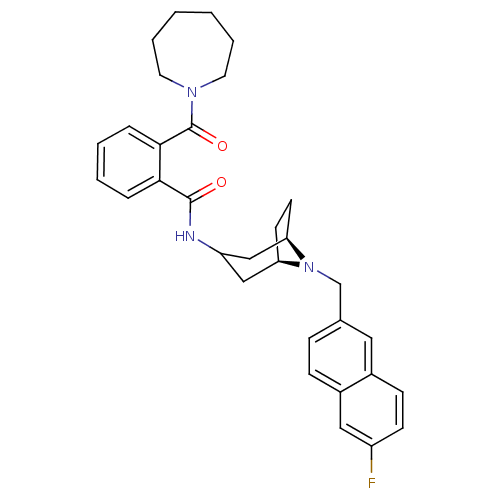 (2-(Azepan-1-ylcarbonyl)-N-{(3-exo)-8-[(6-fluoro-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma. Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR3 receptor expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium influx by spectrophotometry | Bioorg Med Chem 16: 8607-18 (2008) Article DOI: 10.1016/j.bmc.2008.08.006 BindingDB Entry DOI: 10.7270/Q21J9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50394481 (CHEMBL2159868) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR3 receptor | Bioorg Med Chem Lett 22: 6876-81 (2012) Article DOI: 10.1016/j.bmcl.2012.09.035 BindingDB Entry DOI: 10.7270/Q2QC04MN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297187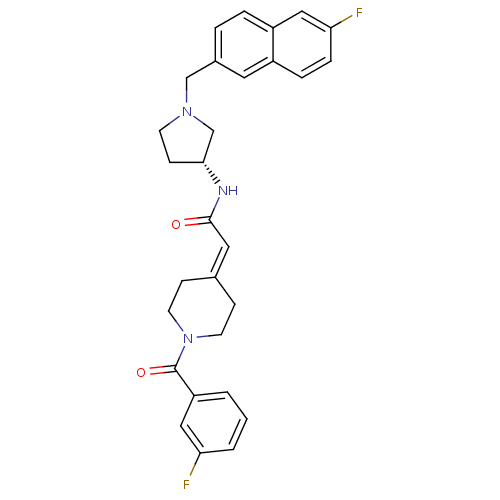 ((R)-2-(1-(3-fluorobenzoyl)piperidin-4-ylidene)-N-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50387661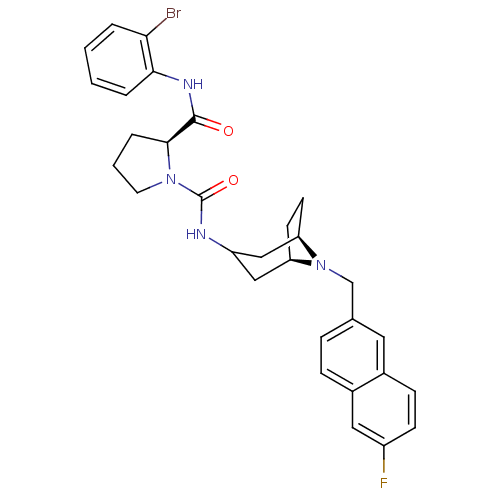 (CHEMBL2057763) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 | Bioorg Med Chem Lett 22: 4951-4 (2012) Article DOI: 10.1016/j.bmcl.2012.06.042 BindingDB Entry DOI: 10.7270/Q2057H06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50264161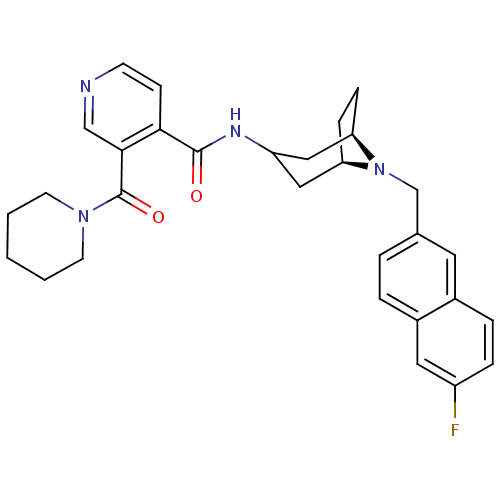 (CHEMBL488929 | N-{(3-exo)-8-[(6-Fluoro-2-naphthyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma. Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR3 receptor expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium influx by spectrophotometry | Bioorg Med Chem 16: 8607-18 (2008) Article DOI: 10.1016/j.bmc.2008.08.006 BindingDB Entry DOI: 10.7270/Q21J9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50387664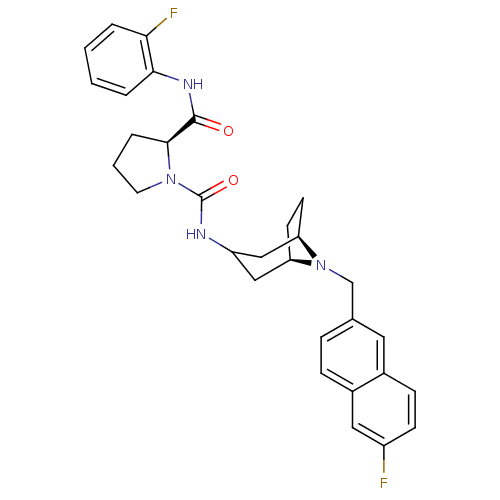 (CHEMBL2057766) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 | Bioorg Med Chem Lett 22: 4951-4 (2012) Article DOI: 10.1016/j.bmcl.2012.06.042 BindingDB Entry DOI: 10.7270/Q2057H06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297176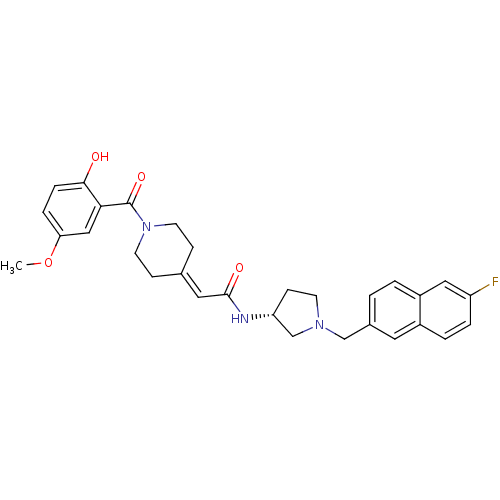 (CHEMBL562574 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fer (Homo sapiens (Human)) | BDBM50501957 (CHEMBL4455220) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His-tagged human FER (SH2 domain to C-terminal) expressed in Escherichia coli assessed as decrease in FL-Peptide... | ACS Med Chem Lett 10: 737-742 (2019) Article DOI: 10.1021/acsmedchemlett.8b00631 BindingDB Entry DOI: 10.7270/Q2WS8XG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50394486 (CHEMBL2159863) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR3 receptor | Bioorg Med Chem Lett 22: 6876-81 (2012) Article DOI: 10.1016/j.bmcl.2012.09.035 BindingDB Entry DOI: 10.7270/Q2QC04MN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50387654 (CHEMBL2057756) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 | Bioorg Med Chem Lett 22: 4951-4 (2012) Article DOI: 10.1016/j.bmcl.2012.06.042 BindingDB Entry DOI: 10.7270/Q2057H06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50264142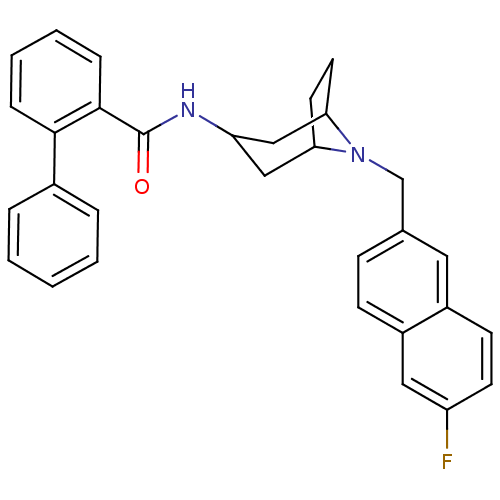 (Biphenyl-2-carboxylic acid [(1R,5R)-8-(6-fluoro-na...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma. Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR3 receptor expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium influx by spectrophotometry | Bioorg Med Chem 16: 8607-18 (2008) Article DOI: 10.1016/j.bmc.2008.08.006 BindingDB Entry DOI: 10.7270/Q21J9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50375019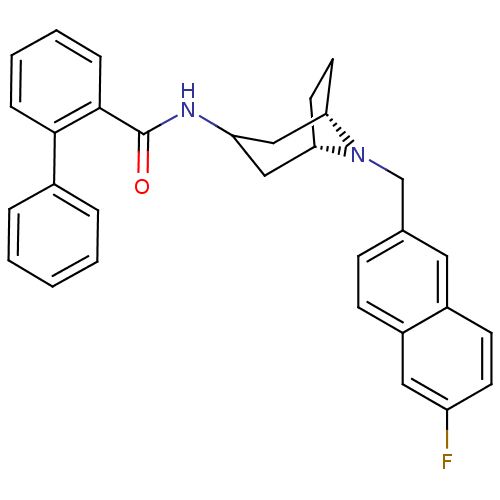 (CHEMBL258996) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR3 expressed in B300-19 cells assessed as inhibition of eotaxin-induced calcium influx | Bioorg Med Chem 16: 144-56 (2008) Article DOI: 10.1016/j.bmc.2007.10.003 BindingDB Entry DOI: 10.7270/Q2445NBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50375019 (CHEMBL258996) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR3 expressed in B300-19 cells assessed as inhibition of eotaxin-induced calcium influx | Bioorg Med Chem 16: 144-56 (2008) Article DOI: 10.1016/j.bmc.2007.10.003 BindingDB Entry DOI: 10.7270/Q2445NBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50264142 (Biphenyl-2-carboxylic acid [(1R,5R)-8-(6-fluoro-na...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50264142 (Biphenyl-2-carboxylic acid [(1R,5R)-8-(6-fluoro-na...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50387642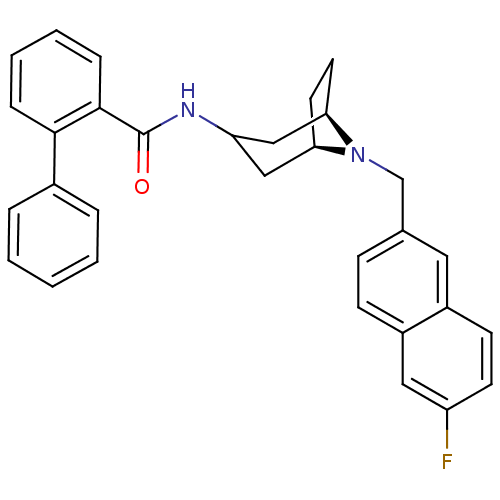 (CHEMBL2057491) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 | Bioorg Med Chem Lett 22: 4951-4 (2012) Article DOI: 10.1016/j.bmcl.2012.06.042 BindingDB Entry DOI: 10.7270/Q2057H06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50264142 (Biphenyl-2-carboxylic acid [(1R,5R)-8-(6-fluoro-na...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma. Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR3 receptor expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium influx by spectrophotometry | Bioorg Med Chem 16: 8607-18 (2008) Article DOI: 10.1016/j.bmc.2008.08.006 BindingDB Entry DOI: 10.7270/Q21J9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297186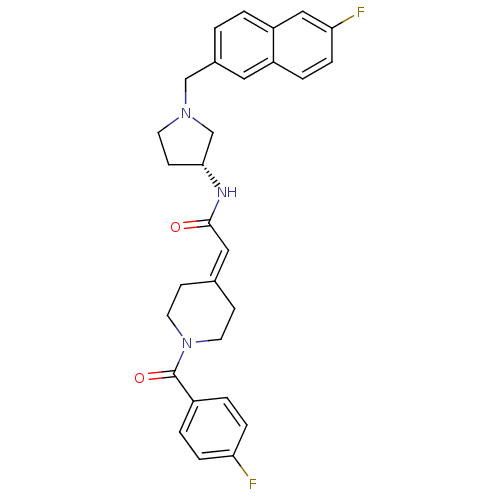 (2-[1-(4-Fluorobenzoyl)piperidin-4-ylidene]-N-{(3R)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50264212 (CHEMBL519042 | N-{(3-exo)-8-[(6-Fluoro-2-naphthyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma. Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR3 receptor expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium influx by spectrophotometry | Bioorg Med Chem 16: 8607-18 (2008) Article DOI: 10.1016/j.bmc.2008.08.006 BindingDB Entry DOI: 10.7270/Q21J9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50394479 (CHEMBL2160097) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR3 receptor | Bioorg Med Chem Lett 22: 6876-81 (2012) Article DOI: 10.1016/j.bmcl.2012.09.035 BindingDB Entry DOI: 10.7270/Q2QC04MN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50264157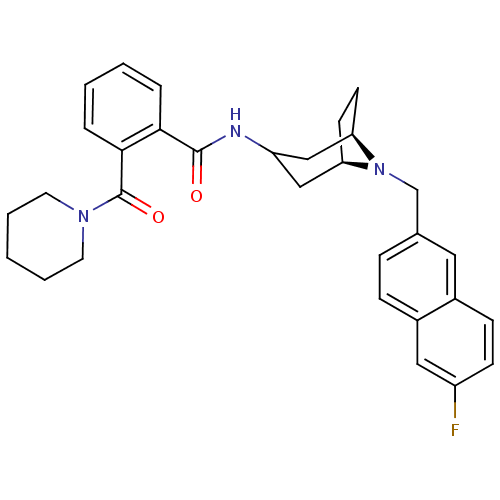 (CHEMBL519750 | N-{(3-exo)-8-[(6-Fluoro-2-naphthyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma. Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR3 receptor expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium influx by spectrophotometry | Bioorg Med Chem 16: 8607-18 (2008) Article DOI: 10.1016/j.bmc.2008.08.006 BindingDB Entry DOI: 10.7270/Q21J9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50264212 (CHEMBL519042 | N-{(3-exo)-8-[(6-Fluoro-2-naphthyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50394480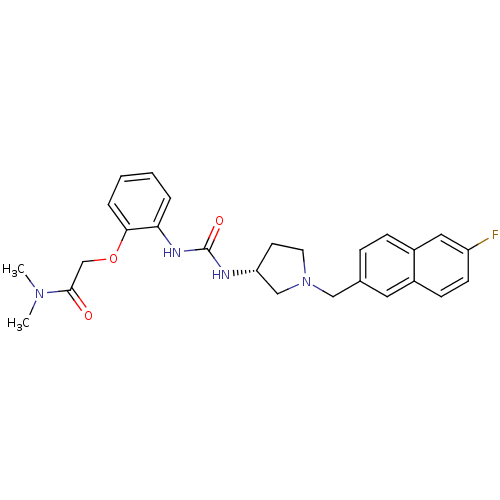 (CHEMBL2159869) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR3 receptor | Bioorg Med Chem Lett 22: 6876-81 (2012) Article DOI: 10.1016/j.bmcl.2012.09.035 BindingDB Entry DOI: 10.7270/Q2QC04MN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fer (Homo sapiens (Human)) | BDBM50501950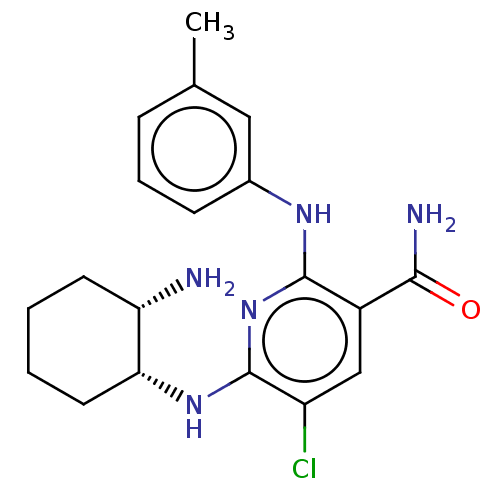 (CHEMBL4548027) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His-tagged human FER (SH2 domain to C-terminal) expressed in Escherichia coli assessed as decrease in FL-Peptide... | ACS Med Chem Lett 10: 737-742 (2019) Article DOI: 10.1021/acsmedchemlett.8b00631 BindingDB Entry DOI: 10.7270/Q2WS8XG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 183 total ) | Next | Last >> |