

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urease [D459Y,K653P] (Canavalia ensiformis (Jack bean)) | BDBM152761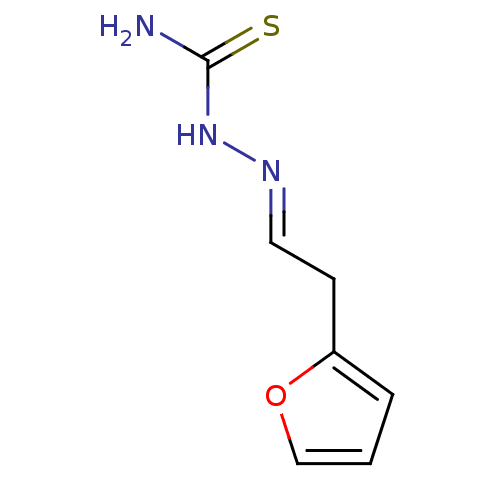 ([(E)-[2-(furan-2-yl)ethylidene]amino]thiourea (3f)) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | 8.2 | n/a |
Quaid-I-Azam University | Assay Description Urease inhibition activity was determined by indophenol method. In brief, each 140 無 assay reaction contained 40 無 buffer (100 mM urea, 0.01 mM K2H... | Chem Biol Drug Des 85: 225-30 (2015) Article DOI: 10.1111/cbdd.12379 BindingDB Entry DOI: 10.7270/Q2P55M71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease [D459Y,K653P] (Canavalia ensiformis (Jack bean)) | BDBM152757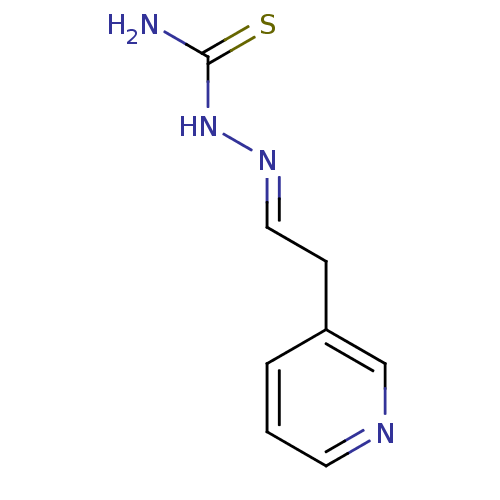 ([(E)-[2-(pyridin-3-yl)ethylidene]amino]thiourea (3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.41E+3 | n/a | n/a | n/a | n/a | 8.2 | n/a |
Quaid-I-Azam University | Assay Description Urease inhibition activity was determined by indophenol method. In brief, each 140 無 assay reaction contained 40 無 buffer (100 mM urea, 0.01 mM K2H... | Chem Biol Drug Des 85: 225-30 (2015) Article DOI: 10.1111/cbdd.12379 BindingDB Entry DOI: 10.7270/Q2P55M71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease [D459Y,K653P] (Canavalia ensiformis (Jack bean)) | BDBM152756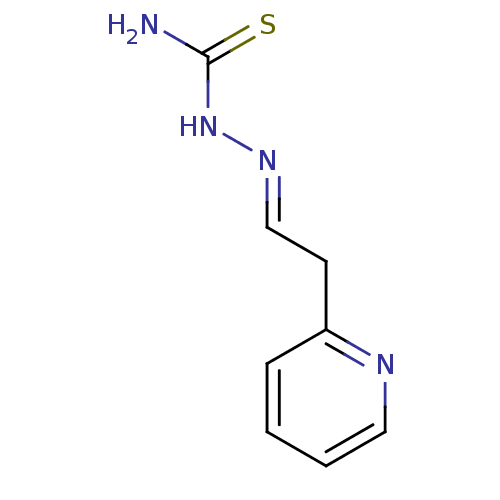 ([(E)-[2-(pyridin-2-yl)ethylidene]amino]thiourea (3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.65E+3 | n/a | n/a | n/a | n/a | 8.2 | n/a |
Quaid-I-Azam University | Assay Description Urease inhibition activity was determined by indophenol method. In brief, each 140 無 assay reaction contained 40 無 buffer (100 mM urea, 0.01 mM K2H... | Chem Biol Drug Des 85: 225-30 (2015) Article DOI: 10.1111/cbdd.12379 BindingDB Entry DOI: 10.7270/Q2P55M71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease [D459Y,K653P] (Canavalia ensiformis (Jack bean)) | BDBM152762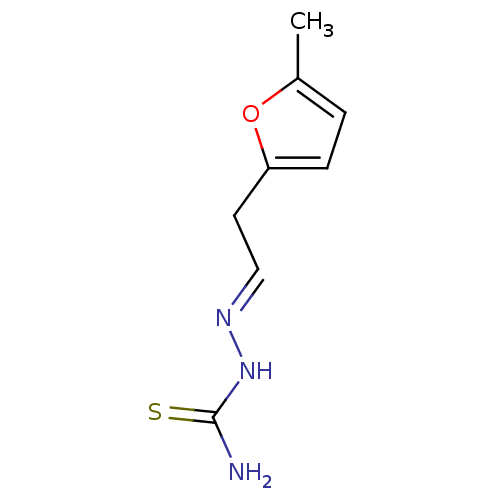 ([(E)-[2-(5-methylfuran-2-yl)ethylidene]amino]thiou...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.23E+3 | n/a | n/a | n/a | n/a | 8.2 | n/a |
Quaid-I-Azam University | Assay Description Urease inhibition activity was determined by indophenol method. In brief, each 140 無 assay reaction contained 40 無 buffer (100 mM urea, 0.01 mM K2H... | Chem Biol Drug Des 85: 225-30 (2015) Article DOI: 10.1111/cbdd.12379 BindingDB Entry DOI: 10.7270/Q2P55M71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease [D459Y,K653P] (Canavalia ensiformis (Jack bean)) | BDBM152766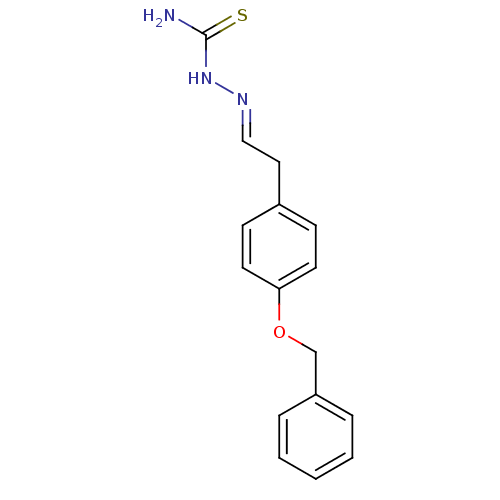 ([(E)-{2-[4-(benzyloxy)phenyl]ethylidene}amino]thio...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.64E+3 | n/a | n/a | n/a | n/a | 8.2 | n/a |
Quaid-I-Azam University | Assay Description Urease inhibition activity was determined by indophenol method. In brief, each 140 無 assay reaction contained 40 無 buffer (100 mM urea, 0.01 mM K2H... | Chem Biol Drug Des 85: 225-30 (2015) Article DOI: 10.1111/cbdd.12379 BindingDB Entry DOI: 10.7270/Q2P55M71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease [D459Y,K653P] (Canavalia ensiformis (Jack bean)) | BDBM152765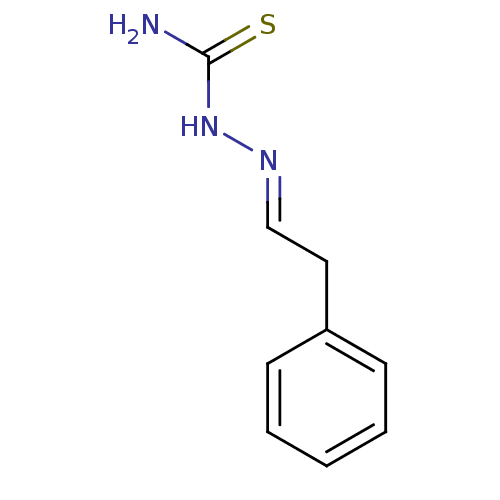 ([(E)-(2-phenylethylidene)amino]thiourea (3j)) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.24E+3 | n/a | n/a | n/a | n/a | 8.2 | n/a |
Quaid-I-Azam University | Assay Description Urease inhibition activity was determined by indophenol method. In brief, each 140 無 assay reaction contained 40 無 buffer (100 mM urea, 0.01 mM K2H... | Chem Biol Drug Des 85: 225-30 (2015) Article DOI: 10.1111/cbdd.12379 BindingDB Entry DOI: 10.7270/Q2P55M71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease [D459Y,K653P] (Canavalia ensiformis (Jack bean)) | BDBM152768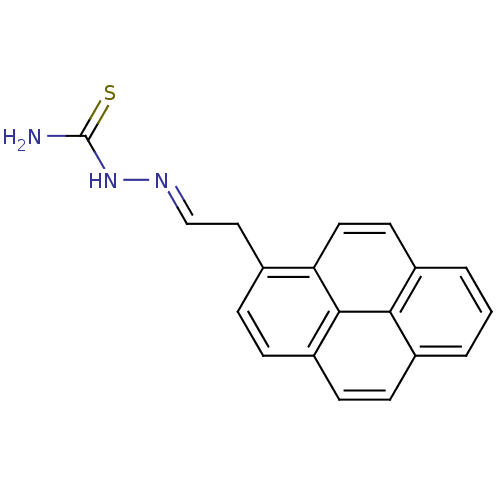 ([(E)-[2-(pyren-1-yl)ethylidene]amino]thiourea (3m)) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.84E+3 | n/a | n/a | n/a | n/a | 8.2 | n/a |
Quaid-I-Azam University | Assay Description Urease inhibition activity was determined by indophenol method. In brief, each 140 無 assay reaction contained 40 無 buffer (100 mM urea, 0.01 mM K2H... | Chem Biol Drug Des 85: 225-30 (2015) Article DOI: 10.1111/cbdd.12379 BindingDB Entry DOI: 10.7270/Q2P55M71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50509618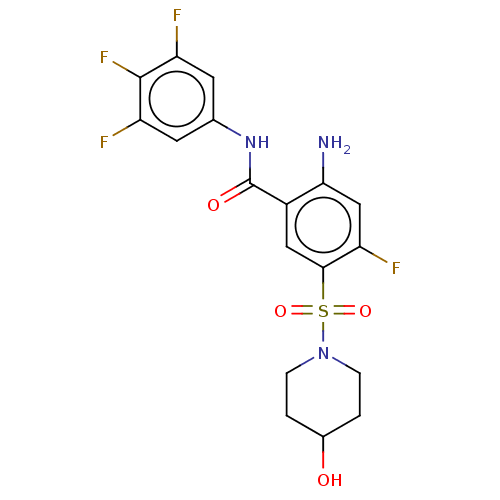 (CHEMBL4463663) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in human HEK293 cells by patch clamp assay | ACS Med Chem Lett 11: 166-171 (2020) Article DOI: 10.1021/acsmedchemlett.9b00550 BindingDB Entry DOI: 10.7270/Q2RF5Z9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50559579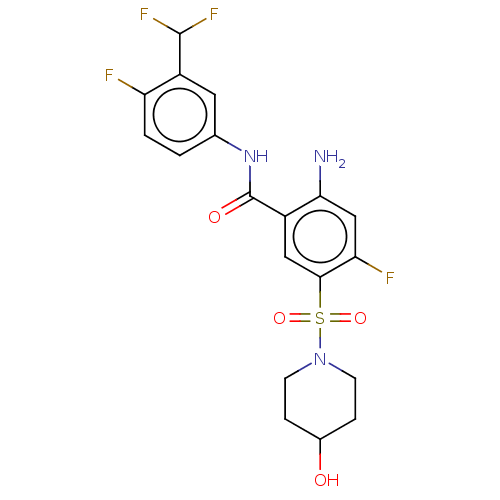 (CHEMBL4754334) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ERG by fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00606 BindingDB Entry DOI: 10.7270/Q2SJ1Q9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50509618 (CHEMBL4463663) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human ERG by competitive fluorescence polarization assay | ACS Med Chem Lett 11: 166-171 (2020) Article DOI: 10.1021/acsmedchemlett.9b00550 BindingDB Entry DOI: 10.7270/Q2RF5Z9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50509618 (CHEMBL4463663) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human CYP2C9 using tolubutamide as substrate by LC/MS/MS analysis | ACS Med Chem Lett 11: 166-171 (2020) Article DOI: 10.1021/acsmedchemlett.9b00550 BindingDB Entry DOI: 10.7270/Q2RF5Z9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50559580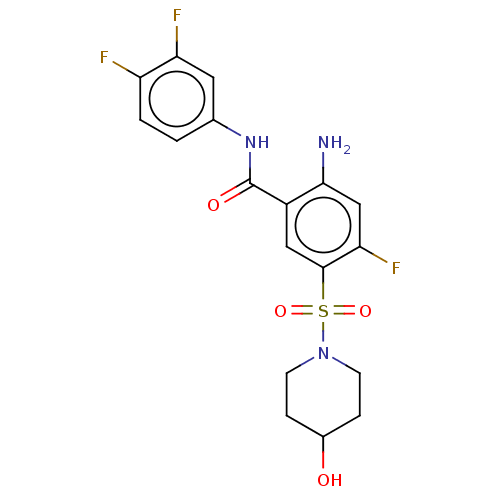 (CHEMBL4761323) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver microsome CYP2C19 using S-mephenytoin as substrate incubated for 20 mins by LC-MS/MS with HPLC analysis | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00606 BindingDB Entry DOI: 10.7270/Q2SJ1Q9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50559579 (CHEMBL4754334) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver microsome CYP2C19 using S-mephenytoin as substrate incubated for 20 mins by LC-MS/MS with HPLC analysis | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00606 BindingDB Entry DOI: 10.7270/Q2SJ1Q9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50559579 (CHEMBL4754334) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver microsome CYP3A4 using sorafenib as substrate incubated for 20 mins by LC-MS/MS with HPLC analysis | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00606 BindingDB Entry DOI: 10.7270/Q2SJ1Q9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease [D459Y,K653P] (Canavalia ensiformis (Jack bean)) | BDBM50229993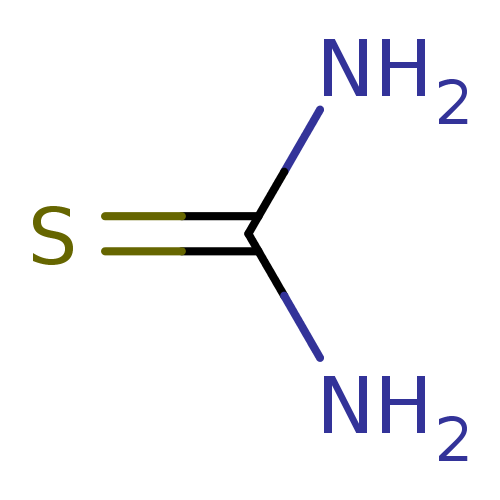 (2-thiourea | CHEMBL260876 | Thiocarbamid | Thiohar...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | 8.2 | n/a |
Quaid-I-Azam University | Assay Description Urease inhibition activity was determined by indophenol method. In brief, each 140 無 assay reaction contained 40 無 buffer (100 mM urea, 0.01 mM K2H... | Chem Biol Drug Des 85: 225-30 (2015) Article DOI: 10.1111/cbdd.12379 BindingDB Entry DOI: 10.7270/Q2P55M71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50509618 (CHEMBL4463663) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human CYP2C19 using S-mephenytoin as substrate by LC/MS/MS analysis | ACS Med Chem Lett 11: 166-171 (2020) Article DOI: 10.1021/acsmedchemlett.9b00550 BindingDB Entry DOI: 10.7270/Q2RF5Z9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50559580 (CHEMBL4761323) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver microsome CYP2C9 using tolbutamide as substrate incubated for 20 mins by LC-MS/MS with HPLC analysis | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00606 BindingDB Entry DOI: 10.7270/Q2SJ1Q9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50559579 (CHEMBL4754334) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver microsome CYP2C9 using tolbutamide as substrate incubated for 20 mins by LC-MS/MS with HPLC analysis | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00606 BindingDB Entry DOI: 10.7270/Q2SJ1Q9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50559579 (CHEMBL4754334) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver microsome CYP2D6 using dextromethorphan as substrate incubated for 20 mins by LC-MS/MS with HPLC analysis | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00606 BindingDB Entry DOI: 10.7270/Q2SJ1Q9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50509618 (CHEMBL4463663) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 using sorafenib as substrate by LC/MS/MS analysis | ACS Med Chem Lett 11: 166-171 (2020) Article DOI: 10.1021/acsmedchemlett.9b00550 BindingDB Entry DOI: 10.7270/Q2RF5Z9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50559580 (CHEMBL4761323) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver microsome CYP2D6 using dextromethorphan as substrate incubated for 20 mins by LC-MS/MS with HPLC analysis | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00606 BindingDB Entry DOI: 10.7270/Q2SJ1Q9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50509618 (CHEMBL4463663) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 using dextromethorphan as substrate by LC/MS/MS analysis | ACS Med Chem Lett 11: 166-171 (2020) Article DOI: 10.1021/acsmedchemlett.9b00550 BindingDB Entry DOI: 10.7270/Q2RF5Z9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50559580 (CHEMBL4761323) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver microsome CYP3A4 using sorafenib as substrate incubated for 20 mins by LC-MS/MS with HPLC analysis | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00606 BindingDB Entry DOI: 10.7270/Q2SJ1Q9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50559579 (CHEMBL4754334) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver microsome CYP1A2 using phenacetin as substrate incubated for 20 mins by LC-MS/MS with HPLC analysis | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00606 BindingDB Entry DOI: 10.7270/Q2SJ1Q9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50559580 (CHEMBL4761323) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ERG by fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00606 BindingDB Entry DOI: 10.7270/Q2SJ1Q9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease [D459Y,K653P] (Canavalia ensiformis (Jack bean)) | BDBM152767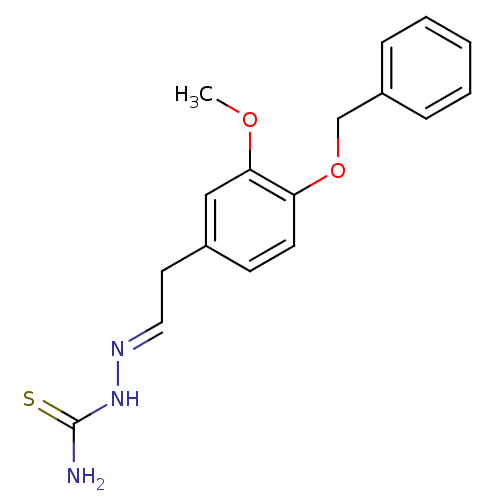 ([(E)-{2-[4-(benzyloxy)-3-methoxyphenyl]ethylidene}...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.51E+4 | n/a | n/a | n/a | n/a | 8.2 | n/a |
Quaid-I-Azam University | Assay Description Urease inhibition activity was determined by indophenol method. In brief, each 140 無 assay reaction contained 40 無 buffer (100 mM urea, 0.01 mM K2H... | Chem Biol Drug Des 85: 225-30 (2015) Article DOI: 10.1111/cbdd.12379 BindingDB Entry DOI: 10.7270/Q2P55M71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50509618 (CHEMBL4463663) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human CYP1A2 using phenacetin as substrate by LC/MS/MS analysis | ACS Med Chem Lett 11: 166-171 (2020) Article DOI: 10.1021/acsmedchemlett.9b00550 BindingDB Entry DOI: 10.7270/Q2RF5Z9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50559580 (CHEMBL4761323) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver microsome CYP1A2 using phenacetin as substrate incubated for 20 mins by LC-MS/MS with HPLC analysis | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00606 BindingDB Entry DOI: 10.7270/Q2SJ1Q9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||