| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Urease [D459Y,K653P] |
|---|
| Ligand | BDBM152762 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | In vitro Urease Inhibition Assay |
|---|
| pH | 8.2±n/a |
|---|
| IC50 | 3.23e+3± 3.6e+2 nM |
|---|
| Comments | extracted |
|---|
| Citation |  Saeed, A; Imran, A; Channar, PA; Shahid, M; Mahmood, W; Iqbal, J 2-(Hetero(aryl)methylene)hydrazine-1-carbothioamides as potent urease inhibitors. Chem Biol Drug Des85:225-30 (2015) [PubMed] Article Saeed, A; Imran, A; Channar, PA; Shahid, M; Mahmood, W; Iqbal, J 2-(Hetero(aryl)methylene)hydrazine-1-carbothioamides as potent urease inhibitors. Chem Biol Drug Des85:225-30 (2015) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Urease [D459Y,K653P] |
|---|
| Name: | Urease [D459Y,K653P] |
|---|
| Synonyms: | UREA_CANEN | Urea amidohydrolase | Urease |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 90763.23 |
|---|
| Organism: | Canavalia ensiformis (Jack bean) |
|---|
| Description: | P07374[D459Y,K653P] |
|---|
| Residue: | 840 |
|---|
| Sequence: | MKLSPREVEKLGLHNAGYLAQKRLARGVRLNYTEAVALIASQIMEYARDGEKTVAQLMCL
GQHLLGRRQVLPAVPHLLNAVQVEATFPDGTKLVTVHDPISRENGELQEALFGSLLPVPS
LDKFAETKEDNRIPGEILCEDECLTLNIGRKAVILKVTSKGDRPIQVGSHYHFIEVNPYL
TFDRRKAYGMRLNIAAGTAVRFEPGDCKSVTLVSIEGNKVIRGGNAIADGPVNETNLEAA
MHAVRSKGFGHEEEKDASEGFTKEDPNCPFNTFIHRKEYANKYGPTTGDKIRLGDTNLLA
EIEKDYALYGDECVFGGGKVIRDGMGQSCGHPPAISLDTVITNAVIIDYTGIIKADIGIK
DGLIASIGKAGNPDIMNGVFSNMIIGANTEVIAGEGLIVTAGAIDCHVHYICPQLVYEAI
SSGITTLVGGGTGPAAGTRATTCTPSPTQMRLMLQSTDYLPLNFGFTGKGSSSKPDELHE
IIKAGAMGLKLHEDWGSTPAAIDNCLTIAEHHDIQINIHTDTLNEAGFVEHSIAAFKGRT
IHTYHSEGAGGGHAPDIIKVCGIKNVLPSSTNPTRPLTSNTIDEHLDMLMVCHHLDREIP
EDLAFAHSRIRKKTIAAEDVLNDIGAISIISSDSQAMGRVGEVISRTWQTADPMKAQTGP
LKCDSSDNDNFRIRRYIAKYTINPAIANGFSQYVGSVEVGKLADLVMWKPSFFGTKPEMV
IKGGMVAWADIGDPNASIPTPEPVKMRPMYGTLGKAGGALSIAFVSKAALDQRVNVLYGL
NKRVEAVSNVRKLTKLDMKLNDALPEITVDPESYTVKADGKLLCVSEATTVPLSRNYFLF
|
|
|
|---|
| BDBM152762 |
|---|
| n/a |
|---|
| Name | BDBM152762 |
|---|
| Synonyms: | [(E)-[2-(5-methylfuran-2-yl)ethylidene]amino]thiourea (3g) |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C8H11N3OS |
|---|
| Mol. Mass. | 197.257 |
|---|
| SMILES | Cc1ccc(C\C=N\NC(N)=S)o1 |
|---|
| Structure | 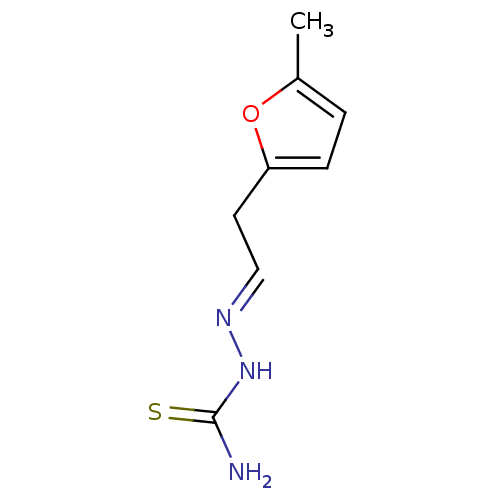
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Saeed, A; Imran, A; Channar, PA; Shahid, M; Mahmood, W; Iqbal, J 2-(Hetero(aryl)methylene)hydrazine-1-carbothioamides as potent urease inhibitors. Chem Biol Drug Des85:225-30 (2015) [PubMed] Article
Saeed, A; Imran, A; Channar, PA; Shahid, M; Mahmood, W; Iqbal, J 2-(Hetero(aryl)methylene)hydrazine-1-carbothioamides as potent urease inhibitors. Chem Biol Drug Des85:225-30 (2015) [PubMed] Article