 Found 249 hits with Last Name = 'ishchenko' and Initial = 'a'
Found 249 hits with Last Name = 'ishchenko' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Renin
(Homo sapiens (Human)) | BDBM29957
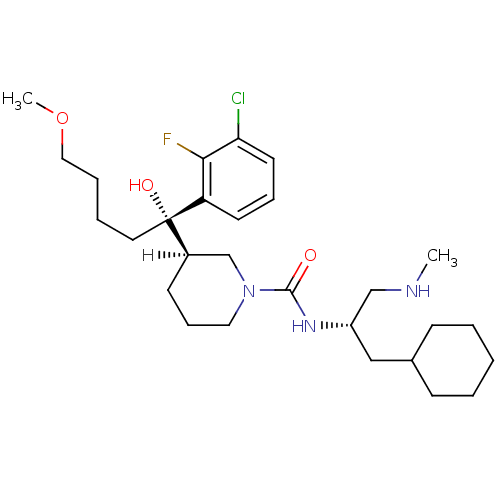
(piperidine-1-carboxamide, 21t)Show SMILES [H][C@]1(CCCN(C1)C(=O)N[C@H](CNC)CC1CCCCC1)[C@@](O)(CCCCOC)c1cccc(Cl)c1F |r| Show InChI InChI=1S/C28H45ClFN3O3/c1-31-19-23(18-21-10-4-3-5-11-21)32-27(34)33-16-9-12-22(20-33)28(35,15-6-7-17-36-2)24-13-8-14-25(29)26(24)30/h8,13-14,21-23,31,35H,3-7,9-12,15-20H2,1-2H3,(H,32,34)/t22-,23+,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | 25 |
Vitae Pharmaceuticals
| Assay Description
The activity of renin inhibitors in vitro was measured using the FRET assay, which was carried out in flat-bottom white opaque microtiter plates. The... |
Bioorg Med Chem Lett 19: 3541-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.140
BindingDB Entry DOI: 10.7270/Q2D21VXV |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM29950
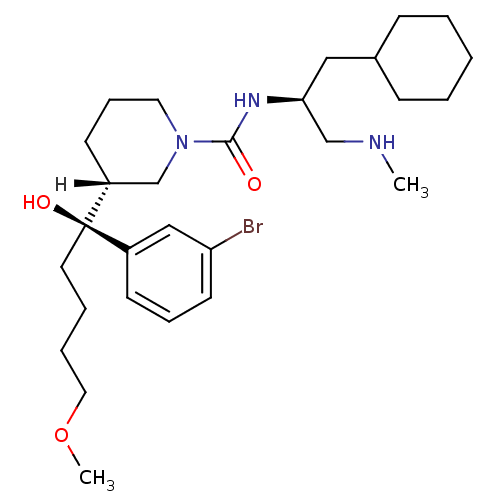
(piperidine-1-carboxamide, 21m)Show SMILES [H][C@]1(CCCN(C1)C(=O)N[C@H](CNC)CC1CCCCC1)[C@@](O)(CCCCOC)c1cccc(Br)c1 |r| Show InChI InChI=1S/C28H46BrN3O3/c1-30-20-26(18-22-10-4-3-5-11-22)31-27(33)32-16-9-13-24(21-32)28(34,15-6-7-17-35-2)23-12-8-14-25(29)19-23/h8,12,14,19,22,24,26,30,34H,3-7,9-11,13,15-18,20-21H2,1-2H3,(H,31,33)/t24-,26+,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Vitae Pharmaceuticals
| Assay Description
The activity of renin inhibitors in vitro was measured using the FRET assay, which was carried out in flat-bottom white opaque microtiter plates. The... |
Bioorg Med Chem Lett 19: 3541-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.140
BindingDB Entry DOI: 10.7270/Q2D21VXV |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM29956
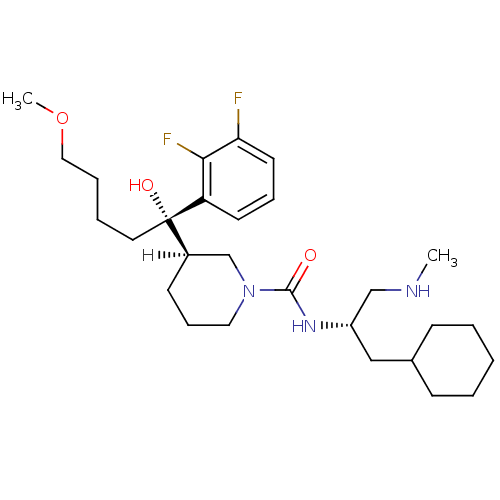
(piperidine-1-carboxamide, 21s)Show SMILES [H][C@]1(CCCN(C1)C(=O)N[C@H](CNC)CC1CCCCC1)[C@@](O)(CCCCOC)c1cccc(F)c1F |r| Show InChI InChI=1S/C28H45F2N3O3/c1-31-19-23(18-21-10-4-3-5-11-21)32-27(34)33-16-9-12-22(20-33)28(35,15-6-7-17-36-2)24-13-8-14-25(29)26(24)30/h8,13-14,21-23,31,35H,3-7,9-12,15-20H2,1-2H3,(H,32,34)/t22-,23+,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | 25 |
Vitae Pharmaceuticals
| Assay Description
The activity of renin inhibitors in vitro was measured using the FRET assay, which was carried out in flat-bottom white opaque microtiter plates. The... |
Bioorg Med Chem Lett 19: 3541-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.140
BindingDB Entry DOI: 10.7270/Q2D21VXV |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50382333

(CHEMBL2023123)Show SMILES CNC[C@@H](NC(=O)N1CCC[C@H](C1)[C@@H](OCCNC(=O)OC)c1cccc(Cl)c1)[C@@H](O)C1CCCCC1 |r| Show InChI InChI=1S/C27H43ClN4O5/c1-29-17-23(24(33)19-8-4-3-5-9-19)31-26(34)32-14-7-11-21(18-32)25(20-10-6-12-22(28)16-20)37-15-13-30-27(35)36-2/h6,10,12,16,19,21,23-25,29,33H,3-5,7-9,11,13-15,17-18H2,1-2H3,(H,30,35)(H,31,34)/t21-,23-,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin using H-Asp-Arg-Val-Tyr-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Asn-OH as substrate assessed as formation of angiot... |
ACS Med Chem Lett 2: 747-751 (2011)
Article DOI: 10.1021/ml200137x
BindingDB Entry DOI: 10.7270/Q2FT8N2Q |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50305450
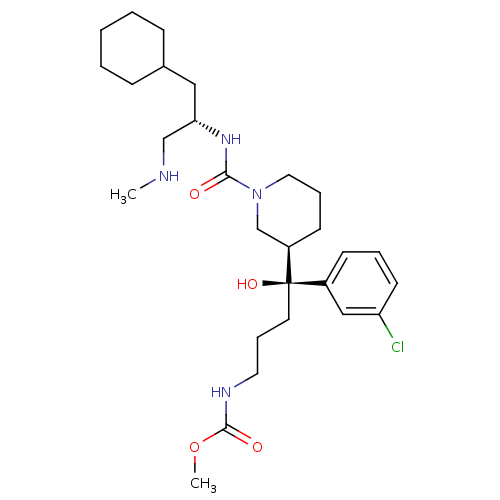
(CHEMBL592763 | methyl (S)-4-(3-chlorophenyl)-4-((R...)Show SMILES CNC[C@H](CC1CCCCC1)NC(=O)N1CCC[C@H](C1)[C@@](O)(CCCNC(=O)OC)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C28H45ClN4O4/c1-30-19-25(17-21-9-4-3-5-10-21)32-26(34)33-16-7-12-23(20-33)28(36,14-8-15-31-27(35)37-2)22-11-6-13-24(29)18-22/h6,11,13,18,21,23,25,30,36H,3-5,7-10,12,14-17,19-20H2,1-2H3,(H,31,35)(H,32,34)/t23-,25+,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-activated human recombinant renin |
Bioorg Med Chem Lett 20: 694-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.066
BindingDB Entry DOI: 10.7270/Q24Q7V20 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50305465
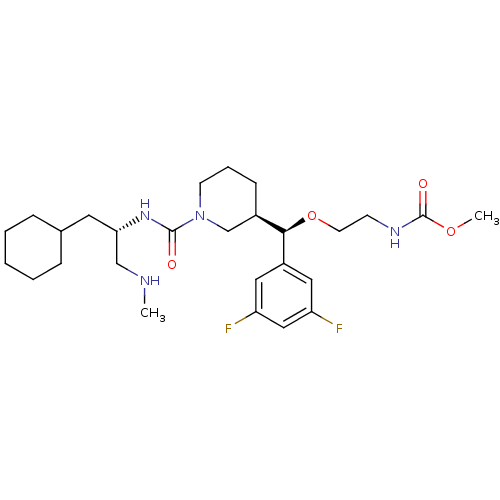
(CHEMBL589647 | methyl 2-((R)-((R)-1-((S)-1-cyclohe...)Show SMILES CNC[C@H](CC1CCCCC1)NC(=O)N1CCC[C@H](C1)[C@@H](OCCNC(=O)OC)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C27H42F2N4O4/c1-30-17-24(13-19-7-4-3-5-8-19)32-26(34)33-11-6-9-20(18-33)25(37-12-10-31-27(35)36-2)21-14-22(28)16-23(29)15-21/h14-16,19-20,24-25,30H,3-13,17-18H2,1-2H3,(H,31,35)(H,32,34)/t20-,24+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-activated human recombinant renin |
Bioorg Med Chem Lett 20: 694-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.066
BindingDB Entry DOI: 10.7270/Q24Q7V20 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50382335
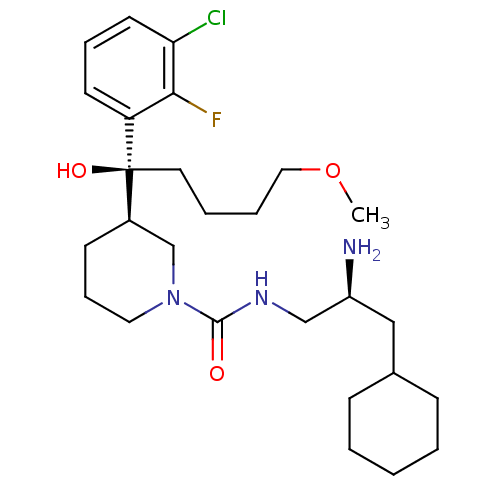
(CHEMBL2024249)Show SMILES COCCCC[C@](O)([C@@H]1CCCN(C1)C(=O)NC[C@@H](N)CC1CCCCC1)c1cccc(Cl)c1F |r| Show InChI InChI=1S/C27H43ClFN3O3/c1-35-16-6-5-14-27(34,23-12-7-13-24(28)25(23)29)21-11-8-15-32(19-21)26(33)31-18-22(30)17-20-9-3-2-4-10-20/h7,12-13,20-22,34H,2-6,8-11,14-19,30H2,1H3,(H,31,33)/t21-,22+,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin using H-Asp-Arg-Val-Tyr-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Asn-OH as substrate assessed as formation of angiot... |
ACS Med Chem Lett 2: 747-751 (2011)
Article DOI: 10.1021/ml200137x
BindingDB Entry DOI: 10.7270/Q2FT8N2Q |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50382334
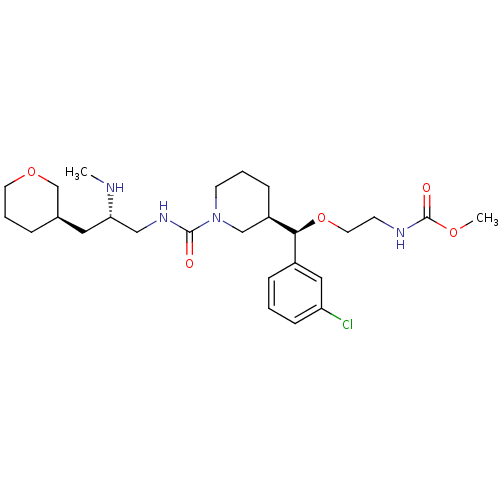
(CHEMBL1276678)Show SMILES CN[C@H](CNC(=O)N1CCC[C@H](C1)[C@@H](OCCNC(=O)OC)c1cccc(Cl)c1)C[C@H]1CCCOC1 |r| Show InChI InChI=1S/C26H41ClN4O5/c1-28-23(14-19-6-5-12-35-18-19)16-30-25(32)31-11-4-8-21(17-31)24(20-7-3-9-22(27)15-20)36-13-10-29-26(33)34-2/h3,7,9,15,19,21,23-24,28H,4-6,8,10-14,16-18H2,1-2H3,(H,29,33)(H,30,32)/t19-,21-,23+,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin using H-Asp-Arg-Val-Tyr-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Asn-OH as substrate assessed as formation of angiot... |
ACS Med Chem Lett 2: 747-751 (2011)
Article DOI: 10.1021/ml200137x
BindingDB Entry DOI: 10.7270/Q2FT8N2Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50382333

(CHEMBL2023123)Show SMILES CNC[C@@H](NC(=O)N1CCC[C@H](C1)[C@@H](OCCNC(=O)OC)c1cccc(Cl)c1)[C@@H](O)C1CCCCC1 |r| Show InChI InChI=1S/C27H43ClN4O5/c1-29-17-23(24(33)19-8-4-3-5-9-19)31-26(34)32-14-7-11-21(18-32)25(20-10-6-12-22(28)16-20)37-15-13-30-27(35)36-2/h6,10,12,16,19,21,23-25,29,33H,3-5,7-9,11,13-15,17-18H2,1-2H3,(H,30,35)(H,31,34)/t21-,23-,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin using DABCYL-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate for 60 mins by fluorimetry |
ACS Med Chem Lett 2: 747-751 (2011)
Article DOI: 10.1021/ml200137x
BindingDB Entry DOI: 10.7270/Q2FT8N2Q |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM17950

((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...)Show SMILES COCCCOc1cc(C[C@@H](C[C@H](N)[C@@H](O)C[C@@H](C(C)C)C(=O)NCC(C)(C)C(N)=O)C(C)C)ccc1OC |r| Show InChI InChI=1S/C30H53N3O6/c1-19(2)22(14-21-10-11-26(38-8)27(15-21)39-13-9-12-37-7)16-24(31)25(34)17-23(20(3)4)28(35)33-18-30(5,6)29(32)36/h10-11,15,19-20,22-25,34H,9,12-14,16-18,31H2,1-8H3,(H2,32,36)(H,33,35)/t22-,23-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin using DABCYL-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate for 60 mins by fluorimetry |
ACS Med Chem Lett 2: 747-751 (2011)
Article DOI: 10.1021/ml200137x
BindingDB Entry DOI: 10.7270/Q2FT8N2Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50382331
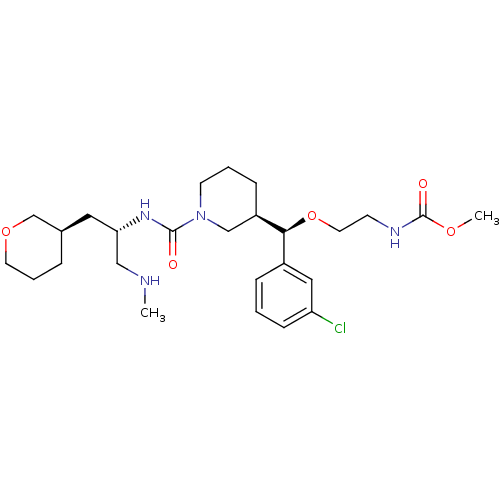
(CHEMBL2024248)Show SMILES CNC[C@H](C[C@H]1CCCOC1)NC(=O)N1CCC[C@H](C1)[C@@H](OCCNC(=O)OC)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C26H41ClN4O5/c1-28-16-23(14-19-6-5-12-35-18-19)30-25(32)31-11-4-8-21(17-31)24(20-7-3-9-22(27)15-20)36-13-10-29-26(33)34-2/h3,7,9,15,19,21,23-24,28H,4-6,8,10-14,16-18H2,1-2H3,(H,29,33)(H,30,32)/t19-,21-,23+,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin using H-Asp-Arg-Val-Tyr-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Asn-OH as substrate assessed as formation of angiot... |
ACS Med Chem Lett 2: 747-751 (2011)
Article DOI: 10.1021/ml200137x
BindingDB Entry DOI: 10.7270/Q2FT8N2Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM29949
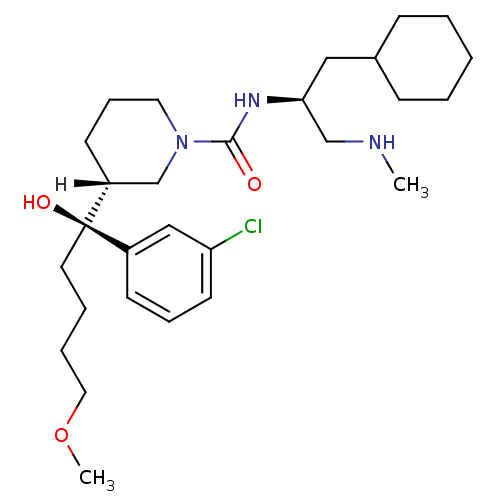
(piperidine-1-carboxamide, 21l)Show SMILES [H][C@]1(CCCN(C1)C(=O)N[C@H](CNC)CC1CCCCC1)[C@@](O)(CCCCOC)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C28H46ClN3O3/c1-30-20-26(18-22-10-4-3-5-11-22)31-27(33)32-16-9-13-24(21-32)28(34,15-6-7-17-35-2)23-12-8-14-25(29)19-23/h8,12,14,19,22,24,26,30,34H,3-7,9-11,13,15-18,20-21H2,1-2H3,(H,31,33)/t24-,26+,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Vitae Pharmaceuticals
| Assay Description
The activity of renin inhibitors in vitro was measured using the FRET assay, which was carried out in flat-bottom white opaque microtiter plates. The... |
Bioorg Med Chem Lett 19: 3541-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.140
BindingDB Entry DOI: 10.7270/Q2D21VXV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50382334

(CHEMBL1276678)Show SMILES CN[C@H](CNC(=O)N1CCC[C@H](C1)[C@@H](OCCNC(=O)OC)c1cccc(Cl)c1)C[C@H]1CCCOC1 |r| Show InChI InChI=1S/C26H41ClN4O5/c1-28-23(14-19-6-5-12-35-18-19)16-30-25(32)31-11-4-8-21(17-31)24(20-7-3-9-22(27)15-20)36-13-10-29-26(33)34-2/h3,7,9,15,19,21,23-24,28H,4-6,8,10-14,16-18H2,1-2H3,(H,29,33)(H,30,32)/t19-,21-,23+,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin using DABCYL-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate for 60 mins by fluorimetry |
ACS Med Chem Lett 2: 747-751 (2011)
Article DOI: 10.1021/ml200137x
BindingDB Entry DOI: 10.7270/Q2FT8N2Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50305454
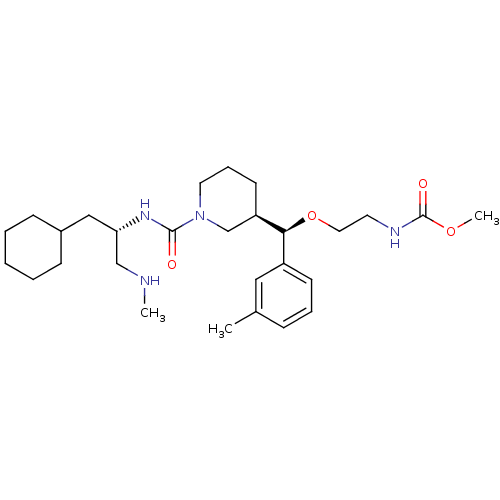
(CHEMBL591342 | methyl 2-((R)-((R)-1-((S)-1-cyclohe...)Show SMILES CNC[C@H](CC1CCCCC1)NC(=O)N1CCC[C@H](C1)[C@@H](OCCNC(=O)OC)c1cccc(C)c1 |r| Show InChI InChI=1S/C28H46N4O4/c1-21-9-7-12-23(17-21)26(36-16-14-30-28(34)35-3)24-13-8-15-32(20-24)27(33)31-25(19-29-2)18-22-10-5-4-6-11-22/h7,9,12,17,22,24-26,29H,4-6,8,10-11,13-16,18-20H2,1-3H3,(H,30,34)(H,31,33)/t24-,25+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-activated human recombinant renin |
Bioorg Med Chem Lett 20: 694-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.066
BindingDB Entry DOI: 10.7270/Q24Q7V20 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM29949

(piperidine-1-carboxamide, 21l)Show SMILES [H][C@]1(CCCN(C1)C(=O)N[C@H](CNC)CC1CCCCC1)[C@@](O)(CCCCOC)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C28H46ClN3O3/c1-30-20-26(18-22-10-4-3-5-11-22)31-27(33)32-16-9-13-24(21-32)28(34,15-6-7-17-35-2)23-12-8-14-25(29)19-23/h8,12,14,19,22,24,26,30,34H,3-7,9-11,13,15-18,20-21H2,1-2H3,(H,31,33)/t24-,26+,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-activated human recombinant renin |
Bioorg Med Chem Lett 20: 694-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.066
BindingDB Entry DOI: 10.7270/Q24Q7V20 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50305456

(CHEMBL605408 | methyl 2-((R)-((R)-1-((S)-1-amino-3...)Show SMILES COC(=O)NCCO[C@H]([C@@H]1CCCN(C1)C(=O)N[C@H](CN)CC1CCCCC1)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C26H41ClN4O4/c1-34-26(33)29-12-14-35-24(20-9-5-11-22(27)16-20)21-10-6-13-31(18-21)25(32)30-23(17-28)15-19-7-3-2-4-8-19/h5,9,11,16,19,21,23-24H,2-4,6-8,10,12-15,17-18,28H2,1H3,(H,29,33)(H,30,32)/t21-,23+,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-activated human recombinant renin |
Bioorg Med Chem Lett 20: 694-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.066
BindingDB Entry DOI: 10.7270/Q24Q7V20 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50305452
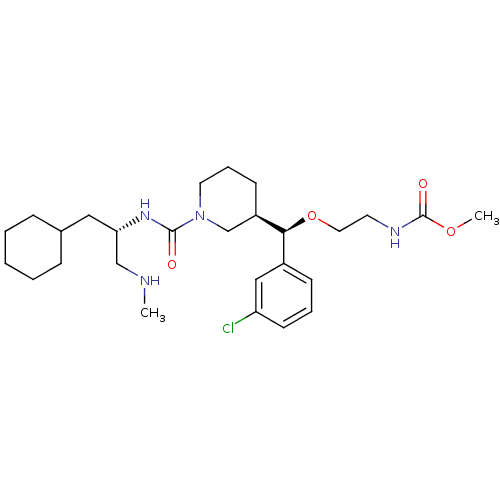
(CHEMBL591578 | methyl 2-((R)-(3-chlorophenyl)((R)-...)Show SMILES CNC[C@H](CC1CCCCC1)NC(=O)N1CCC[C@H](C1)[C@@H](OCCNC(=O)OC)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C27H43ClN4O4/c1-29-18-24(16-20-8-4-3-5-9-20)31-26(33)32-14-7-11-22(19-32)25(21-10-6-12-23(28)17-21)36-15-13-30-27(34)35-2/h6,10,12,17,20,22,24-25,29H,3-5,7-9,11,13-16,18-19H2,1-2H3,(H,30,34)(H,31,33)/t22-,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-activated human recombinant renin |
Bioorg Med Chem Lett 20: 694-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.066
BindingDB Entry DOI: 10.7270/Q24Q7V20 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50305452

(CHEMBL591578 | methyl 2-((R)-(3-chlorophenyl)((R)-...)Show SMILES CNC[C@H](CC1CCCCC1)NC(=O)N1CCC[C@H](C1)[C@@H](OCCNC(=O)OC)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C27H43ClN4O4/c1-29-18-24(16-20-8-4-3-5-9-20)31-26(33)32-14-7-11-22(19-32)25(21-10-6-12-23(28)17-21)36-15-13-30-27(34)35-2/h6,10,12,17,20,22,24-25,29H,3-5,7-9,11,13-16,18-19H2,1-2H3,(H,30,34)(H,31,33)/t22-,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin using DABCYL-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate for 60 mins by fluorimetry |
ACS Med Chem Lett 2: 747-751 (2011)
Article DOI: 10.1021/ml200137x
BindingDB Entry DOI: 10.7270/Q2FT8N2Q |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50382331

(CHEMBL2024248)Show SMILES CNC[C@H](C[C@H]1CCCOC1)NC(=O)N1CCC[C@H](C1)[C@@H](OCCNC(=O)OC)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C26H41ClN4O5/c1-28-16-23(14-19-6-5-12-35-18-19)30-25(32)31-11-4-8-21(17-31)24(20-7-3-9-22(27)15-20)36-13-10-29-26(33)34-2/h3,7,9,15,19,21,23-24,28H,4-6,8,10-14,16-18H2,1-2H3,(H,29,33)(H,30,32)/t19-,21-,23+,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of renin in human plasma assessed as formation of angiotensin1 product after 90 mins by competitive radioimmunoassay |
ACS Med Chem Lett 2: 747-751 (2011)
Article DOI: 10.1021/ml200137x
BindingDB Entry DOI: 10.7270/Q2FT8N2Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM17950

((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...)Show SMILES COCCCOc1cc(C[C@@H](C[C@H](N)[C@@H](O)C[C@@H](C(C)C)C(=O)NCC(C)(C)C(N)=O)C(C)C)ccc1OC |r| Show InChI InChI=1S/C30H53N3O6/c1-19(2)22(14-21-10-11-26(38-8)27(15-21)39-13-9-12-37-7)16-24(31)25(34)17-23(20(3)4)28(35)33-18-30(5,6)29(32)36/h10-11,15,19-20,22-25,34H,9,12-14,16-18,31H2,1-8H3,(H2,32,36)(H,33,35)/t22-,23-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | 25 |
Vitae Pharmaceuticals
| Assay Description
The activity of renin inhibitors in vitro was measured using the FRET assay, which was carried out in flat-bottom white opaque microtiter plates. The... |
Bioorg Med Chem Lett 19: 3541-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.140
BindingDB Entry DOI: 10.7270/Q2D21VXV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM29949

(piperidine-1-carboxamide, 21l)Show SMILES [H][C@]1(CCCN(C1)C(=O)N[C@H](CNC)CC1CCCCC1)[C@@](O)(CCCCOC)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C28H46ClN3O3/c1-30-20-26(18-22-10-4-3-5-11-22)31-27(33)32-16-9-13-24(21-32)28(34,15-6-7-17-35-2)23-12-8-14-25(29)19-23/h8,12,14,19,22,24,26,30,34H,3-7,9-11,13,15-18,20-21H2,1-2H3,(H,31,33)/t24-,26+,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
USA.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human renin using of DABCYL-c-Abu-IleHis-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate after 60 to 360 mins by fluores... |
Bioorg Med Chem Lett 21: 4836-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.043
BindingDB Entry DOI: 10.7270/Q25M663G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50305466
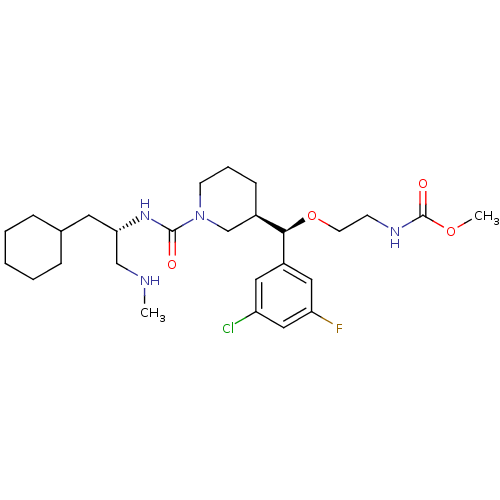
(CHEMBL592765 | methyl 2-((R)-(3-chloro-5-fluorophe...)Show SMILES CNC[C@H](CC1CCCCC1)NC(=O)N1CCC[C@H](C1)[C@@H](OCCNC(=O)OC)c1cc(F)cc(Cl)c1 |r| Show InChI InChI=1S/C27H42ClFN4O4/c1-30-17-24(13-19-7-4-3-5-8-19)32-26(34)33-11-6-9-20(18-33)25(37-12-10-31-27(35)36-2)21-14-22(28)16-23(29)15-21/h14-16,19-20,24-25,30H,3-13,17-18H2,1-2H3,(H,31,35)(H,32,34)/t20-,24+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-activated human recombinant renin |
Bioorg Med Chem Lett 20: 694-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.066
BindingDB Entry DOI: 10.7270/Q24Q7V20 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM17950

((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...)Show SMILES COCCCOc1cc(C[C@@H](C[C@H](N)[C@@H](O)C[C@@H](C(C)C)C(=O)NCC(C)(C)C(N)=O)C(C)C)ccc1OC |r| Show InChI InChI=1S/C30H53N3O6/c1-19(2)22(14-21-10-11-26(38-8)27(15-21)39-13-9-12-37-7)16-24(31)25(34)17-23(20(3)4)28(35)33-18-30(5,6)29(32)36/h10-11,15,19-20,22-25,34H,9,12-14,16-18,31H2,1-8H3,(H2,32,36)(H,33,35)/t22-,23-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-activated human recombinant renin |
Bioorg Med Chem Lett 20: 694-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.066
BindingDB Entry DOI: 10.7270/Q24Q7V20 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM17950

((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...)Show SMILES COCCCOc1cc(C[C@@H](C[C@H](N)[C@@H](O)C[C@@H](C(C)C)C(=O)NCC(C)(C)C(N)=O)C(C)C)ccc1OC |r| Show InChI InChI=1S/C30H53N3O6/c1-19(2)22(14-21-10-11-26(38-8)27(15-21)39-13-9-12-37-7)16-24(31)25(34)17-23(20(3)4)28(35)33-18-30(5,6)29(32)36/h10-11,15,19-20,22-25,34H,9,12-14,16-18,31H2,1-8H3,(H2,32,36)(H,33,35)/t22-,23-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin using H-Asp-Arg-Val-Tyr-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Asn-OH as substrate assessed as formation of angiot... |
ACS Med Chem Lett 2: 747-751 (2011)
Article DOI: 10.1021/ml200137x
BindingDB Entry DOI: 10.7270/Q2FT8N2Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50382331

(CHEMBL2024248)Show SMILES CNC[C@H](C[C@H]1CCCOC1)NC(=O)N1CCC[C@H](C1)[C@@H](OCCNC(=O)OC)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C26H41ClN4O5/c1-28-16-23(14-19-6-5-12-35-18-19)30-25(32)31-11-4-8-21(17-31)24(20-7-3-9-22(27)15-20)36-13-10-29-26(33)34-2/h3,7,9,15,19,21,23-24,28H,4-6,8,10-14,16-18H2,1-2H3,(H,29,33)(H,30,32)/t19-,21-,23+,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin using DABCYL-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate for 60 mins by fluorimetry |
ACS Med Chem Lett 2: 747-751 (2011)
Article DOI: 10.1021/ml200137x
BindingDB Entry DOI: 10.7270/Q2FT8N2Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50305457
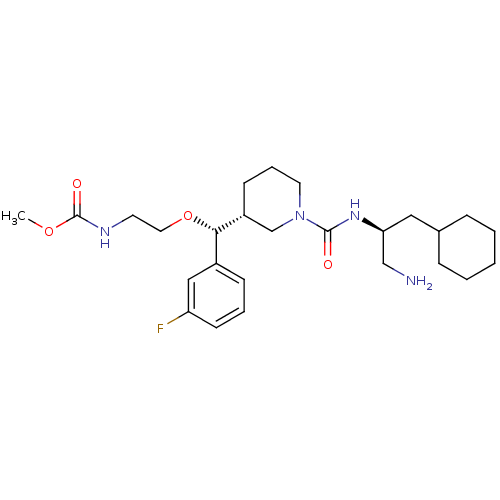
(CHEMBL591340 | methyl 2-((R)-((R)-1-((S)-1-amino-3...)Show SMILES COC(=O)NCCO[C@H]([C@@H]1CCCN(C1)C(=O)N[C@H](CN)CC1CCCCC1)c1cccc(F)c1 |r| Show InChI InChI=1S/C26H41FN4O4/c1-34-26(33)29-12-14-35-24(20-9-5-11-22(27)16-20)21-10-6-13-31(18-21)25(32)30-23(17-28)15-19-7-3-2-4-8-19/h5,9,11,16,19,21,23-24H,2-4,6-8,10,12-15,17-18,28H2,1H3,(H,29,33)(H,30,32)/t21-,23+,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-activated human recombinant renin |
Bioorg Med Chem Lett 20: 694-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.066
BindingDB Entry DOI: 10.7270/Q24Q7V20 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50305453
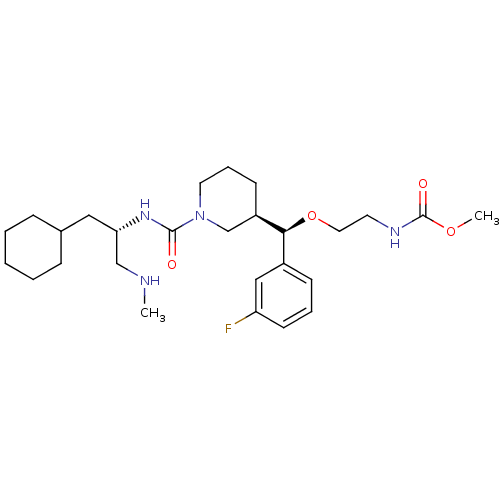
(CHEMBL592762 | methyl 2-((R)-((R)-1-((S)-1-cyclohe...)Show SMILES CNC[C@H](CC1CCCCC1)NC(=O)N1CCC[C@H](C1)[C@@H](OCCNC(=O)OC)c1cccc(F)c1 |r| Show InChI InChI=1S/C27H43FN4O4/c1-29-18-24(16-20-8-4-3-5-9-20)31-26(33)32-14-7-11-22(19-32)25(21-10-6-12-23(28)17-21)36-15-13-30-27(34)35-2/h6,10,12,17,20,22,24-25,29H,3-5,7-9,11,13-16,18-19H2,1-2H3,(H,30,34)(H,31,33)/t22-,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-activated human recombinant renin |
Bioorg Med Chem Lett 20: 694-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.066
BindingDB Entry DOI: 10.7270/Q24Q7V20 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50305462
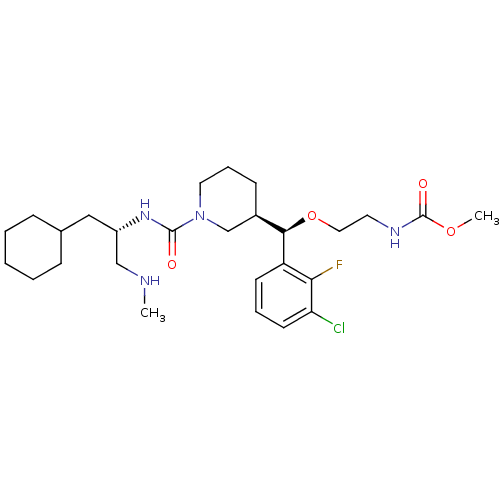
(CHEMBL590623 | methyl 2-((R)-(3-chloro-2-fluorophe...)Show SMILES CNC[C@H](CC1CCCCC1)NC(=O)N1CCC[C@H](C1)[C@@H](OCCNC(=O)OC)c1cccc(Cl)c1F |r| Show InChI InChI=1S/C27H42ClFN4O4/c1-30-17-21(16-19-8-4-3-5-9-19)32-26(34)33-14-7-10-20(18-33)25(37-15-13-31-27(35)36-2)22-11-6-12-23(28)24(22)29/h6,11-12,19-21,25,30H,3-5,7-10,13-18H2,1-2H3,(H,31,35)(H,32,34)/t20-,21+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-activated human recombinant renin |
Bioorg Med Chem Lett 20: 694-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.066
BindingDB Entry DOI: 10.7270/Q24Q7V20 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50305461
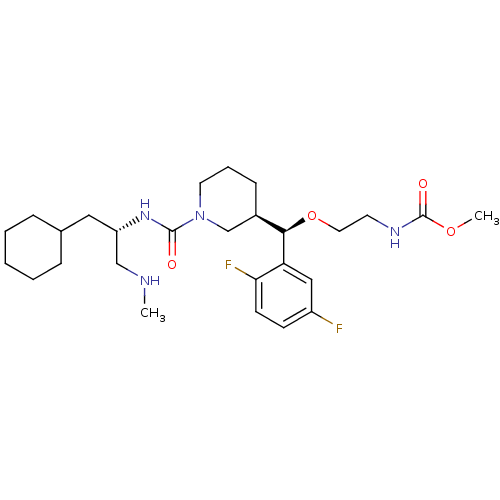
(CHEMBL606238 | methyl 2-((R)-((R)-1-((S)-1-cyclohe...)Show SMILES CNC[C@H](CC1CCCCC1)NC(=O)N1CCC[C@H](C1)[C@@H](OCCNC(=O)OC)c1cc(F)ccc1F |r| Show InChI InChI=1S/C27H42F2N4O4/c1-30-17-22(15-19-7-4-3-5-8-19)32-26(34)33-13-6-9-20(18-33)25(37-14-12-31-27(35)36-2)23-16-21(28)10-11-24(23)29/h10-11,16,19-20,22,25,30H,3-9,12-15,17-18H2,1-2H3,(H,31,35)(H,32,34)/t20-,22+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-activated human recombinant renin |
Bioorg Med Chem Lett 20: 694-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.066
BindingDB Entry DOI: 10.7270/Q24Q7V20 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50305463
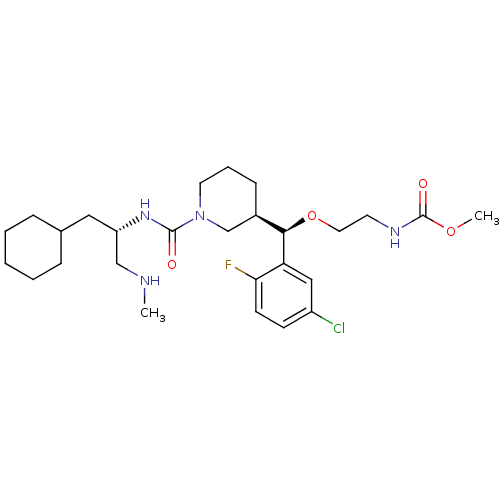
(CHEMBL590388 | methyl 2-((R)-(5-chloro-2-fluorophe...)Show SMILES CNC[C@H](CC1CCCCC1)NC(=O)N1CCC[C@H](C1)[C@@H](OCCNC(=O)OC)c1cc(Cl)ccc1F |r| Show InChI InChI=1S/C27H42ClFN4O4/c1-30-17-22(15-19-7-4-3-5-8-19)32-26(34)33-13-6-9-20(18-33)25(37-14-12-31-27(35)36-2)23-16-21(28)10-11-24(23)29/h10-11,16,19-20,22,25,30H,3-9,12-15,17-18H2,1-2H3,(H,31,35)(H,32,34)/t20-,22+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-activated human recombinant renin |
Bioorg Med Chem Lett 20: 694-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.066
BindingDB Entry DOI: 10.7270/Q24Q7V20 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM17950

((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...)Show SMILES COCCCOc1cc(C[C@@H](C[C@H](N)[C@@H](O)C[C@@H](C(C)C)C(=O)NCC(C)(C)C(N)=O)C(C)C)ccc1OC |r| Show InChI InChI=1S/C30H53N3O6/c1-19(2)22(14-21-10-11-26(38-8)27(15-21)39-13-9-12-37-7)16-24(31)25(34)17-23(20(3)4)28(35)33-18-30(5,6)29(32)36/h10-11,15,19-20,22-25,34H,9,12-14,16-18,31H2,1-8H3,(H2,32,36)(H,33,35)/t22-,23-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of renin in human plasma assessed as formation of angiotensin1 product after 90 mins by competitive radioimmunoassay |
ACS Med Chem Lett 2: 747-751 (2011)
Article DOI: 10.1021/ml200137x
BindingDB Entry DOI: 10.7270/Q2FT8N2Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM17950

((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...)Show SMILES COCCCOc1cc(C[C@@H](C[C@H](N)[C@@H](O)C[C@@H](C(C)C)C(=O)NCC(C)(C)C(N)=O)C(C)C)ccc1OC |r| Show InChI InChI=1S/C30H53N3O6/c1-19(2)22(14-21-10-11-26(38-8)27(15-21)39-13-9-12-37-7)16-24(31)25(34)17-23(20(3)4)28(35)33-18-30(5,6)29(32)36/h10-11,15,19-20,22-25,34H,9,12-14,16-18,31H2,1-8H3,(H2,32,36)(H,33,35)/t22-,23-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin assessed as decrease in plasma renin activity by competitive radioimmunoassay in presence of human plasma |
Bioorg Med Chem Lett 20: 694-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.066
BindingDB Entry DOI: 10.7270/Q24Q7V20 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50350915
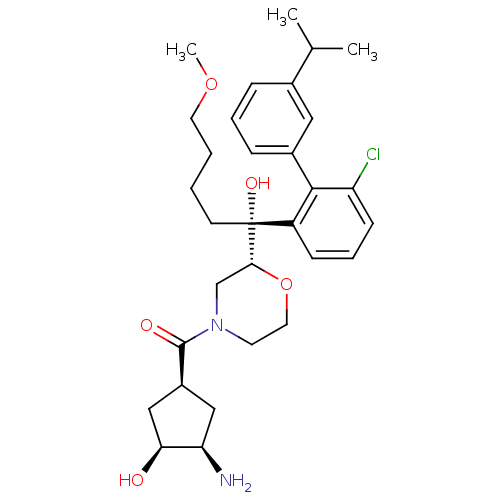
(CHEMBL1818070)Show SMILES COCCCC[C@](O)([C@H]1CN(CCO1)C(=O)[C@H]1C[C@@H](N)[C@@H](O)C1)c1cccc(Cl)c1-c1cccc(c1)C(C)C |r| Show InChI InChI=1S/C31H43ClN2O5/c1-20(2)21-8-6-9-22(16-21)29-24(10-7-11-25(29)32)31(37,12-4-5-14-38-3)28-19-34(13-15-39-28)30(36)23-17-26(33)27(35)18-23/h6-11,16,20,23,26-28,35,37H,4-5,12-15,17-19,33H2,1-3H3/t23-,26+,27-,28+,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
USA.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human renin using of DABCYL-c-Abu-IleHis-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate after 60 to 360 mins by fluores... |
Bioorg Med Chem Lett 21: 4836-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.043
BindingDB Entry DOI: 10.7270/Q25M663G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM29957

(piperidine-1-carboxamide, 21t)Show SMILES [H][C@]1(CCCN(C1)C(=O)N[C@H](CNC)CC1CCCCC1)[C@@](O)(CCCCOC)c1cccc(Cl)c1F |r| Show InChI InChI=1S/C28H45ClFN3O3/c1-31-19-23(18-21-10-4-3-5-11-21)32-27(34)33-16-9-12-22(20-33)28(35,15-6-7-17-36-2)24-13-8-14-25(29)26(24)30/h8,13-14,21-23,31,35H,3-7,9-12,15-20H2,1-2H3,(H,32,34)/t22-,23+,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin using DABCYL-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate for 60 mins by fluorimetry |
ACS Med Chem Lett 2: 747-751 (2011)
Article DOI: 10.1021/ml200137x
BindingDB Entry DOI: 10.7270/Q2FT8N2Q |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50305452

(CHEMBL591578 | methyl 2-((R)-(3-chlorophenyl)((R)-...)Show SMILES CNC[C@H](CC1CCCCC1)NC(=O)N1CCC[C@H](C1)[C@@H](OCCNC(=O)OC)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C27H43ClN4O4/c1-29-18-24(16-20-8-4-3-5-9-20)31-26(33)32-14-7-11-22(19-32)25(21-10-6-12-23(28)17-21)36-15-13-30-27(34)35-2/h6,10,12,17,20,22,24-25,29H,3-5,7-9,11,13-16,18-19H2,1-2H3,(H,30,34)(H,31,33)/t22-,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin assessed as decrease in plasma renin activity by competitive radioimmunoassay in presence of human plasma |
Bioorg Med Chem Lett 20: 694-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.066
BindingDB Entry DOI: 10.7270/Q24Q7V20 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50305452

(CHEMBL591578 | methyl 2-((R)-(3-chlorophenyl)((R)-...)Show SMILES CNC[C@H](CC1CCCCC1)NC(=O)N1CCC[C@H](C1)[C@@H](OCCNC(=O)OC)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C27H43ClN4O4/c1-29-18-24(16-20-8-4-3-5-9-20)31-26(33)32-14-7-11-22(19-32)25(21-10-6-12-23(28)17-21)36-15-13-30-27(34)35-2/h6,10,12,17,20,22,24-25,29H,3-5,7-9,11,13-16,18-19H2,1-2H3,(H,30,34)(H,31,33)/t22-,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of renin in human plasma assessed as formation of angiotensin1 product after 90 mins by competitive radioimmunoassay |
ACS Med Chem Lett 2: 747-751 (2011)
Article DOI: 10.1021/ml200137x
BindingDB Entry DOI: 10.7270/Q2FT8N2Q |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50305458
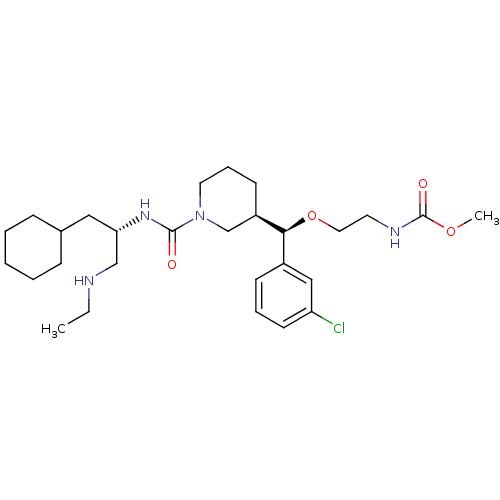
(CHEMBL591105 | methyl 2-((R)-(3-chlorophenyl)((R)-...)Show SMILES CCNC[C@H](CC1CCCCC1)NC(=O)N1CCC[C@H](C1)[C@@H](OCCNC(=O)OC)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C28H45ClN4O4/c1-3-30-19-25(17-21-9-5-4-6-10-21)32-27(34)33-15-8-12-23(20-33)26(22-11-7-13-24(29)18-22)37-16-14-31-28(35)36-2/h7,11,13,18,21,23,25-26,30H,3-6,8-10,12,14-17,19-20H2,1-2H3,(H,31,35)(H,32,34)/t23-,25+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-activated human recombinant renin |
Bioorg Med Chem Lett 20: 694-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.066
BindingDB Entry DOI: 10.7270/Q24Q7V20 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM29959
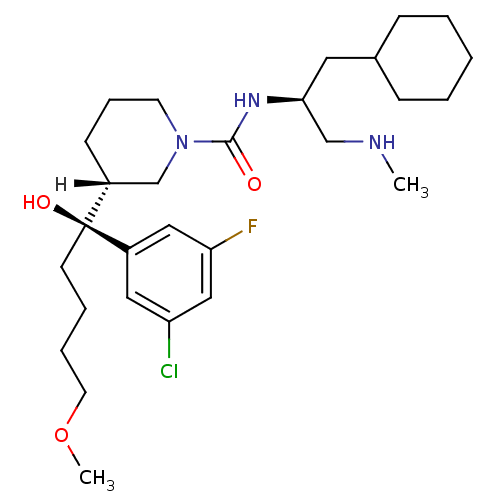
(piperidine-1-carboxamide, 21v)Show SMILES [H][C@]1(CCCN(C1)C(=O)N[C@H](CNC)CC1CCCCC1)[C@@](O)(CCCCOC)c1cc(F)cc(Cl)c1 |r| Show InChI InChI=1S/C28H45ClFN3O3/c1-31-19-26(15-21-9-4-3-5-10-21)32-27(34)33-13-8-11-22(20-33)28(35,12-6-7-14-36-2)23-16-24(29)18-25(30)17-23/h16-18,21-22,26,31,35H,3-15,19-20H2,1-2H3,(H,32,34)/t22-,26+,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | 25 |
Vitae Pharmaceuticals
| Assay Description
The activity of renin inhibitors in vitro was measured using the FRET assay, which was carried out in flat-bottom white opaque microtiter plates. The... |
Bioorg Med Chem Lett 19: 3541-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.140
BindingDB Entry DOI: 10.7270/Q2D21VXV |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50042863
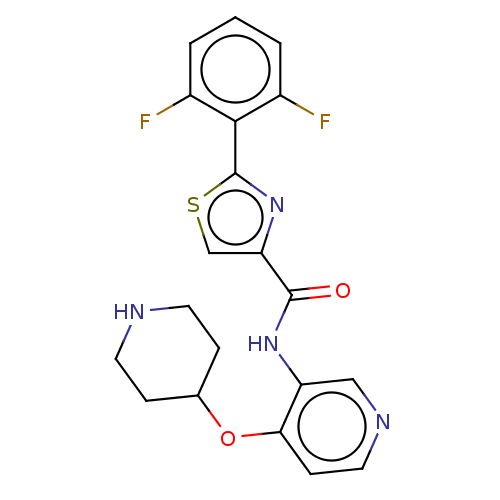
(CHEMBL3354513)Show SMILES Fc1cccc(F)c1-c1nc(cs1)C(=O)Nc1cnccc1OC1CCNCC1 Show InChI InChI=1S/C20H18F2N4O2S/c21-13-2-1-3-14(22)18(13)20-26-16(11-29-20)19(27)25-15-10-24-9-6-17(15)28-12-4-7-23-8-5-12/h1-3,6,9-12,23H,4-5,7-8H2,(H,25,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) |
Bioorg Med Chem Lett 25: 474-80 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.041
BindingDB Entry DOI: 10.7270/Q2VQ34BW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50042871
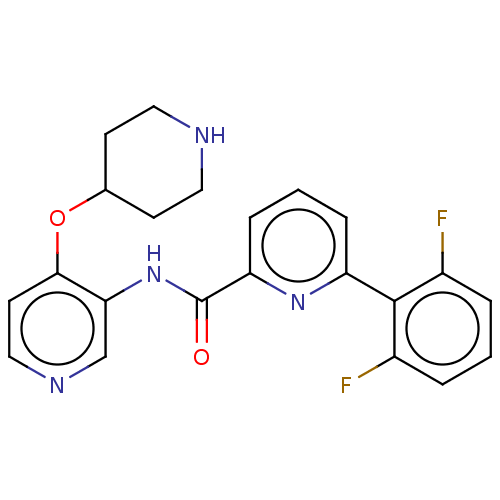
(CHEMBL3354521)Show SMILES Fc1cccc(F)c1-c1cccc(n1)C(=O)Nc1cnccc1OC1CCNCC1 Show InChI InChI=1S/C22H20F2N4O2/c23-15-3-1-4-16(24)21(15)17-5-2-6-18(27-17)22(29)28-19-13-26-12-9-20(19)30-14-7-10-25-11-8-14/h1-6,9,12-14,25H,7-8,10-11H2,(H,28,29) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PIM3 (unknown origin) |
Bioorg Med Chem Lett 25: 474-80 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.041
BindingDB Entry DOI: 10.7270/Q2VQ34BW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50042868
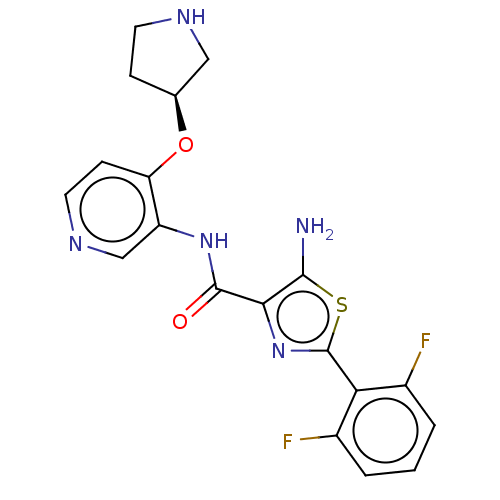
(CHEMBL3354518)Show SMILES Nc1sc(nc1C(=O)Nc1cnccc1O[C@H]1CCNC1)-c1c(F)cccc1F |r| Show InChI InChI=1S/C19H17F2N5O2S/c20-11-2-1-3-12(21)15(11)19-26-16(17(22)29-19)18(27)25-13-9-24-7-5-14(13)28-10-4-6-23-8-10/h1-3,5,7,9-10,23H,4,6,8,22H2,(H,25,27)/t10-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PIM3 (unknown origin) |
Bioorg Med Chem Lett 25: 474-80 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.041
BindingDB Entry DOI: 10.7270/Q2VQ34BW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50042863

(CHEMBL3354513)Show SMILES Fc1cccc(F)c1-c1nc(cs1)C(=O)Nc1cnccc1OC1CCNCC1 Show InChI InChI=1S/C20H18F2N4O2S/c21-13-2-1-3-14(22)18(13)20-26-16(11-29-20)19(27)25-15-10-24-9-6-17(15)28-12-4-7-23-8-5-12/h1-3,6,9-12,23H,4-5,7-8H2,(H,25,27) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PIM3 (unknown origin) |
Bioorg Med Chem Lett 25: 474-80 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.041
BindingDB Entry DOI: 10.7270/Q2VQ34BW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-2
(Homo sapiens (Human)) | BDBM50042868

(CHEMBL3354518)Show SMILES Nc1sc(nc1C(=O)Nc1cnccc1O[C@H]1CCNC1)-c1c(F)cccc1F |r| Show InChI InChI=1S/C19H17F2N5O2S/c20-11-2-1-3-12(21)15(11)19-26-16(17(22)29-19)18(27)25-13-9-24-7-5-14(13)28-10-4-6-23-8-10/h1-3,5,7,9-10,23H,4,6,8,22H2,(H,25,27)/t10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PIM2 (unknown origin) |
Bioorg Med Chem Lett 25: 474-80 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.041
BindingDB Entry DOI: 10.7270/Q2VQ34BW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50042868

(CHEMBL3354518)Show SMILES Nc1sc(nc1C(=O)Nc1cnccc1O[C@H]1CCNC1)-c1c(F)cccc1F |r| Show InChI InChI=1S/C19H17F2N5O2S/c20-11-2-1-3-12(21)15(11)19-26-16(17(22)29-19)18(27)25-13-9-24-7-5-14(13)28-10-4-6-23-8-10/h1-3,5,7,9-10,23H,4,6,8,22H2,(H,25,27)/t10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) |
Bioorg Med Chem Lett 25: 474-80 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.041
BindingDB Entry DOI: 10.7270/Q2VQ34BW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50042867
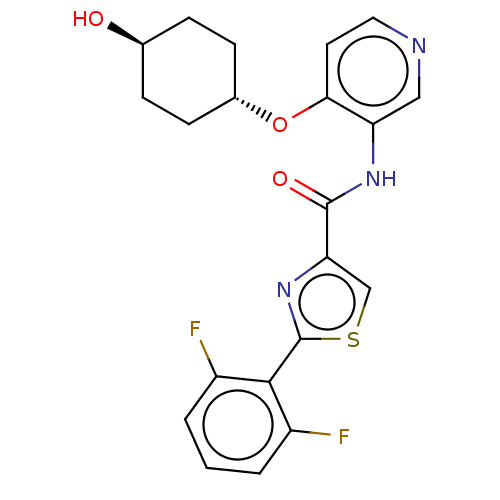
(CHEMBL3354517)Show SMILES O[C@H]1CC[C@@H](CC1)Oc1ccncc1NC(=O)c1csc(n1)-c1c(F)cccc1F |r,wU:4.7,wD:1.0,(22.36,-34.59,;21.11,-33.68,;21.28,-32.14,;20.03,-31.23,;18.63,-31.87,;18.46,-33.4,;19.7,-34.3,;17.39,-30.97,;17.55,-29.44,;18.95,-28.81,;19.11,-27.27,;17.85,-26.37,;16.45,-27.01,;16.3,-28.53,;14.9,-29.16,;13.65,-28.26,;13.8,-26.73,;12.24,-28.89,;10.91,-28.13,;9.77,-29.16,;10.4,-30.57,;11.93,-30.4,;9.63,-31.9,;10.41,-33.23,;11.95,-33.23,;9.64,-34.58,;8.09,-34.58,;7.32,-33.24,;8.1,-31.91,;7.33,-30.57,)| Show InChI InChI=1S/C21H19F2N3O3S/c22-14-2-1-3-15(23)19(14)21-26-17(11-30-21)20(28)25-16-10-24-9-8-18(16)29-13-6-4-12(27)5-7-13/h1-3,8-13,27H,4-7H2,(H,25,28)/t12-,13- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) |
Bioorg Med Chem Lett 25: 474-80 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.041
BindingDB Entry DOI: 10.7270/Q2VQ34BW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50042866
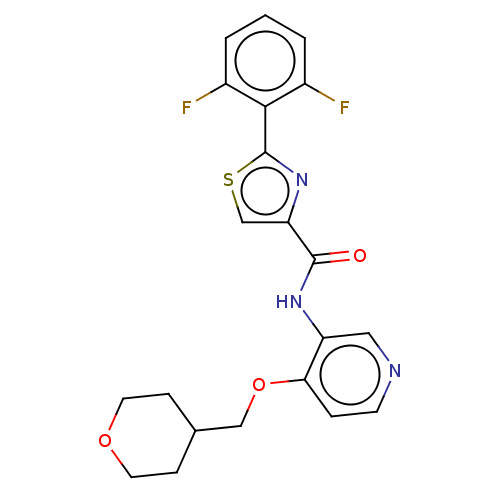
(CHEMBL3354516)Show SMILES Fc1cccc(F)c1-c1nc(cs1)C(=O)Nc1cnccc1OCC1CCOCC1 Show InChI InChI=1S/C21H19F2N3O3S/c22-14-2-1-3-15(23)19(14)21-26-17(12-30-21)20(27)25-16-10-24-7-4-18(16)29-11-13-5-8-28-9-6-13/h1-4,7,10,12-13H,5-6,8-9,11H2,(H,25,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) |
Bioorg Med Chem Lett 25: 474-80 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.041
BindingDB Entry DOI: 10.7270/Q2VQ34BW |
More data for this
Ligand-Target Pair | |
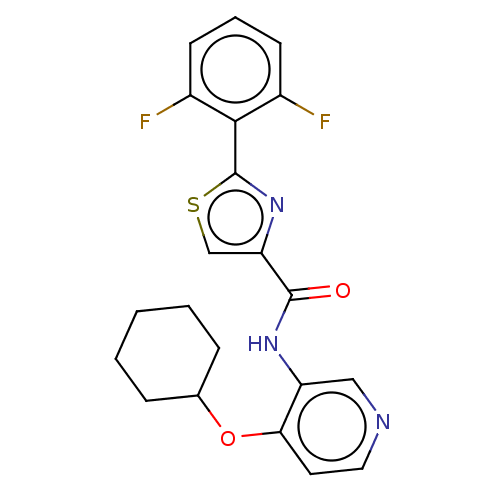
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50042864
(CHEMBL3354514)Show SMILES Fc1cccc(F)c1-c1nc(cs1)C(=O)Nc1cnccc1OC1CCCCC1 Show InChI InChI=1S/C21H19F2N3O2S/c22-14-7-4-8-15(23)19(14)21-26-17(12-29-21)20(27)25-16-11-24-10-9-18(16)28-13-5-2-1-3-6-13/h4,7-13H,1-3,5-6H2,(H,25,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) |
Bioorg Med Chem Lett 25: 474-80 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.041
BindingDB Entry DOI: 10.7270/Q2VQ34BW |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50382333

(CHEMBL2023123)Show SMILES CNC[C@@H](NC(=O)N1CCC[C@H](C1)[C@@H](OCCNC(=O)OC)c1cccc(Cl)c1)[C@@H](O)C1CCCCC1 |r| Show InChI InChI=1S/C27H43ClN4O5/c1-29-17-23(24(33)19-8-4-3-5-9-19)31-26(34)32-14-7-11-21(18-32)25(20-10-6-12-22(28)16-20)37-15-13-30-27(35)36-2/h6,10,12,16,19,21,23-25,29,33H,3-5,7-9,11,13-15,17-18H2,1-2H3,(H,30,35)(H,31,34)/t21-,23-,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.06 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of renin in human plasma assessed as formation of angiotensin1 product after 90 mins by competitive radioimmunoassay |
ACS Med Chem Lett 2: 747-751 (2011)
Article DOI: 10.1021/ml200137x
BindingDB Entry DOI: 10.7270/Q2FT8N2Q |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50350916
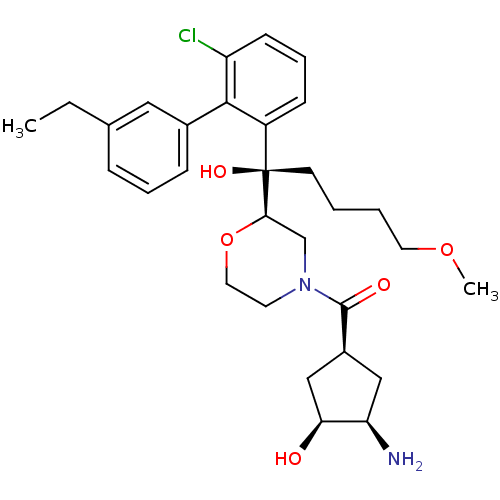
(CHEMBL1818069)Show SMILES CCc1cccc(c1)-c1c(Cl)cccc1[C@](O)(CCCCOC)[C@H]1CN(CCO1)C(=O)[C@H]1C[C@@H](N)[C@@H](O)C1 |r| Show InChI InChI=1S/C30H41ClN2O5/c1-3-20-8-6-9-21(16-20)28-23(10-7-11-24(28)31)30(36,12-4-5-14-37-2)27-19-33(13-15-38-27)29(35)22-17-25(32)26(34)18-22/h6-11,16,22,25-27,34,36H,3-5,12-15,17-19,32H2,1-2H3/t22-,25+,26-,27+,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
USA.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human renin using of DABCYL-c-Abu-IleHis-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate after 60 to 360 mins by fluores... |
Bioorg Med Chem Lett 21: 4836-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.043
BindingDB Entry DOI: 10.7270/Q25M663G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50382334

(CHEMBL1276678)Show SMILES CN[C@H](CNC(=O)N1CCC[C@H](C1)[C@@H](OCCNC(=O)OC)c1cccc(Cl)c1)C[C@H]1CCCOC1 |r| Show InChI InChI=1S/C26H41ClN4O5/c1-28-23(14-19-6-5-12-35-18-19)16-30-25(32)31-11-4-8-21(17-31)24(20-7-3-9-22(27)15-20)36-13-10-29-26(33)34-2/h3,7,9,15,19,21,23-24,28H,4-6,8,10-14,16-18H2,1-2H3,(H,29,33)(H,30,32)/t19-,21-,23+,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of renin in human plasma assessed as formation of angiotensin1 product after 90 mins by competitive radioimmunoassay |
ACS Med Chem Lett 2: 747-751 (2011)
Article DOI: 10.1021/ml200137x
BindingDB Entry DOI: 10.7270/Q2FT8N2Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data